Abstract
Using multiple murine foster-nursing protocols, thereby eliminating placental transfer and allowing a distinction between dam- and pup-derived cells, we show that foster nursing by an immunized dam results in development of CD8+ T cells in nonimmunized foster pups that are specific for Ags against which the foster dam was immunized (Mycobacterium tuberculosis or Candida albicans). We have dubbed this process “maternal educational immunity” to distinguish it from passive cellular immunity. Of the variety of maternal immune cells present in milk, only T cells were detected in pup tissues. Maternal T cells, a substantial percentage of which were CD4+MHC class II+, accumulated in the pup thymus and spleen during the nursing period. Further analysis of maternal cells in the pup thymus showed that a proportion was positive for maternal immunogen-specific MHC class II tetramers. To determine the outcome of Ag presentation in the thymus, the maternal or foster pup origin of immunogen-responding CD8+ cells in foster pup spleens was assessed. Whereas ∼10% were maternally derived in the first few weeks after weaning, all immunogen-responding CD8+ T cells were pup derived by 12 wk of age. Pup-derived immunogen-responsive CD8+ cells persisted until at least 1 y of age. Passive cellular immunity is well accepted and has been demonstrated in the human population. In this study, we show an arguably more important role for transferred immune cells: the direction of offspring T cell development. Harnessing maternal educational immunity through prepregnancy immunization programs has potential for improvement of infant immunity.
Introduction
Breast feeding has many benefits, among which are protection against gastrointestinal infection and positive influences on offspring immunity (1, 2). Milk contains a variety of immunoactive components, including milk protein peptides, oligosaccharides, cytokines, hormones, Igs, soluble receptors for bacterial LPS, microRNA-containing exosomes, and viable immune cells (3, 4). Notably, the beneficial effects of breastfeeding continue after weaning and well beyond the lifetime of Igs and other soluble factors from milk (3, 5, 6). Others have demonstrated dam to pup transfer of milk immune cells and suggested that they conferred short-term, passive, cellular immunity (7–10). However, previous work from this laboratory, showing persistence of lactationally transferred immunity to adulthood (11), suggested the existence of a more active mechanism through which transferred cells instructed development of the neonate’s T cell repertoire. In this study, we provide evidence in support of such a mechanism.
Materials and Methods
Mice
C57BL/6J (CD45.2+CD45.1−), congenic B6-SJL-Ptprca Pepcb/BoyJ (CD45.1+CD45.2−), MHC class II (MHCII) knockout (KO) (B6.129-S2-H2dlAb1-Ea/J), and GFP transgenic (UBI-GFP/BL6GFP) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animal use was approved by the Institutional Animal Care and Use Committee.
Immunization
Adult female mice were immunized either by i.p. injection of heat-killed Mycobacterium tuberculosis H37 Ra (BD Diagnostic Systems, Franklin Lakes, NJ) (150 μg in 200 μl of Dulbecco’s PBS) or intradermal injection of fixed Candida albicans (100 μl of suspension per flank) into both flanks. After 7 d, mice were challenged in the footpad with 50 μl (2.5 United States tuberculin units) of tuberculin purified protein derivative (PPD) (Sanofi Pasteur, Toronto, ON, Canada) or 50 μl of C. albicans protein Ag (1 mg/ml; Alerchek, Portland, ME). These challenged mice, along with control injected groups, were mated 7 d later. Timed matings allowed for coordinated cross fosterings of immunized dams with nonimmunized pups such that days of lactation were equivalent to pup age. Pup litter sizes were normalized to ±1.
Flow cytometry and immunohistochemistry
All reagents and Abs were purchased from eBioscience (San Diego, CA) or BD Biosciences (San Jose, CA) unless otherwise indicated: anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD11c (HL3), anti-CD44 (IM7), anti-CD45.1 (A20), anti-CD45.2 (104), anti–IFN-γ (XMG1.2), anti-CD62L (MEL-14), anti-CD127 (A7R34), anti-MHCII (I-A) (NIMR-4), anti–IL-2 (JES6-5H4), anti-B220 (RA3-6B2), and mouse IgG2a and rat IgG2b isotype controls. Fc receptors were blocked using purified rat anti-mouse CD16/CD32 Ab (2.4G2) (0.5 μg/100 μl). Flow cytometry acquired viable cells, which were further gated on the basis of forward versus side scatter and then analyzed after staining with conjugated Abs. To assess GFP positivity, FITC beads and cells from non-GFP mice were used for gating and to carefully, distinctly, and conservatively distinguish a positive from a negative cell. For conjugated Abs, fluorochrome conjugates were used to stain BD Biosciences CompBeads to set the equipment for compensation controls along with nonstained controls. In each Ab analysis, appropriate isotype controls were used to define gate settings. The control tetramer was CLIP 103–117 (PVSKMRMATPLLMQA) and the specific tetramers were Ag 85B FF15 240-254 (FQDAYNAAGGHNAVF) for M. tuberculosis (12) and agglutinin-like protein peptide 236–253 (KGLNDWNYPVSSESFSYT) for C. albicans (13). Stained cells were fixed in 2% paraformaldehyde, acquired using a FACSAria (BD Biosciences), and analyzed by FlowJo software, version 9 (Tree Star, Ashland, OR). No data were excluded from analysis.
After processing for frozen sectioning, 20-μm sections were cut, postfixed in acetone, and blocked with 5% FBS, rabbit IgG (1:1000; Sigma-Aldrich, St. Louis, MO), and mouse IgG, Fc fragment (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA) in Dulbecco’s PBS, and stained as described previously (11).
