Abstract
Background:
Rising incidence of human papillomavirus (HPV) infection and cervical cancer can be reduced by effective vaccination. Saudi Food and Drug Administration approved prophylactic HPV vaccine in 2010 for females of 11–26 years.
Objectives:
To determine the awareness of HPV infection, its health sequel and the attitude and barriers to the acceptance of HPV vaccine by young women in Saudi Arabia. Dynamics influencing the decision of patients and parents regarding vaccination were assessed to foster effective and strategically focused interventions.
Materials and Methods:
All patients of Family Medicine department, King Faisal Specialist Hospital and Research Center, Riyadh were invited to participate in this study from January 2012 to June 2014. A culturally sensitive and specially designed questionnaire was administered using an interview-based model to assess the knowledge, perception, and associated sociodemographic factors of HPV.
Results:
A total of 325 patients participated as per the inclusion criteria: 87.4% were Saudis, 53.5% had university or higher education and 65.2% were adolescents (age 11-19 years). The questionnaire was answered by participants (50.8%) or guardians (49.2%). About 34.5% of the population was aware of HPV infection, and 27.4% were aware of its relation with cervical cancer. However, awareness of the HPV vaccine, perception of its prevention of cervical cancer and other HPV-related disease was relatively low (32.3%), Saudis (29.9%) versus non-Saudis (48.8%) (P = 0.016). More guardians (41.2%) were aware of the HPV vaccine and its impact than participants (27.9%) (P = 0.01). Higher educational background (43.1%) increased the knowledge of HPV compared to less than high school education (24.5%) (odds ratio: 2.33; 95% confidence interval: 1.44–3.76). Nearly 64.3% of participants agreed, and 35.7% refused to receive the HPV vaccine.
Conclusion:
Knowledge and perception of HPV infection as an sexually transmitted infections and its vaccine was significantly low in this cohort of patients. Higher age and educational levels directly correlated with increased knowledge of HPV infection and its complications. It is recommended that awareness should be raised, and access to HPV vaccination increased to help reduce the health care burden of HPV sequelae in the Kingdom.
Key words: Adolescents, attitude, human papillomavirus vaccination, perception, Saudi Arabia
INTRODUCTION
The human papillomavirus (HPV) is a group of >150 related viruses and precursor to nearly all cervical cancers and genital warts. More than 40 HPV types can be transmitted from person to person through sexual contact and can occur in the genitals, anal, or oral regions. According to World Health Organization (WHO), with up to 70% coverage, the HPV vaccine can prevent >4 million deaths in women in low to middle-income countries over the next decade.[1]
The prevalence of HPV infection is about 31% in the general population of Saudi Arabia, 80% of women with cytological abnormalities and >92% of women with cervical carcinoma.[2,3] The population of Saudi Arabia at risk for cervical cancer (female population aged >15 years) is 6.51 million as per the World Health Statistics Report 2010 of the WHO.[4] As per the 2010 Saudi Cancer Registry report, the number of newly diagnosed cervical cancers is rising significantly: 153 new cases of cervical cancer were diagnosed in 2010 in the Kingdom of Saudi Arabia. It is the 4th most common cancer among non-Saudi women residing in Saudi Arabia. The most common age group for cervical cancer among Saudis was 45–49 years and for non-Saudis 40–44 years. Cervical cancer was the 8th most common cancer in Saudi women aged from 30 to 44 years [Figure 1].[5] Cervical cancer in Saudi Arabia is associated with HPV infection caused by various genotypes. Most of the HPV-positive tumors are infected with HPV-16/18, which causes cancer to appear about 5 years earlier than the combined HPV-negative and other HPV genotypes.[6,7] More data are needed to map out the HPV infection load in the female population of the country.
Figure 1.
