Abstract
Background and Aims:
Preoperative anxiety in children leading to postoperative negative changes and long-term behavioral problems needs better preanesthetic sedation. Across the world, midazolam is the most commonly used premedicant in pediatric patients. The fact that no single route has achieved universal acceptance for its administration suggests that each route has its own merits and demerits. This study compares oral midazolam syrup and intranasal midazolam spray as painless and needleless systems of drug administration for preanesthetic sedation in children.
Material and Methods:
With randomization, Group O (30 children): Received oral midazolam syrup 0.5 mg/kg and Group IN (30 children): Received intranasal midazolam spray 0.2 mg/kg. Every child was observed for acceptance of drug, response to drug administration, sedation scale, separation score, acceptance to mask, recovery score and side effects of drug. Data were analyzed using Student's t-test, standard error of the difference between two means and Chi-square test.
Results:
In Group O and IN, 15/30 children (50%) and 7/30 children (23%) accepted drug easily (P < 0.05); 4/22 children (18%) in Group O and 11/20 children (55%) in Group IN cried after drug administration (P < 0.05). In both the groups, sedation at 20 min after premedication (Group O [80%] 24/30 vs. Group IN [77%] 23/30), parental separation and acceptance to mask were comparable (P > 0.05); 12/30 children (40%) in Group IN showed transient nasal irritation.
Conclusion:
Oral midazolam and intranasal midazolam spray produce similar anxiolysis and sedation, but acceptance of drug and response to drug administration is better with oral route.
Key words: Children, intranasal spray, midazolam, oral, preanesthetic sedation
Introduction
In preschool children, preoperative anxiety is likely to predispose to emergence delirium, sleep disturbances and behavioral changes postoperatively. To prevent preoperative anxiety of parental separation, pharmacological intervention by adequate pediatric sedation before induction of anesthesia was found to be more effective than behavioral intervention.[1,2,3] Midazolam, an ideal anxiolytic removes fear and anxiety in children; and makes child calm and sedated for smooth induction of anesthesia and rapid recovery in postoperative period.[4] Though it is administered by intravenous, intramuscular, rectal, sublingual, oral, and nasal routes, oral and intranasal routes are preferred for pediatric sedation.[5,6,7,8]
Earlier studies on midazolam by oral and intranasal routes used parenteral preparation of the drug. For intranasal route, it was used in the form of drops. Their results showed equal efficacy oral and intranasal midazolam or superiority of intranasal midazolam.[7,9,10] Bitter taste of parenteral form of midazolam, a limitation factor and cause for rejection and low compliance by oral route, is overcome by giving midazolam in syrup form.[10,11] For intranasal route, the problem of volume retention in nasal cavity and effective dose availability is solved using concentrated, atomized nasal midazolam spray to have slow and uniform spraying of drug.[12,13] However, comparative studies of these two forms are meager.
We used oral midazolam syrup and intranasal midazolam spray as a needleless, painless, user friendly system of drug administration and aimed to compare the efficacy of midazolam for preanesthetic sedation in pediatric patients. We compared effects of oral midazolam syrup and intranasal midazolam spray on sedation and anxiety of child before induction of anesthesia. Hypothesis put forward was that, there would not be difference in sedation level, anxiety level, acceptance of drug, drug response, hemodynamic changes, recovery, and complications when oral midazolam syrup or intranasal midazolam spray was used for pediatric sedation.
Material and Methods
After obtaining approval from Hospital Ethics Committee, a pilot study was conducted on 10 children from each group (oral and intranasal) to determine sample size. Sample size was determined with the help of software based on WHO publication “Sample size determination in Health Studies” by Lwanga and Lameshow.[14] Outcome parameter considered was satisfactory sedation score at 20 min. Six out of 10 children in oral group and 9 of 10 children in intranasal group developed satisfactory sedation score of 3 of more at 20 min of administration. A sample size of 18 for each group was adequate to allow power of 80 to detect a difference of 15 between the groups. To generalize the results, we selected a larger size of 30 children in each group.