Splenocyte stimulation
Freshly isolated (within 1 h) splenocytes with >95% viability were incubated at 1 × 106 cells/ml in RPMI 1640 supplemented with 10% FBS (Life Technologies, Grand Island, NY) in the presence of purified anti-CD28 Ab (37.51; 1 μg/ml) (BD Biosciences) in the presence or absence of the PPD Ag (0.25 tuberculin units/ml). After 1 h, BD GolgiStop was added (final concentration of 2.0 μM) for an additional 16–18 h before harvesting. Cells were then stained for surface Ags, fixed and permeabilized (BD Biosciences), and stained for intracellular molecules. For this functional assay, a response was considered positive when the degree was at least twice that of the background negative value and >0.05%. Staphylococcal enterotoxin B and/or purified anti-CD3 Abs were used as positive controls. No data were excluded from analysis.
Deletion of CD4+ maternal cells
Dams received 250 μg of either anti-CD4 Ab (clone GK1.5) or control Ab (clone LTF-2) 1 or 2 d prior to parturition, on the day of parturition, and then at weekly intervals until weaning of the pups. The dose was delivered i.p. This protocol had no effect on CD8+ cells and eliminated all CD4+ cells from milk by postnatal day 3.
Statistical analysis
Because of the small number of maternal cells being tracked in any given foster pup, a generalized Poisson mixed model with an unstructured error assumption was used to model the number of GFP cells in a sample using the GLIMMIX procedure from the SAS Institute (http://www.sas.com/en_us/home.html). Probability and quantitative correlation statistical analyses to follow the route of cells were conducted by the University of California Statistical Consulting Collaboratory (http://collaboratory.ucr.edu/). To model the probability of MHCII+ out of the number of GFP+ cells, and further, MHCII+CD4+ out of the number of MHCII+, a generalized linear mixed model with the logit link function for binomial count data and an unstructured error assumption was used. The generalized linear mixed model was estimated using the GLIMMIX procedure (SAS Institute). In all instances, pairwise comparisons were further conducted for any significant effect. For other analyses, statistical significance was determined using a Student t test, with Bonferroni corrections, where appropriate. Data are expressed as means ± SD, and p < 0.05 was considered statistically significant.
Results
Migration patterns of maternal milk cells show specific trafficking to the thymus and spleen
In the foster experiments, we have used a variety of dam and foster pup pairs so that we could build confidence in data where only a small number of maternal cells move into pup tissues. We began with GFP+ dams suckled by non-GFP foster pups because in the GFP+ dams, all cells were GFP+, allowing us to capture all maternal cells in the non-GFP foster pups. Identification of the cells as maternal without the use of an Ab also allowed us to reduce the potential for steric hindrance of multiple surface Ab labeling when further categorizing the cells. CD45.1/2 combinations, although suitable for most cells in the hematopoietic lineage and suitable for our study once we had established the nature of the cells traveling to the thymus and spleen, would not have allowed for tracking of some potentially important cells, including some differentiation states of immune cells (14, 15). Previous work using transfer of cells from a GFP+ dam to a non-GFP suckling foster pup demonstrated that resultant GFP+ cells within the pup thymus were capable of synthesizing GFP and were not GFP+ as a result of heterophagy (11).
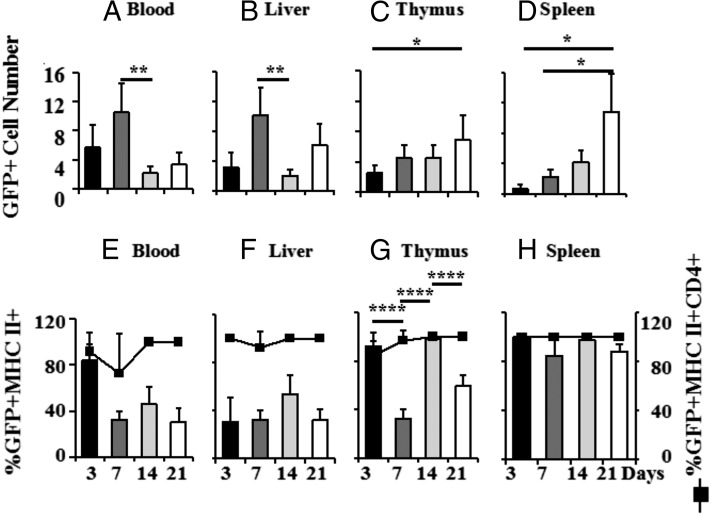
Fig. 1A–D show the patterns of maternal GFP+ cells transferred via milk to the blood, liver, thymus, and spleen of non-GFP foster pups as a function of pup age/days of lactation. The data are plotted with constant denominators in each tissue so that one can compare as a function of pup age, but with a different denominator per tissue because of the very different numbers of cells in each tissue and the desire to illustrate comparative patterns. Because the total number of cells in the thymus and spleen also increases with age, total accumulation per organ is greater than that illustrated. The number of GFP+ dam cells in pup blood and liver was highest at day 7 and declined thereafter. In contrast, the number of GFP+ cells increased gradually in the pup thymus and exponentially in the spleen to maxima at day 21. Thus, when compared with blood and liver, there was evidence of specific uptake and/or retention of maternal cells in the thymus and spleen.
FIGURE 1.
Maternal cells in mouse pup body during nursing. GFP+ dams were used as foster dams for non-GFP+ pups from equivalently timed matings. To illustrate relative numbers of maternal GFP+ cells in pup tissues as a function of pup age/time since the onset of nursing, numbers of GFP+ maternal cells are plotted per 10,000 (blood) (A), 40,000 (liver) (B), 950,000 (thymus) (C), and 450,000 (spleen) (D) non-GFP pup cells. Maternal GFP+ cells in the different tissues of the pup were further characterized by flow cytometry using Abs against MHCII and CD4 [(E), blood; (F), liver; (G), thymus; (H), spleen]. This figure compiles data from eight independent experiments, with four to nine pups at each time point. Data are expressed as means ± SD, and *p < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ****p < 0.0001.