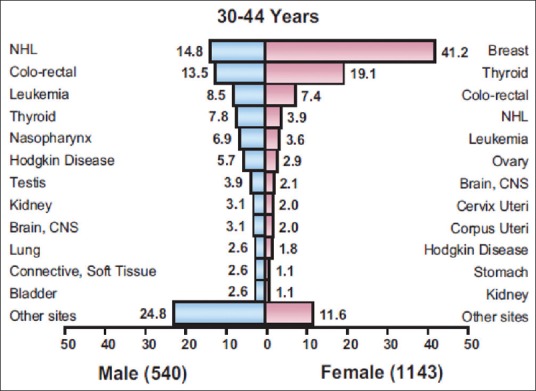
Percentage distribution of most frequent types of cancer among 30-44 year old Saudis by Sex, 2010 (Courtesy of Saudi Cancer Registry)
Prophylactic HPV vaccines, bivalent vaccine (Cervarix), and quadrivalent vaccine (Gardasil) became available in the Kingdom of Saudi Arabia in 2010 and was approved for females between the ages of 11 and 26 years. The vaccine works by preventing the most common types of HPV that causes cervical cancer and genital warts.[8] Acceptability of the HPV vaccine varies worldwide and is age dependent.[9,10] The vaccine that is available at King Faisal Specialist Hospital and Research Center (KFSH and RC) is Gardasil and is offered at routine office visits to Family Medicine and Pediatric clinics.
The primary purpose of this study was to determine the awareness of HPV infection, its health sequel including the attitude and barriers for acceptance of the HPV vaccine by young women in Saudi Arabia. The secondary purpose was to highlight the factors influencing decisions on HPV vaccine initiation and provide direction for developing interventions.
MATERIALS AND METHODS
All females aged between 11 and 26 who came to the Family Medicine department, KFSH&RC, Riyadh were invited to participate in this study from Jan 2012 to June 2014, according to the eligibility criteria. The criterion for inclusion was female patients aged between 11 and 26 years. The exclusion criteria included pregnant or lactating women, any patient who had an allergic, life-threatening reaction to HPV vaccine or any of its components, any patient with history of severe allergies or allergy to yeast, and patients with any moderate or severe illness. A totally anonymous, culturally sensitive, and specially designed questionnaire was administered using the interview-based model. The questionnaire included questions related to awareness of HPV infection, its link with cervical cancer and other health consequences, knowledge about the HPV vaccine and its ability to give protection against HPV infection, and related diseases. The questionnaire also included the sociodemographic data and the reasons for acceptance or rejection of the HPV vaccine.
This study was approved by the Ethics Committee at KFSH&RC. A study-specific verbal informed consent was obtained from each participant before enrollment in the study. The Institutional Review Board (IRB) exempted this study from written consent for less than minimum risk. Participants’ consent was documented in their medical record according to the IRB guidelines. The verbal informed consent was obtained from the parents or guardians on behalf of minors.
The questionnaire was distributed by the treating physicians in the Family Medicine outpatient clinics of KFSH&RC, Riyadh, Saudi Arabia. Participants answered coded and open-ended questions. The forms were completed in the presence of the physicians to ensure precision and accuracy of the results. This was also an opportunity for physicians to relay in-depth information about HPV to the patients. Anonymity and confidentiality were maintained. The study included 325 participants of varying ages and levels of education.
All data were analyzed using the software package SPSS version 20, by IBM (IBM Corp. Released 2011. IBMSPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Descriptive statistics for the continuous variables were reported as mean ± standard deviation (SD) while categorical variables were expressed as frequencies and compared by Chi-square test. The level of significance for all variables was set at 0.05. Participants were divided into groups based on different demographic variables (nationality, participants, education, and age group). The Chi-square test was performed to examine the demographic differences between participants who knew about the HPV, its relation with cervical cancer, availability of vaccine for the prevention of cervical cancer, and acceptance of the HPV vaccine.
RESULTS
Of 325 patients enrolled according to the inclusion criteria, 87.4% were Saudis and 12.6% were non-Saudis. Of these 46.5% had high school education or less, and 53.5% participants had university education or higher. About 65% of participants were adolescents and about 35% were adults (range 11–26 years, mean 17.14, and SD 5.25). The questionnaire was answered by either the patients (50.8%) or the parents/guardians (49.2%).