This prospective, randomized, clinical study was carried out at a tertiary health care hospital during Sept 2011 to April 2012. Sixty American Society of Anesthesiologists physical status I children of 1-5 years age group, weighing 8-18 kg scheduled for routine surgeries lasting for <75 min were included in this study. Preanesthetic evaluation was done and patients having upper gastrointestinal tract pathology, nasal infection or any nasal pathology, cyanotic spells, allergy to study drug, taking any other sedative or anti-convulsant medicine were excluded as response to drug may be altered. Investigations like hemoglobin concentration, blood cell count, and urine examination were carried out in all the patients. Blood urea and serum creatinine levels were obtained in patients who were posted for genitourinary surgical procedures. Written informed consent was obtained from the child's parents. To avoid selection bias, odd number children received intranasal midazolam spray and even number children received oral midazolam syrup.
All the patients were fasted for 4 h for breast milk and 6 h for nonhuman milk or light meal.[15] No child received any form of sedation before arrival in the operating room. Baseline sedation score was noted using 5-point scale.[16] After recording baseline pulse rate, respiratory rate, blood pressure and arterial oxygen saturation, Group O received oral midazolam syrup 0.5 mg/kg (2 mg/ml); Group IN received intranasal midazolam spray 0.2 mg/kg with half the dose administered in each nostril (5 mg/ml, each spray delivered 0.1 ml or 0.5 mg). The premedication was given either by the attending anesthesiologist or one of the parents using measuring cap or atomized nasal spray. While receiving oral midazolam or intranasal midazolam spray, the child sat facing forward on the parent's lap while their arms were gently restrained by one parental hand and the other hand used to tilt the forehead back 15°.
Acceptance of drug was noted as poor (refused to accept medication) - Score 1, moderate (accepted medication with difficulty) - Score 2, or good (accepted medication without complaint) - Score 3.[17] Children who were crying before drug administration were excluded from the observation of response to drug administration. Response to drug administration was noted as yes or no, depending on whether the child started crying after administration of drug.
The child was observed in the preoperative room for 20 min and state of sedation, and vital parameters were recorded at 5 min interval. Sedation score was labeled as 1, 2, 3, 4 or 5 by observing the sedation status of child as agitated, alert, calm, drowsy or asleep respectively. Sedation Score 1 and 2 were considered as unsatisfactory while sedation Score 3, 4, and 5 were considered as satisfactory.[16] Complications such as nausea, vomiting, nasal irritation, hypoxia and hypertension were noted in both the groups and treated accordingly.
20 min after drug administration the child was separated from the parent and ease of separation noted as excellent, good, fair and poor. Separation Score 1 (patient un-afraid, co-operative) and Score 2 (slight fear or crying, quiet with assurance) were considered as satisfactory or acceptable and Score 3 (moderate fear, crying not quiet with assurance) and Score 4 (crying, need for restraint) were considered as unsatisfactory or said to have a difficult separation.[18]
In the operation theater, to set up an intravenous line, gaseous induction was done uniformly with oxygen and sevoflurane (4-8%) mask. Acceptance to mask or response to gaseous induction was noted using 3-point criteria. The quality of anesthetic induction was evaluated as excellent, good or poor and score given was 1, 2, or 3. Score 1 and 2 were considered as satisfactory and score 3 was considered as unsatisfactory acceptance of mask.[17] In all patients, surgery was carried out under general anesthesia with endotracheal intubation. Vital parameters (pulse rate, blood pressure, SpO2) were recorded during intraoperative period. After extubation, patient was shifted to recovery room.
In the recovery room, vital signs (pulse rate, respiratory rate, blood pressure, SpO2) were monitored until the child was fully awake. Using a 10-point recovery room score (which included respiration, activity, consciousness, temperature and circulation, each on a scale of 0-2, to give a maximum cumulative total of 10,[19]) recovery assessment was done at 10 min intervals for 30 min from the time of extubation. Recovery score of 8 or more considered satisfactory and time taken for it was noted.[20] In postoperative period, the children were followed up for 24 hours to observe nausea, vomiting, nasal irritation, ulceration, etc.