Because we have previously shown that there was lactationally transferred immunity (11) and expected this to be associated with MHCII+ cells, we determined the percentage of GFP+ cells that were MHCII+. In pup blood, 80–100% of lactationally transferred maternal cells were also MHCII+ at day 3, but the percentage fell to ∼40% or below thereafter (Fig. 1E, left ordinate). In the pup thymus, 90–100% of maternal cells were also MHCII+ on day 3. As in the blood, the percentage fell at day 7, but unlike the blood, it rose again to 90–100% at day 14 of nursing (Fig. 1G), indicative of a second influx of maternal MHCII+ cells. In the spleen, essentially all cells at all time points were both GFP+ and MHCII+ (Fig. 1H). The changes in percentage of MHCII+ maternal cells in the pup thymus and spleen throughout the nursing period therefore do not simply reflect proportional or indiscriminate uptake from the circulation. Statistical analysis of the kinetics of the GFP+ cell subsets (MHCII+/− and CD4+/−) in different pup tissue compartments in eight foster litters determined a high likelihood that at least most GFP+ cells found in the thymus traveled directly there and not via the spleen (R2 = 0.81, p < 0.015). Essentially all GFP+MHCII+ cells in all pup tissue compartments were also CD4+ (right axis of Fig. 1E–H). Of the pups containing sufficient maternal cells in the thymus to allow further analysis (40%), up to 100% of GFP+MHCII+CD4+ cells were also CD44+ (47 ± 31%). Maternal GFP+MHCII− cells in the thymus were a mixture of CD4+ and CD8+ T cells, as described previously (11).
Maternal cells in the pup stomach, thymus, and spleen
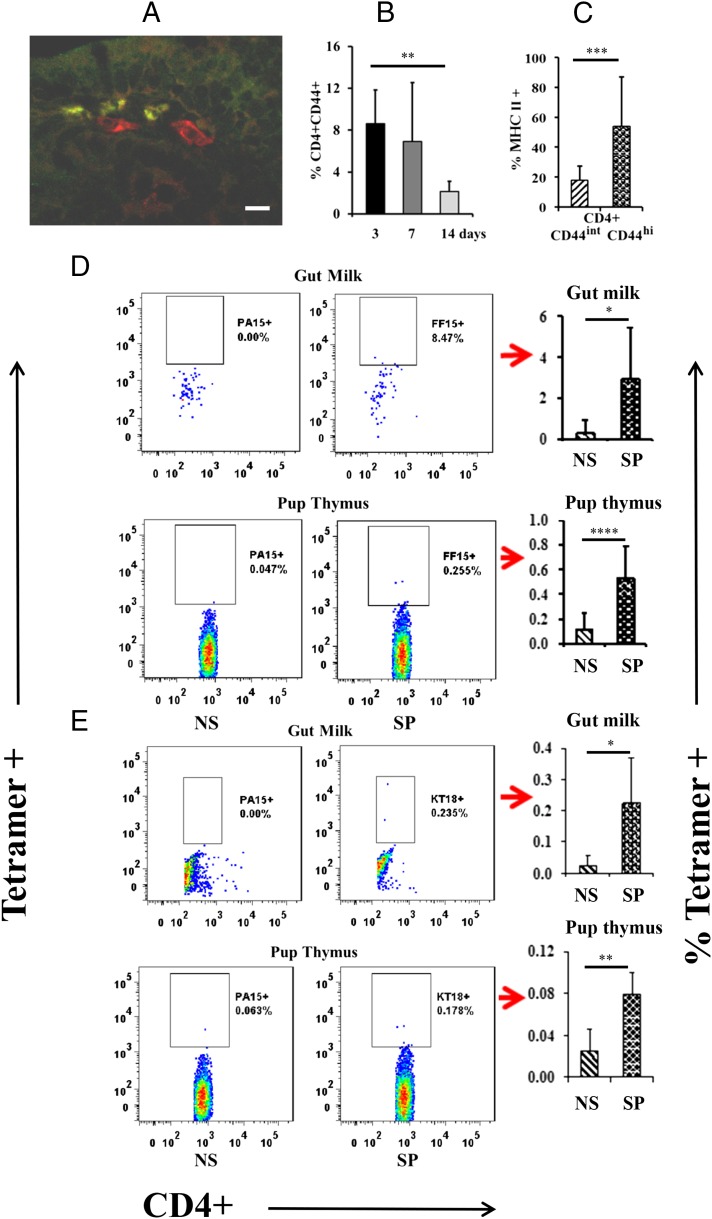
Many cells have been shown to express MHCII under certain conditions (16), but few have been reported to also express CD4. Among those that do are dendritic cells and macrophages (17). Although dendritic cells and macrophages were present in milk (on days 1, 3, and 14, the whey clot in the pup stomachs collectively averaged 1.73 ± 1.72% CD11chi dendritic cells and 4.04 ± 3.12% CD11b+ macrophages), we found no evidence that maternal dendritic cells or macrophages crossed into pup tissues. Five different fostering experiments were analyzed and immunofluorescence staining of slides was conducted on tissue sections from six different animals in each. For each animal, two sections were completely examined at ×1000 magnification. This kind of immunohistochemical analysis, as well as flow cytometry, has been used previously by this laboratory to demonstrate successful transepithelial transfer of both CD4+ and CD8+ maternal cells and lack of transfer of F4/80+ macrophages (11). Fig. 2A illustrates an example from the immunohistochemical analysis. It shows a region of lamina propria from pup small intestine in which one can appreciate the presence of two CD11c+ cells (red fluorescence) and three maternal GFP+ cells that have made their way across the epithelium from the lumen of the pup’s gastrointestinal tract. However, no double-positive cells that would have indicated the presence of maternal dendritic cells were seen.