Overall, the awareness of HPV was significantly low; only 34.5% participants were aware of it compared to 65.5% who had never heard about it. The majority of the patients (72.6%) did not know that HPV had been established as an etiology of most cervical cancers. Across the sample, only 32.3% participants were aware of the ability of the HPV vaccine to prevent cervical cancer and other HPV-related diseases. However, the rate of acceptance of the vaccine was significantly higher (64.3%) among all the participants.
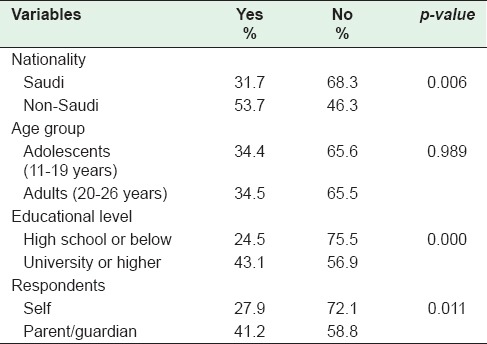
When the HPV awareness was substratified by nationality, it was significantly lower among Saudis (31.7%) compared to non-Saudis (53.7%) (P = 0.006). There was no difference in awareness when stratified by age groups into adolescents (34.4%) versus adults (34.5%). Awareness of HPV varied widely by educational level; high school and below (24.5%) versus university and higher education (43.1%) (P = 0.000). More parents/guardians were aware of HPV vaccine than the patients themselves (41.2% vs. 27.9%) (P = 0.01) [Table 1].
Table 1.
Awareness of human papillomavirus among patients by sociodemographic characteristic
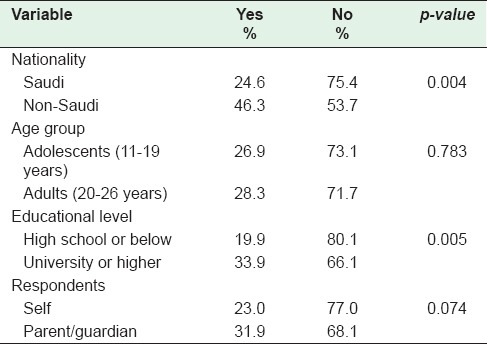
When the awareness of HPV as an etiology of most cervical cancers was substratified by nationality, it was significantly lower among Saudis (24.6%) compared to non-Saudis (46.3%) (P = 0.004). There was no difference in awareness when stratified by age groups into adolescents (26.9%) versus adults (28.3%). Awareness of HPV as an etiology for most cervical cancers varied widely by educational level: High school and below (19.9%) versus university and higher education (33.9%) (P = 0.005). There was no difference in the awareness of HPV as an etiology of cervical cancers among patients and parents/guardians (23% vs. 31.9%) (P = 0.07) [Table 2].
Table 2.
Patients’ awareness of human papillomavirus as an etiology of cervical cancer by sociodemographic characteristics
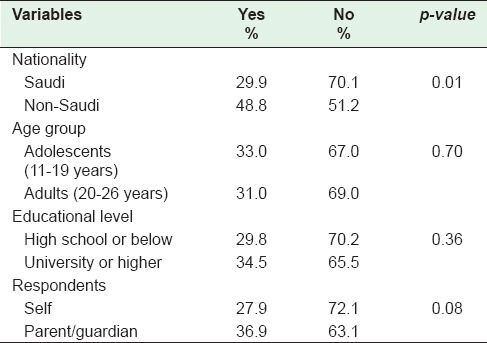
The knowledge of the ability of the HPV vaccine to prevent cervical cancer and other HPV-related morbidities was evaluated by nationality; it was significantly lower among Saudis (29.9%) than non-Saudis (48.8%) (P = 0.01). There was no significant difference in knowledge by age (adolescents [33%] vs. adults [31%]), and by education level (high school and below [29.8%] vs. university and higher education [34.5%]) [Table 3].
Table 3.
Awareness human papillomavirus vaccine's prevention of cervical cancer and other human papillomavirus-related diseases
Approximately, 64% of the participants indicated an intention to receive an HPV vaccine while 35.7% rejected the idea of getting the vaccine. When the acceptance of HPV vaccination was substratified by nationality, age, and educational level, no significant differences were found.