All data are presented as mean ± standard deviation, and/or number. Demographic data were analyzed by t-test and Chi-square test. Acceptance of drug, response to drug administration, sedation scale, parental separation and response to gaseous induction was analyzed by Chi-square test. Hemodynamic changes and anesthesia recovery was assessed by standard error of difference between two means and t-test. P < 0.05 was considered statistically significant.
Results
In demographic data, age, weight and duration of anesthesia were comparable (P > 0.05) in both the groups [Table 1]. Male children outnumbered female children (43 vs. 17) in this study.
Table 1.
Demographic data
In both the groups, crying children except 1 child in Group IN were settled down at 15 min of drug administration.
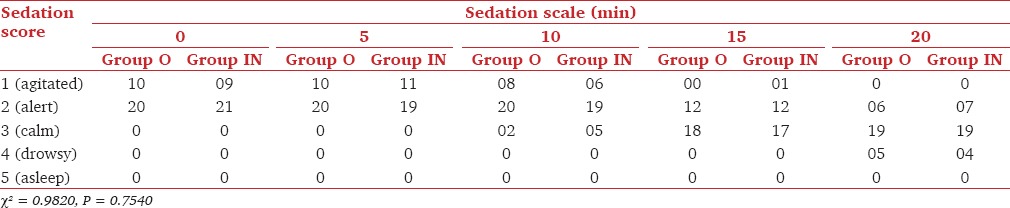
Baseline sedation score was comparable in both the groups and it ranged in between 1 and 2. At 20 min after premedication, 24/30 (80%) patients in Group O and 23/30 (77%) patients in Group IN had showed satisfactory sedation that is, sedation score of 3 or more [Table 2].
Table 2.
Number of children at different time interval of midazolam sedation
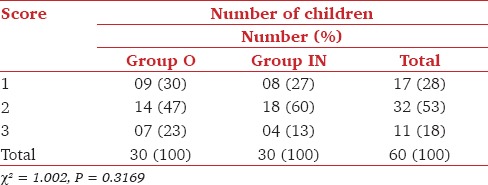
Totally, 23/30 (77%) patients in Group O and 22/30 (73%) patients in Group IN showed acceptable parental separation (Score 1, 2) and 23/30 (77%) patients in Group O while 26/30 (87%) patients in Group IN showed satisfactory acceptance to mask (Score 1, 2) (P > 0.05) [Table 3 and 4].
Table 3.
Separation score at 20 min of midazolam sedation
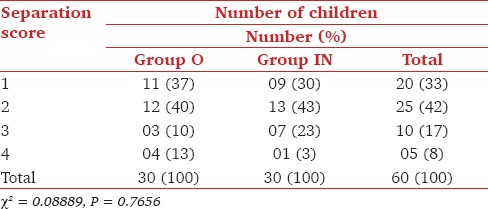
Table 4.
Comparison of acceptance to mask
There were no significant differences in baseline vital parameters. Changes that occurred in vital parameters after sedation, during intraoperative period and postoperative period were statistically nonsignificant (P > 0.05). Recovery score at 10 min, 20 min, and 30 min was similar in both the groups and all children in both the groups attained score of 10 at 30 min postoperative period.
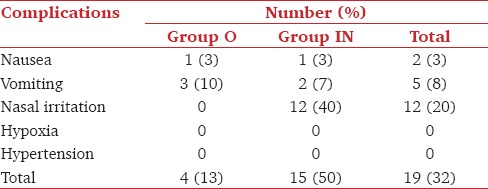
Transient nasal irritation in the form of rubbing of the nose, watering, sneezing and lacrimation was observed in 12/30 (40%) patients of Group IN. There was no redness or ulceration observed in postoperative period [Table 5].
Table 5.