FIGURE 2.
Phenotype and Ag-specificity of maternal cells in pup gut and thymus. An example from the immunofluorescence analysis, examining the possibility that some of the maternal GFP+ cells crossing into the pup were dendritic cells, is shown in (A). The dendritic cell marker, anti-CD11c (red), is labeling two cells in the lamina propria of the small intestine of the pup where one can also see three foster maternal GFP+ cells. Scale bar, 20 μm. No colocalization of GFP with CD11c labeling was observed. The percentage of maternal milk CD4+ cells expressing CD44, as assessed by flow cytometry, was highest at early time points (B), and when CD44 was high, the cells were more likely to express MHCII (all ages analyzed together) (C). These experiments were repeated five times, with seven to nine pups per time point. Ag-specific M. tuberculosis (D) and C. albicans (E) tetramer staining of CD4+ cells in the milk and pup thymus, with quantitative data derived from four to seven pups on each of two occasions. Data are expressed as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NS, nonspecific tetramer; SP, specific tetramer.
CD4 positivity is most commonly associated with a subset of T cells. CD4+ T cells averaged 1.96 ± 1.7% and CD8+ T cells averaged 1.17 ± 1.68% of total maternal cells in the whey clots collected on days 1, 3, and 14 from the foster pup stomachs. If information relevant to maternal immunization were being passed by T cells, they would likely be memory cells. The frequency of CD4+ T cells expressing the memory marker, CD44+ (18, 19), was higher at earlier lactation time points (Fig. 2B). Furthermore, ∼60% of CD4+ cells expressing high levels of CD44 also expressed MHCII (Fig. 2C).
To determine whether maternal CD4+MHCII+ cells transferred to nonimmunized pups were capable of presenting Ags against which the foster dam had been immunized, we used MHCII tetramer staining. A small, but reproducible, proportion of CD4+ T cells in the gut milk and thymus of the nonimmunized foster pup was specific for M. tuberculosis or C. albicans, the immunogens administered to the foster dams (Fig. 2D, 2E). Thus, Ag-specific T cells are present in milk and pass into the neonate thymus. Frequencies of specific MHCII tetramer+ cells would be expected to be low and in the range of one to a few per million (20).
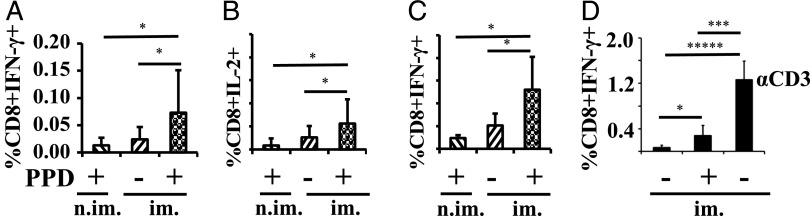
Stimulation of splenocytes from 3-wk-old nonimmunized foster pups, nursed by dams immunized against M. tuberculosis Ag, revealed the presence of Ag-specific, IFN-γ– and IL-2–producing CD8+ T cells (Fig. 3A, 3B). These responses were not detected in splenocytes from pups fostered to nonimmunized dams (Fig. 3A, 3B). Examination at 5 wk showed a similar percentage response (Fig. 3C), which is within the expected range for a constituent of the splenic memory repertoire (21). The response by CD4+ T cells was not very robust (data not shown). In a separate set of experiments, immunized dams were sacrificed and the ex vivo responses of their splenocytes were compared. Ag-responding cells were present at ∼3-fold the frequency in the immunized maternal spleens versus the spleens of the nonimmunized foster pups nursed by immunized dams (Fig. 3D).
FIGURE 3.
Splenocytes from the foster pups respond ex vivo to the Ag used to immunize the foster dam. Splenocytes were exposed to anti-CD28 in the absence (−) or presence (+) of PPD for 16–18 h (A–C). The percentage is of Ag-responsive CD8+ splenocytes when dams were last exposed (or not) to M. tuberculosis 4 wk before the onset of lactation; pup splenocytes were analyzed at 3 (A and B) and 5 (C) wk of age; n = 11–24 pups derived from three (A and B) or two (C) independent fosterings. The percentage of Ag-responsive CD8+ splenocytes in the directly immunized dam spleens (D) is shown for comparison after an 18-h incubation in anti-CD28 in the absence (−) or presence (+) of PPD or anti-CD3 [α-CD3, only for right bar of (D)], the latter as a pan-positive control. All analyses were by flow cytometry. Data are expressed as means ± SD. *p < 0.05, ***p < 0.001, ****p < 0.0001. im., foster dams were immunized with BCG; n.im., foster dams were not immunized with BCG.