Intention to receive an HPV vaccine was directly correlated with the knowledge of HPV and other HPV-related diseases. Of the patients who refused to take the HPV vaccination, the most common reason was concern about the possible side effects and need for more information about the vaccine. The other reasons for refusal were need to discuss the vaccine with their family, concerns about safety, and efficacy of this new vaccine. A small minority perceived themselves as not at all at risk of HPV infection.
DISCUSSION
HPV is globally the most common sexually transmitted infections (STI). There are over 100 genotypes of HPV, 40 of which can infect the mouth and genital areas of both men and women. Genital HPV infection is extremely common. It most often causes no symptoms, and most infections resolve over time. Most people infected with HPV pass the virus on to a partner without knowing they have done so. A proportion of individuals infected with low-risk HPV types such as HPV-6 or HPV-11 will develop genital warts whereas a subset of women with high-risk HPVs such as HPV-16 or HPV-18 will develop preneoplastic lesions of cervical intraepithelial neoplasia.[11,12] Low-grade cervical dysplasia is common and most regress spontaneously. In contrast, the minority of lesions that progress to high-grade dysplasia tend to persist and/or progress to carcinomas in situ before becoming invasive cancers. Virtually, all cervical cancers together with 90% of anal cancers, >60% of certain subtypes of oro-pharyngeal cancers, and 40% of vagina, vulva, and penile cancers are due to HPV infection.[13] Most recent estimates suggest that each year there are more than a quarter of a million deaths from cervical cancer and over 500,000 new cases in the world, most of which could have been prevented. The WHO projects that without immediate action the global number of deaths from this disease will increase by nearly 80% by 2030, mostly in low- and middle-income countries.[4,14]
The rate of HPV infection prevalent in cervical cancers in Saudi Arabia is comparable to international rates.[2] Cervical cancer is the 2nd most common cancer among women worldwide[4] and among the top twelve most common cancers in women in the Kingdom of Saudi Arabia.[5] About 89% of cervical cancers in Saudi Arabia were associated with HPV infection while 78.7% of HPV-positive tumors were infected with HPV-16/18.[6,7]
With routine cervical cancer screening and implementation of HPV vaccination programs, primary care physicians have the potential to eliminate nearly all cervical cancers through the prevention of HPV transmission. The most likely strategy for the prevention of the risk of genital HPV infection among sexually active patients is a long-term, mutually monogamous relationship with an uninfected partner. However, it is difficult to determine whether a partner who has been sexually active in the past is currently infected. Research has shown that correct and consistent condom use can reduce the transmission of HPV between sexual partners.[15] However, because areas not covered by a condom can be infected by the virus, condoms are unlikely to provide complete protection against transmission of infection. Efforts are needed to increase HPV vaccine coverage in Saudi Arabia.
The United States Food and Drug Administration has approved three vaccines for the prevention of HPV infection: Gardasil® (quadrivalent and 9-valent vaccines) and Cervarix® (bivalent vaccine). All three vaccines are highly effective in preventing infections with HPV types 16 and 18, two high-risk HPVs that cause about 70% of cervical and anal cancers. These vaccines are available in the market as protection against certain types of HPV that can lead to cervical cancer. Gardasil, the quadrivalent vaccine also prevents infection with HPV types 6 and 11, which cause 90% of genital warts.[16] The Gardasil 9-valent vaccine provides coverage for additional five types of HPV that contribute to 10% of genital cancers.[17,18] There are no formal screening programs for these cancers, so vaccination has the potential to greatly reduce deaths from these cancers also. Both Gardasil and Cervarix are designed to be given to people in three doses over a 6-month period. However, a recent study showed that women who received only two doses of Cervarix had just as much protection from persistent HPV 16/18 infection as women who received all three doses, and the protection was observed over 4 years of follow-up.[19] Even one dose provided protection; however, these findings need to be evaluated with more research to determine whether fewer than three doses of the vaccine will provide adequate duration of protection. Overall, about 30% of cervical cancers will not be prevented by these vaccines. Neither vaccine prevents other sexually transmitted diseases, nor do they treat HPV infection or cervical cancer.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of girls and boys, aged 11 or 12 years.[20,21,22,23,24] The goals of the current vaccination recommendations for adolescents are to prevent persistent HPV infections and the occurrence of anogenital warts beginning in young adulthood, and cervical, vaginal, vulvar, and anal cancers that occur later in life.