Incidence of complications
Discussion
Oral midazolam in the dose of 0.5 mg/kg is a safe and effective mode of premedication than that of 0.75 mg/kg and 1 mg/kg which gives no additional benefit, may cause more side effects.[18] Nasal administration of midazolam may be in the form of drops, nasal spray or nebulization.[12,13,16,21] Intranasal midazolam has been used in the doses of 0.2, and 0.3 mg/kg, but found no additional benefits from higher dosage and recommended the lower dose of midazolam 0.2 mg/kg.[16,22] Similarly in other studies, intranasal midazolam spray was used in the dose of 0.2 mg/kg[23] while a mucosal atomizer device was used to administer midazolam intranasally in the dose of 0.4 mg/kg.[13] Concentrated, atomized midazolam spray ensures accurate drug delivery, covers larger nasal mucosal area and increases bioavailability maximally.[12] We used oral midazolam syrup in the dose of 0.5 mg/kg and intranasal midazolam spray in the dose of 0.2 mg/kg of body weight.
We observed good acceptance and better response to drug administration in Group O than in Group IN. In similar comparative study, while accepting the drug, less anxiety was observed in oral midazolam group than in intranasal midazolam group as 44% children in oral group were quiet. Nasal midazolam was also found to be more irritating than rectal and sublingual route.[7] Children were even distressed by instillation of normal saline into nasal cavity though they were settled very rapidly in presence of their parents.[16]
Earlier study has stated that child can be separated as early as 10 min after oral midazolam (0.5 mg/kg).[24] Significant anxiolytic effects of oral midazolam were observed at 15 ± 4 min of its administration.[25] With intranasal midazolam, significant changes in sedation were found as early at 5 min[16] and separation from parents was said to be possible at 10 min. Demonstrable high plasma concentration was at 14 ± 5 min.[12] Others found maximum sedation and anxiolysis at 20 min in intranasal group while at 30 min for the oral group.[7] To avoid bias and incongruence with the earlier studies, we observed children for sedation level for 20 min and then separated them from their parents.
Earlier assessors used a 3-, 4- or 5-point sedation scales to assign sedation score.[8,16,18] In our study, 5-point sedation scale was used to measure sedation. Satisfactory sedation score at 20 min of oral or intranasal midazolam premedication was comparable. The sedation status in our study was comparable with observed 91% and 85% sedation of earlier studies.[23,25]
The most important criterion of a satisfactory premedicant for preschool children is its ability to facilitate the separation of child from parents. Other studies reported, 78%, 89%, 70% satisfactory separation with 0.5 mg/kg oral midazolam and 91%, 80% with intranasal midazolam.[22,24,25,26] In our study, comparable satisfactory acceptance to mask was observed. In earlier studies, similar response was there to oral midazolam (80-90%)[18] and to intranasal midazolam (60%, 80%).[22,27] Reduced anxiety and similar acceptance to mask (75%) with either oral or intranasal midazolam has also been noted.[7,10]
Monitoring throughout the period of drug action is often necessary. Postsedation vitals, intraoperative vitals and duration of surgery were comparable in both the groups. While studying effects of oral and intranasal midazolam, investigators observed that vital signs remained stable with medication before and after surgery.[7,9] Others found stable vitals during study on oral midazolam.[18]
Detectable preoperative sedation is predictive of delayed emergence and midazolam premedication does not affect intraoperative BIS, emergence times or recovery times.[28] In our study, recovery score for oral versus intranasal midazolam at 10 min (8.8 ± 1.0 vs. 8.5 ± 1.1), 20 min (9.6 ± 0.6 vs. 9.2 ± 0.8), and 30 min (10 ± 0 vs. 10 ± 0) was comparable (P > 0.05). In one study on intranasal midazolam, recovery assessment using 10-point recovery room score at 10 min interval for 30 min found score 6 at 10 min, score 9 at 20 min and score 9 at 30 min.[16] Another study observed recovery score of 7.2 ± 0.4 (10 min), 8.9 ± 0.8 (20 min) and 10 ± 0 (30 min).[22]
In our study, nasal irritation was observed in patients of Group IN. Nasal side effects have been reported differently in intranasal midazolam studies such as nasal irritation (20/31), nasal discomfort 17/38 (45%), intense burning.[22,29] One study observed nasal irritation in up to 77% of patient.[7] We observed low incidence of nausea and vomiting as in other studies.[16] Hypoxia and hypertension were not observed in any of the groups of present study.