Most Ag-responding CD8+ cells in the nonimmunized pup spleen were derived from the pup thymus
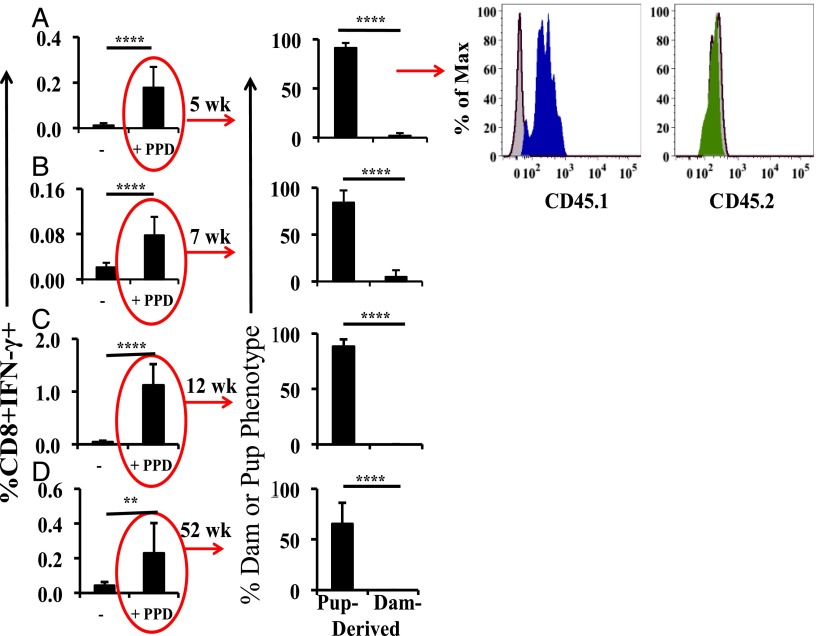
To demonstrate the origin (maternal versus suckling pup) of Ag-responsive CD8+ cells in the spleen of the nonimmunized foster pup, we employed congenic CD45.1 and CD45.2 mice (22) as M. tuberculosis–immunized dams and nonimmunized foster pups in both combinations. Splenocytes from pups at different ages were stimulated with PPD and cytokine production was measured. Fig. 4A shows representative staining from an experiment utilizing CD45.1 pups fostered by CD45.2 dams at 5 wk of age. More than 90% of the responding CD8+ cells were derived from the pup thymus (positive CD45.1 staining in blue and control staining in gray). The numbers were essentially the same at 7 wk (Fig. 4B), and all responding CD8+ cells were pup derived at 12 and 52 wk (Fig. 4C, 4D). The reverse CD45 combination showed very similar results (data not shown).
FIGURE 4.
Dam versus pup derivation of PPD-responding cells in the pup spleen. Cross fostering of animals expressing the congenic markers CD45.1 and CD45.2 was used to identify the responding dam and pup CD8+ cells in the pup splenocyte preparations at 5 (A), 7 (B), 12 (C) and 52 (D) wk of age. Gating for production of IFN-γ by CD8+ cells is shown. Dams were CD45.2 (green) and pups CD45.1 (blue), with an n = 8 pups at each age. The median responding CD8+IFN-γ+ number from five CD45.1/CD45.2 BCG-Foster experiments including a total of 35 pups was 28. The cross-fostering combinations (i.e., including the reverse CD45.1/2 combination) were conducted two or more times each with the same result. All analyses were by flow cytometry. Data are expressed as means ± SD. **p < 0.01, ****p < 0.0001.
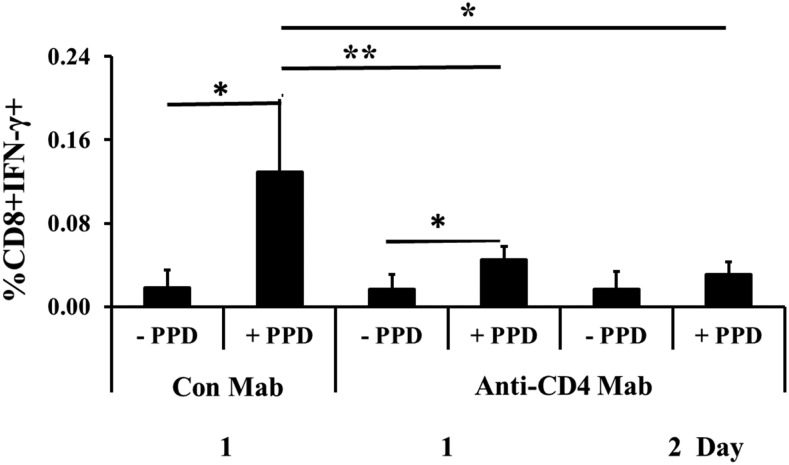
Transfer of CD4+ cells from immunized dam to nonimmunized pup is required for the generation of most Ag-responsive CD8+ cells in the pup spleen
Treatment of dams with anti-CD4 or control Ab 1 or 2 d prior to birth, the day of parturition, and then at weekly intervals until weaning did not immediately eliminate all CD4+ cells from the milk, but CD4+ cells were gone by day 3. As can be seen in Fig. 5, treatment of the immunized dams with anti-CD4 reduced the number of Ag-responding CD8+ cells in the nonimmunized and nontreated foster pups at 5 wk of age by 60–70% compared with the situation where the dam was instead treated with a control Ab. Thus, maternal CD4+ cells are important to the generation of Ag-responsive CD8+ cells in the pup at a time point when we know that >90% of Ag-responsive cells in the pup spleen are of pup origin.
FIGURE 5.
Transfer of CD4+ cells from immunized dam to nonimmunized pup is required for the generation of most Ag-responsive CD8+ cells in the pup spleen. Control mAb (Con Mab) or Anti-CD4 mAb was first injected in the dams 1 or 2 d before parturition (indicated as 1 or 2 d). Ab was again injected on the day of birth and at weekly intervals until weaning. At 5 wk of age, Ag-responsive CD8+ cells were determined in the nonimmunized foster pups. Results from two treatments with anti-CD4 are shown (with six pups each) and one control Ab treatment with five pups. All analyses were by flow cytometry. Data are expressed as means ± SD. *p < 0.05, **p < 0.01.