Although there are different ways of preventing HPV infection such as abstinence from sexual intercourse, the use of barrier methods of contraception, education and lifestyle modification, and HPV vaccination, it has been found that there is HPV transmission rate of 8-15% before initiation of sexual contact.[25] It is important to target the younger age group as it is vital to give the vaccine before exposure since younger people have higher immunogenicity.
Both Gardasil and Cervarix are proven to be effective only if given before infection with HPV, so it is recommended that they are given before an individual is sexually active. For females, ACIP recommends that Gardasil or Cervarix vaccination be given routinely at ages 11 or 12, although the series may be started for girls as early as 9 years of age. Vaccination is also recommended for girls and women aged 13–26 who have not been vaccinated already or who did not complete the three-dose series. If a woman reaches the age of 26 before completing the three-dose series, as per ACIP recommendations, she can still receive the remaining doses. Women who have abnormal Pap test results, which may indicate HPV infection, should still receive HPV vaccination if they are in the appropriate age group because the vaccine may protect them against high-risk HPV types that they have not yet acquired. However, these women should be told that the vaccination will not cure them of current HPV infections and that it will not treat the abnormal results of their Pap test.[23]
Cervical cancer mortality can be reduced by up to two-thirds, provided all women are vaccinated with long-term protection. In addition, the vaccines can reduce the health care burden and associated sequelae.[26]
Primary health care physicians, pediatricians, and nurses are all crucial in educating and encouraging patients to initiate and complete the HPV vaccine series. Patients are more likely to accept the vaccine if their health care providers recommend it to them.[27,28,29,30] The most common parental determinants effecting vaccination are knowledge, physician's recommendation, and the belief that vaccination will protect against genital warts and cervical cancer. The most common adolescent factors are knowledge and awareness of HPV, beliefs and attitudes about HPV vaccine, access to health care facilities, transportation, and health care priority. Young women may still rely on parental input or support for vaccination.
Although the proportion of participants who had prior knowledge of HPV vaccine was low, the majority of participants consented to initiate the HPV vaccine series. This was predominantly the result of their physician's encouragement and the free availability of vaccine. Therefore, the health care providers are a quintessential source of education and advocates of this relatively new vaccine.
Those who refused the HPV vaccine wanted more information about this novel vaccine. Some of the participants wanted to discuss this option with their family first. This underlines the highly family-oriented patient population and the fact that young patients depend on parents to make major healthcare decisions for them.
Due to increased incidence rates of some HPV-associated cancers and low vaccination coverage of adolescents, intensified prevention efforts including increased vaccination coverage are required for HPV-associated cancers.[31]
CONCLUSION
Knowledge and perception of HPV attributed cervical cancer risk, and HPV vaccination was low. Saudi women should be provided with more information, education, and awareness of HPV infection and its link to cervical cancer and genital warts to improve HPV vaccination rates. These measures will help raise acceptance of the vaccine, dispel myths, and eliminate stigma attached to STI prevention resulting in a reduction of HPV infection rates. Early education and improvement of accessibility can help decrease the risk of HPV in the kingdom. Culturally, sensitive interventions and an integrated approach are recommended to enhance knowledge, perception, and awareness of the HPV vaccine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to gratefully acknowledge the skillful assistance provided by Sahar Iqbal and the research staff in the preparation of this manuscript.
REFERENCES
- 1.WHO Fact Sheet: Sexually Transmitted Infections (STIs) [Last accessed on 2105 Jul 02]. Available from: http://www.who.int/mediacentre/factsheets/fs110/en/
- 2.Al-Muammar T, Al-Ahdal MN, Hassan A, Kessie G, Dela Cruz DM, Mohamed GE. Human papilloma virus-16/18 cervical infection among women attending a family medical clinic in Riyadh. Ann Saudi Med. 2007;27:1–5. doi: 10.5144/0256-4947.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsbeih G. HPV infection in cervical and other cancers in Saudi Arabia: Implication for prevention and vaccination. Front Oncol. 2014;4:65. doi: 10.3389/fonc.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Saudi Cancer Registry. 2010. [Last accessed on 2015 Jul 05]. Available from: http://www.chs.gov.sa/Ar/mediacenter/NewsLetter/2010%20Report%20(1).pdf .