Acceptability of nasal drops as well as nasal spray is poor and is not recommended in children. The recall of horrible, unpleasant response could make the child more fearful of another visit to hospital.[30] Four percent topical lignocaine spray[29] and formulation in aqueous cyclodextrin buffer solution have been tried to reduce the nasal irritation due to acidic midazolam.[31]
Lack of blinding could be a cause for bias in our study.
Thus, we conclude that, oral midazolam and intranasal midazolam spray produce similar sedation and anxiolysis in preschool children, but acceptance of drug and response to drug administration is better in oral route. Nasal irritation is the issue of concern in case of intranasal midazolam use, which warrants future studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–54. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 2.Kain ZN, Mayes LC, O'Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150:1238–45. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 3.Kain ZN, Mayes LC, Wang SM, Caramico LA, Hofstadter MB. Parental presence during induction of anesthesia versus sedative premedication: Which intervention is more effective? Anesthesiology. 1998;89:1147–56. doi: 10.1097/00000542-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Madej TH, Paasuke RT. Anaesthetic premedication: Aims, assessment and methods. Can J Anaesth. 1987;34:259–73. doi: 10.1007/BF03015163. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman E, Davidson E, Sheinkman Z, Magora F. Comparison between intranasal and intravenous midazolam sedation (with or without patient control) in a dental phobia clinic. J Oral Maxillofac Surg. 1994;52:840–3. doi: 10.1016/0278-2391(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 6.Rita L, Seleny FL, Mazurek A, Rabins SY. Intramuscular midazolam for pediatric preanesthetic sedation: A double-blind controlled study with morphine. Anesthesiology. 1985;63:528–31. doi: 10.1097/00000542-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: A comparison of four routes of administration. Paediatr Anaesth. 2002;12:685–9. doi: 10.1046/j.1460-9592.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 8.McCann ME, Kain ZN. The management of preoperative anxiety in children: An update. Anesth Analg. 2001;93:98–105. doi: 10.1097/00000539-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Lee-Kim SJ, Fadavi S, Punwani I, Koerber A. Nasal versus oral midazolam sedation for pediatric dental patients. J Dent Child (Chic) 2004;71:126–30. [PubMed] [Google Scholar]
- 10.Connors K, Terndrup TE. Nasal versus oral midazolam for sedation of anxious children undergoing laceration repair. Ann Emerg Med. 1994;24:1074–9. doi: 10.1016/s0196-0644(94)70236-5. [DOI] [PubMed] [Google Scholar]
- 11.Coté CJ. Preoperative preparation and premedication. Br J Anaesth. 1999;83:16–28. doi: 10.1093/bja/83.1.16. [DOI] [PubMed] [Google Scholar]
- 12.Knoester PD, Jonker DM, van der Hoven RT, Vermeij TA, Edelbroek PM, Brekelmans GJ, et al. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Clin Pharmacol. 2002;53:501–7. doi: 10.1046/j.1365-2125.2002.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane RD, Schunk JE. Atomized intranasal midazolam use for minor procedures in the pediatric emergency department. Pediatr Emerg Care. 2008;24:300–3. doi: 10.1097/PEC.0b013e31816ecb6f. [DOI] [PubMed] [Google Scholar]
- 14.Lwanga SK, Lameshow S. World Health Organization. Geneva, Switzerland: WHO Library Cataloguing in Publication Data; 1991. Sample Size Determination in Health Studies — A Practical Manual. [Google Scholar]