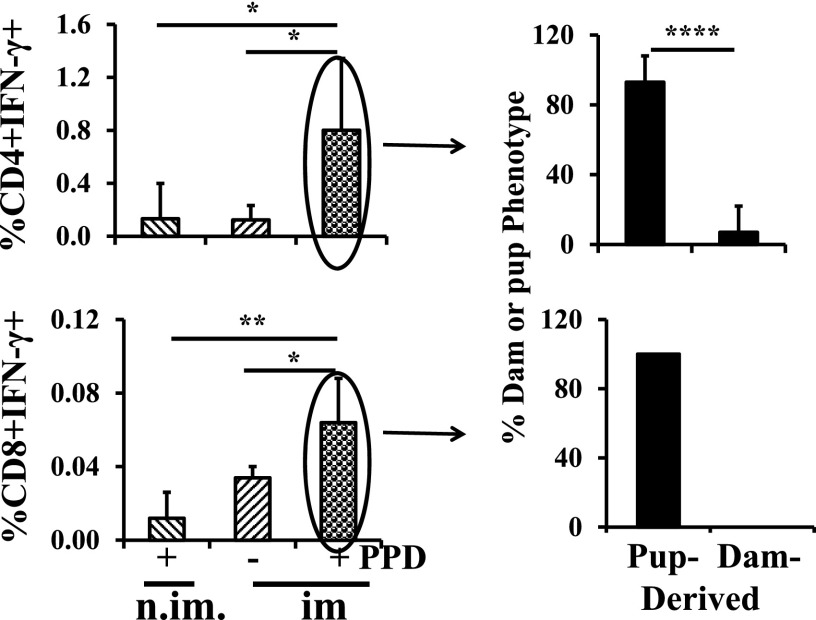
Maternal MHCII+CD4+ T cells that went directly to the spleen contribute to passive cellular immunity
To determine whether the maternal MHCII+CD4+ cells that homed to the spleen were capable of contributing to passive cellular immunity, we characterized Ag-responding cells originating from immunized MHCII+ dams that were present in MHCII-null foster pups (nonimmunized). Fig. 6 shows the result at 5 wk. Both CD4+ and CD8+ cells in the pup spleen responded to PPD by the production of IFN-γ (left upper and lower panels). Of the Ag-responding CD4+ cells, ∼10% were MHCII+ (upper right panel) and therefore of maternal origin. No responding CD8+ cells were MHCII+ (lower right panel). For CD8+ cells, these data are directly comparable to percentages in the other graphs because the percentage of splenocytes that is CD8+ is similar between normal C57BL/6J and MHCII-null C57BL/6J mice. This is not true for the CD4+ cells because the percentage of CD4+ cells in the MHCII-null mouse spleens is only 2.7% of normal. A relatively robust percentage of responding CD4+ cells is therefore more a function of total CD4+ cell number than degree of response.
FIGURE 6.
Maternal MHCII+ CD4+ T cells that went directly to the spleen contribute to passive cellular immunity. MHCII-null pups were fostered to MHCII+, M. tuberculosis–immunized dams and the MHCII status of ex vivo–responding pup splenocytes was assessed at 5 wk of age. Data are derived from nine pups. All analyses were by flow cytometry. Data are expressed as means ± SD. *p < 0.05, **p < 0.01, ****p < 0.0001. im., foster dams were immunized with BCG; n.im., foster dams were not immunized with BCG.
Discussion
In a previous publication, we demonstrated that cell-mediated immunity was transferred from dam to pup via suckling (11). Because the immunity lasted well into adulthood and we could not detect any maternal cells in adult animals, an effect on development of the pup’s T cell repertoire was suggested. In this study, we show that maternal immunization and foster pup suckling results not only in transfer of passive cellular immunity to the pup, but also in the development of Ag-specific T cells in foster pups. In other words, we show that suckling affected specific and not just general development of the pup’s T cell repertoire.
Because of their role in Ag presentation and their presence in milk, one might logically expect the maternal cell traveling from the milk to the pup thymus to be a dendritic cell. However, we were unable to demonstrate the presence of maternal dendritic cells in the pup. Furthermore, although dendritic cells can express CD4, expression in mice has been reported to be limited to subsets found in the spleen (17). They were therefore unlikely to comprise any of the maternal MHCII+CD4+ cells in the thymus. Also, although CD44 can be expressed on dendritic cells, the level of expression is low and in the same range as for a naive T cell (23). In contrast, high expression of the T cell memory marker, CD44, correlated best with MHCII positivity. Additionally, IFN-γ was produced in response to Ag in CD4+ maternal cells also shown to be MHCII+. Finally, given that dendritic cells are relatively short-lived (days to possibly weeks) (24) and suckling began 4 wk after immunization in the present study and 12 wk after in a previous study (11), Ag-bearing dendritic cells in the dam would be extremely few. Collectively, the data and literature indicate that the MHCII+ cells transferred from dam to pup were CD4+ T cells, previously termed Th APCs (25).
Why choose a T cell for delivery of Ag to the thymus? Although classically the thymus is thought of as a primary lymphoid organ, mature effector T cells also recycle from the periphery back through the thymus (26). Thus, maternal cells can use this normal pathway to gain entry into the pup’s thymus. Recycling cells express the memory/migration marker CD44 (26), as did a variable proportion but up to 100% of maternal GFP+MHCII+CD4+ cells in the thymus. A small, but appropriate, proportion of maternal cells in the thymus was also specific tetramer+.