- 6.Alsbeih G, Ahmed R, Al-Harbi N, Venturina LA, Tulbah A, Balaraj K. Prevalence and genotypes’ distribution of human papillomavirus in invasive cervical cancer in Saudi Arabia. Gynecol Oncol. 2011;121:522–6. doi: 10.1016/j.ygyno.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Al-Badawi IA, Al-Suwaine A, Al-Aker M, Asaad L, Alaidan A, Tulbah A, et al. Detection and genotyping of human papilloma virus in cervical cancer specimens from Saudi patients. Int J Gynecol Cancer. 2011;21:907–10. doi: 10.1097/IGC.0b013e318214219f. [DOI] [PubMed] [Google Scholar]
- 8.Lowndes CM. Vaccines for cervical cancer. Epidemiol Infect. 2006;134:1–12. doi: 10.1017/S0950268805005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: A randomized intervention study of written information about HPV. Pediatrics. 2006;117:1486–93. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- 10.Walsh CD, Gera A, Shah M, Sharma A, Powell JE, Wilson S. Public knowledge and attitudes towards human papilloma virus (HPV) vaccination. BMC Public Health. 2008;8:368. doi: 10.1186/1471-2458-8-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong DK, Handley JM. Anogenital warts in prepubertal children: Pathogenesis, HPV typing and management. Int J STD AIDS. 1997;8:78–81. doi: 10.1258/0956462971919598. [DOI] [PubMed] [Google Scholar]
- 12.Sellors JW, Law C. Anogenital human papillomavirus infection. Changes in understanding and management. Can Fam Physician. 1994;40:93–101. [PMC free article] [PubMed] [Google Scholar]
- 13.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 15.Winer RL, Hughes JP, Feng Q, O’Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 16.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 17.Petrosky E, Bocchini JA, Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Cuzick J. Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines. 2015;14:1047–9. doi: 10.1586/14760584.2015.1051470. [DOI] [PubMed] [Google Scholar]
- 19.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 21.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 22.Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, et al. Human papillomavirus vaccine introduction - The first five years. Vaccine. 2012;30(Suppl 5):F139–48. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–9. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males - Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 25.Sait KH. Attitudes, knowledge, and practices in relation to cervical cancer and its screening among women in Saudi Arabia. Saudi Med J. 2009;30:1208–12. [PubMed] [Google Scholar]
- 26.Steinbrook R. The potential of human papillomavirus vaccines. N Engl J Med. 2006;354:1109–12. doi: 10.1056/NEJMp058305. [DOI] [PubMed] [Google Scholar]
- 27.Madhivanan P, Krupp K, Yashodha MN, Marlow L, Klausner JD, Reingold AL. Attitudes toward HPV vaccination among parents of adolescent girls in Mysore, India. Vaccine. 2009;27:5203–8. doi: 10.1016/j.vaccine.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 28.Jaspers L, Budiningsih S, Wolterbeek R, Henderson FC, Peters AA. Parental acceptance of human papillomavirus (HPV) vaccination in Indonesia: A cross-sectional study. Vaccine. 2011;29:7785–93. doi: 10.1016/j.vaccine.2011.07.107. [DOI] [PubMed] [Google Scholar]
- 29.Gordon D, Waller J, Marlow LA. Attitudes to HPV vaccination among mothers in the British Jewish community: Reasons for accepting or declining the vaccine. Vaccine. 2011;29:7350–6. doi: 10.1016/j.vaccine.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 30.Al-Dubai SA, Alshagga MA, Al-Naggar RA, Al-Jashamy K, Baobaid MF, Tuang CP, et al. Knowledge, attitudes and barriers for human papilloma virus (HPV) vaccines among Malaysian women. Asian Pac J Cancer Prev. 2010;11:887–92. [PubMed] [Google Scholar]
- 31.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]