- 15.American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495–511. doi: 10.1097/ALN.0b013e3181fcbfd9. [DOI] [PubMed] [Google Scholar]
- 16.Wilton NC, Leigh J, Rosen DR, Pandit UA. Preanesthetic sedation of preschool children using intranasal midazolam. Anesthesiology. 1988;69:972–5. doi: 10.1097/00000542-198812000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Parnis SJ, Foate JA, van der Walt JH, Short T, Crowe CE. Oral midazolam is an effective premedication for children having day-stay anaesthesia. Anaesth Intensive Care. 1992;20:9–14. doi: 10.1177/0310057X9202000102. [DOI] [PubMed] [Google Scholar]
- 18.McMillan CO, Spahr-Schopfer IA, Sikich N, Hartley E, Lerman J. Premedication of children with oral midazolam. Can J Anaesth. 1992;39:545–50. doi: 10.1007/BF03008315. [DOI] [PubMed] [Google Scholar]
- 19.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 20.Viitanen H, Annila P, Viitanen M, Tarkkila P. Premedication with midazolam delays recovery after ambulatory sevoflurane anesthesia in children. Anesth Analg. 1999;89:75–9. doi: 10.1097/00000539-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 21.McCormick AS, Thomas VL, Berry D, Thomas PW. Plasma concentrations and sedation scores after nebulized and intranasal midazolam in healthy volunteers. Br J Anaesth. 2008;100:631–6. doi: 10.1093/bja/aen072. [DOI] [PubMed] [Google Scholar]
- 22.Bhakta P, Ghosh BR, Roy M, Mukherjee G. Evaluation of intranasal midazolam for preanaesthetic sedation in paediatric patients. Indian J Anaesth. 2007;51:111–6. [Google Scholar]
- 23.Ljungman G, Kreuger A, Andréasson S, Gordh T, Sörensen S. Midazolam nasal spray reduces procedural anxiety in children. Pediatrics. 2000;105:73–8. doi: 10.1542/peds.105.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Levine MF, Spahr-Schopfer IA, Hartley E, Lerman J, MacPherson B. Oral midazolam premedication in children: The minimum time interval for separation from parents. Can J Anaesth. 1993;40:726–9. doi: 10.1007/BF03009769. [DOI] [PubMed] [Google Scholar]
- 25.Kain ZN, Hofstadter MB, Mayes LC, Krivutza DM, Alexander G, Wang SM, et al. Midazolam: Effects on amnesia and anxiety in children. Anesthesiology. 2000;93:676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Feld LH, Negus JB, White PF. Oral midazolam preanesthetic medication in pediatric outpatients. Anesthesiology. 1990;73:831–4. doi: 10.1097/00000542-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Davis PJ, Tome JA, McGowan FX, Jr, Cohen IT, Latta K, Felder H. Preanesthetic medication with intranasal midazolam for brief pediatric surgical procedures. Effect on recovery and hospital discharge times. Anesthesiology. 1995;82:2–5. doi: 10.1097/00000542-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Brosius KK, Bannister CF. Oral midazolam premedication in preadolescents and adolescents. Anesth Analg. 2002;94:31–6. doi: 10.1097/00000539-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Lugo RA, Fishbein M, Nahata MC, Lininger B. Complication of intranasal midazolam. Pediatrics. 1993;92:638. [PubMed] [Google Scholar]
- 30.Griffith N, Howell S, Mason DG. Intranasal midazolam for premedication of children undergoing day-case anaesthesia: Comparison of two delivery systems with assessment of intra-observer variability. Br J Anaesth. 1998;81:865–9. doi: 10.1093/bja/81.6.865. [DOI] [PubMed] [Google Scholar]
- 31.Gudmundsdottir H, Sigurjonsdottir JF, Masson M, Fjalldal O, Stefansson E, Loftsson T. Intranasal administration of midazolam in a cyclodextrin based formulation: Bioavailability and clinical evaluation in humans. Pharmazie. 2001;56:963–6. [PubMed] [Google Scholar]