The mechanisms by which CD4+ T cells can acquire MHCII seem to be fewer in mice than humans (27). One mechanism is the acquisition of MHCII by trogocytosis during interaction with an MHCII+ cell at an immunological synapse (28–30). This mechanism is consistent with the observation that a significant proportion of transferred maternal cells: 1) had a memory phenotype; 2) were Ag-specific by tetramer staining; or 3) when in the spleen, responded to Ag by the production of appropriate cytokines. Importantly, Umeshappa et al. (25) and others have shown that T cell acquisition of MHCII and MHC class I at an immunological synapse can include the antigenic peptide and costimulatory molecules.
After gaining Ag-bearing MHCs during an immune response in the dam, maternal Th APCs would have to cross mammary, intestinal, and thymic epithelial barriers to present Ags within the pup thymus. On the basis that much of the detected MHCII was intracellular in the dam cells present in milk and in the pup, we propose a working model in which the MHC/Ag complex is protected by internalization in a nondegradative vesicular compartment during migration.
Even though the analyses showed the transfer of only a small number of maternal cells into the pup thymus, confidence in the results is greatly enhanced by demonstration in four different dam and foster pup combinations. Furthermore, when CD4+ cells were depleted from maternal milk, the production of Ag-specific CD8+ cells in the nonimmunized pup was markedly reduced. In combination with the presence of specific MHCII+tetramer+ maternal cells in the thymus, the results support the transport of Ag into the pup thymus by maternal Th APCs. How proper presentation to effect positive selection then occurs in the thymus can only be the subject of hypothesis at present, but could involve a second trogocytosis event or release and local APC uptake of the Ag.
Whatever the intrathymic mechanism of transported Ag presentation, the results unequivocally demonstrate that nursing influences development of the pup’s CD8+ T cell repertoire in an Ag-specific way. We have dubbed this process maternal educational immunity. In vivo, the number and activity of these Ag-specific CD8+ pup cells, once in the spleen, could be augmented by interaction with the maternal MHCII+CD4+ Ag-presenting T cells that came directly to the spleen from the milk. In this regard, Th APCs have been shown to stimulate proliferation of naive CD8+ T cells and their differentiation into cytotoxic T lymphocytes (25). In other words, we propose that the Th APCs from bacillus Calmette–Guérin (BCG)–immunized foster dams that migrated to the foster recipient spleens ensure the requisite cytokine environment for the newly formed pup T cells to be functionally competent and not anergic (31).
The results also illustrate passive cellular immunity because there were maternally derived Ag-responding cells. At least some of these were MHCII+, further indicating that the cells capable of presenting Ag had a memory phenotype. However, Ag-responding maternal cells disappeared as a function of time, whereas the cells produced by maternal educational immunity persisted.
The existence of passive cellular immunity is well accepted and has been demonstrated in the human population. Thus, breast milk from mothers infected with M. tuberculosis contains cells that produce IFN-γ in response to PPD (32), and tuberculin-reactive T cells are transferred through breastfeeding to the infant (33). However, to our knowledge, our work is the first to show a second and arguably more important role for transferred immune cells: the direction of offspring T cell development. Thus, nature has produced two complementary mechanisms for ensuring early cell-mediated immune protection against intracellular pathogens to which the mother has been exposed. First, there is passive cellular immunity, which provides immediate protection while the thymic educational process is ongoing. This is then replaced by the outcome of maternal educational immunity.
We suggest that programs aimed at harnessing maternal educational immunity through prepregnancy immunization have potential for improvement of vaccination strategies against diseases requiring a cell-mediated immune response.
Acknowledgments
Tetramers were synthesized by the National Institutes of Health core facility at Emory University (contract number HHSN272201300006C). We thank Dr. Ilhem Messaoudi (University of California, Riverside) for critiquing the manuscript and Hans Raué and Mark Slifka (Oregon Health and Science University) for the anti-CD4 Ab.
This work was supported by National Institutes of Health Grant HD 65099.
- BCG
- bacillus Calmette–Guérin
- MHCII
- MHC class II
- PPD
- purified protein derivative.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Oddy W. H. 2001. Breastfeeding protects against illness and infection in infants and children: a review of the evidence. Breastfeed. Rev. 9: 11–18. [PubMed] [Google Scholar]
- 2.van Odijk J., Kull I., Borres M. P., Brandtzaeg P., Edberg U., Hanson L. A., Høst A., Kuitunen M., Olsen S. F., Skerfving S., et al. 2003. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 58: 833–843. [DOI] [PubMed] [Google Scholar]
- 3.Head J. R., Beer A. E., Billingham R. E. 1977. Significance of the cellular component of the maternal immunologic endowment in milk. Transplant. Proc. 9: 1465–1471. [PubMed] [Google Scholar]
- 4.Van de Perre P. 2003. Transfer of antibody via mother’s milk. Vaccine 21: 3374–3376. [DOI] [PubMed] [Google Scholar]
- 5.Pereira P. F., Alfenas Rde. C., Araújo R. M. 2014. Does breastfeeding influence the risk of developing diabetes mellitus in children? A review of current evidence. J. Pediatr. (Rio J.) 90: 7–15. [DOI] [PubMed] [Google Scholar]
- 6.Campbell D. A., Jr., Lorber M. I., Sweeton J. C., Turcotte J. G., Niederhuber J. E., Beer A. E. 1984. Breast feeding and maternal-donor renal allografts. Possibly the original donor-specific transfusion. Transplantation 37: 340–344. [DOI] [PubMed] [Google Scholar]
- 7.Seelig L. L., Jr., Billingham R. E. 1981. Concerning the natural transplantation of maternal lymphocytes via milk. Transplant. Proc. 13: 1245–1249. [PubMed] [Google Scholar]
- 8.Tuboly S., Bernáth S., Glávits R., Kovács A., Megyeri Z. 1995. Intestinal absorption of colostral lymphocytes in newborn lambs and their role in the development of immune status. Acta Vet. Hung. 43: 105–115. [PubMed] [Google Scholar]
- 9.Jain L., Vidyasagar D., Xanthou M., Ghai V., Shimada S., Blend M. 1989. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch. Dis. Child. 64: 930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheldrake R. F., Husband A. J. 1985. Intestinal uptake of intact maternal lymphocytes by neonatal rats and lambs. Res. Vet. Sci. 39: 10–15. [PubMed] [Google Scholar]
- 11.Ma L. J., Walter B., Deguzman A., Muller H. K., Walker A. M. 2008. Trans-epithelial immune cell transfer during suckling modulates delayed-type hypersensitivity in recipients as a function of gender. PLoS One 3: e3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandra L., Smialek J. L., Shank S. S., Convery M., Boom W. H., Harding C. V. 2005. Phagosomal processing of Mycobacterium tuberculosis antigen 85B is modulated independently of mycobacterial viability and phagosome maturation. Infect. Immun. 73: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bär E., Gladiator A., Bastidas S., Roschitzki B., Acha-Orbea H., Oxenius A., LeibundGut-Landmann S. 2012. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J. Immunol. 188: 5636–5643. [DOI] [PubMed] [Google Scholar]
- 14.Hu V. H., Luthert P. J., Derrick T., Pullin J., Weiss H. A., Massae P., Mtuy T., Makupa W., Essex D., Mabey D. C., et al. 2016. Immunohistochemical analysis of scarring trachoma indicates infiltration by natural killer and undefined CD45 negative cells. PLoS Negl. Trop. Dis. 10: e0004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellat-Deceunynck C., Bataille R. 2004. Normal and malignant human plasma cells: proliferation, differentiation, and expansions in relation to CD45 expression. Blood Cells Mol. Dis. 32: 293–301. [DOI] [PubMed] [Google Scholar]
- 16.Kambayashi T., Laufer T. M. 2014. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat. Rev. Immunol. 14: 719–730. [DOI] [PubMed] [Google Scholar]
- 17.Schlitzer A., Ginhoux F. 2014. Organization of the mouse and human DC network. Curr. Opin. Immunol. 26: 90–99. [DOI] [PubMed] [Google Scholar]
- 18.Baaten B. J., Li C.-R., Deiro M. F., Lin M. M., Linton P. J., Bradley L. M. 2010. CD44 regulates survival and memory development in Th1 cells. Immunity 32: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baaten B. J., Tinoco R., Chen A. T., Bradley L. M. 2012. Regulation of antigen-experienced T cells: lessons from the quintessential memory marker CD44. Front. Immunol. 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nepom G. T. 2012. MHC class II tetramers. J. Immunol. 188: 2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacher P., Scheffold A. 2013. Flow-cytometric analysis of rare antigen-specific T cells. Cytometry A 83: 692–701. [DOI] [PubMed] [Google Scholar]
- 22.Mardiney M., III, Malech H. L. 1996. Enhanced engraftment of hematopoietic progenitor cells in mice treated with granulocyte colony-stimulating factor before low-dose irradiation: implications for gene therapy. Blood 87: 4049–4056. [PubMed] [Google Scholar]
- 23.Termeer C., Averbeck M., Hara H., Eibel H., Herrlich P., Sleeman J., Simon J. C. 2003. Targeting dendritic cells with CD44 monoclonal antibodies selectively inhibits the proliferation of naive CD4+ T-helper cells by induction of FAS-independent T-cell apoptosis. Immunology 109: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merad M., Manz M. G. 2009. Dendritic cell homeostasis. Blood 113: 3418–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umeshappa C. S., Huang H., Xie Y., Wei Y., Mulligan S. J., Deng Y., Xiang J. 2009. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J. Immunol. 182: 193–206. [DOI] [PubMed] [Google Scholar]
- 26.Kirberg J., Bosco N., Deloulme J. C., Ceredig R., Agenès F. 2008. Peripheral T lymphocytes recirculating back into the thymus can mediate thymocyte positive selection. J. Immunol. 181: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 27.Holling T. M., Schooten E., van Den Elsen P. J. 2004. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum. Immunol. 65: 282–290. [DOI] [PubMed] [Google Scholar]
- 28.Patel D. M., Arnold P. Y., White G. A., Nardella J. P., Mannie M. D. 1999. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J. Immunol. 163: 5201–5210. [PubMed] [Google Scholar]
- 29.Arnold P. Y., Mannie M. D. 1999. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur. J. Immunol. 29: 1363–1373. [DOI] [PubMed] [Google Scholar]
- 30.Davis D. M. 2007. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat. Rev. Immunol. 7: 238–243. [DOI] [PubMed] [Google Scholar]
- 31.Friesen T. J., Ji Q., Fink P. J. 2016. Recent thymic emigrants are tolerized in the absence of inflammation. J. Exp. Med. 213: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cranmer L. M., Kanyugo M., Lohman-Payne B., Tapia K., John-Stewart G. C. 2015. Tuberculosis interferon-γ responses in the breast milk of human immunodeficiency virus infected mothers. Int. J. Tuberc. Lung Dis. 19: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlesinger J. J., Covelli H. D. 1977. Evidence for transmission of lymphocyte responses to tuberculin by breast-feeding. Lancet 2: 529–532. [DOI] [PubMed] [Google Scholar]