Abstract
Objectives:
To study the clinical presentation and treatment outcome of molar pregnancy at a Tertiary Care Hospital in Dammam, Saudi Arabia.
Materials and Methods:
Reviewed medical records of all molar pregnancy cases among all the deliveries at a tertiary care hospital in Dammam from 2005 to 2014, after approval by institutional ethical review committee. Data abstracted included patient's age, parity, presenting symptoms, gestational age at diagnosis, uterine size, ultrasonographic findings, BhCG level at the time of diagnosis and at follow-up after evacuation, and blood loss during evacuation. Data was entered and analyzed using Excel; frequency distribution for categorical variables and descriptive statistics for continuous variables were computed.
Results:
Of a total of 25,000 deliveries in ten years, 22 cases of complete molar pregnancy were encountered: 0.9 cases of molar pregnancy per 1000 pregnancies. Majority of patients (63.7%) were older than 35 years, and were nulliparous (45.5%). The commonest symptom was vaginal bleeding (86.4%) followed by hyperemesis gravidarum (41.0%); Hyperthyroidism was seen in 1 patient (4.5%). Ovarian enlargement by theca-lutin cyst was seen in 3 patients (13.6%). The majority of patients (63.6%) had normal BhCG within 9 weeks (63 days) after suction curettage. The majority of the cases followed a benign course.
Conclusion:
Aged older than 35 years seems a risk factor and vaginal bleeding is the commonest presenting symptom. Early booking of pregnant women to antenatal care clinics and routine first trimester ultrasound made diagnosis easier and earlier before complications appear.
Key words: Complete mole, GTD, hydatidform mole, molar pregnancy, Saudi Arabia
INTRODUCTION
Gestational trophoblastic disease (GTD) is a group of rare tumors resulting from abnormal growth of cells of trophoblastic epithelium of the placenta. The most common type of GTD is called a hydatidiform mole (HM), also known as a molar pregnancy. HM can be complete or partial: Complete mole (CM) accounts for the majority of HM which develops when either 1 or 2 sperm cells fertilize an egg containing a nucleus or DNA, with no identifiable fetus, whereas the partial mole contains some fetal tissue but no viable fetus.[1,2]
There is a vast difference in the incidence of molar pregnancy in the different regions of the world. The incidence of molar pregnancy is 0.5-1 per 1000 pregnancies in North America and Europe, 2 per 1000 pregnancies in Southeast Asia, 1 per 250 pregnancies in Philippines, and much higher in Taiwan, 1 per 125 pregnancies.[3,4] These variations may be attributed to the difference in the reporting data source, whether population-based or hospital-based. The other factors that may be responsible for this variation in the occurrence of molar pregnancy include socioeconomic and nutritional factors. In South Korea, the incidence of molar pregnancies has dropped from 4.4:1000 pregnancies in the 1960s to 1.6:1000 pregnancies in1990s mainly due to an improvement in living standards, socioeconomic and nutritional factors.[5] Low dietary carotene and animal fat consumption is associated with increased risk of molar pregnancy.[6,7] Two reports from Saudi Arabia in 1988, reported incidence of molar pregnancy 1:446 and 1:676 pregnancies;[8,9] whereas, a study in 2003 reported incidence as 0.9 per 1000 pregnancies.[10] This decrease in incidence may be related to an improvement in socioeconomic and dietary factors as animal studies have shown that diet can reset genetic outline.[11]
Among various reported risk factors, the most common are extreme maternal age, and previous history of HM. In complete mole, markedly elevated BhCG causes symptoms and complications.[12] The most common presenting symptom is vaginal bleeding. The diagnosis of molar pregnancy is usually made during the second trimester, and classical signs and symptoms include large uterine size, toxemia, anemia, hyperemesis, respiratory distress, and hypothyroidism.[12] In recent years, clinical presentation of molar pregnancy has changed largely because of diagnosis of CM at early gestational age.[12]
The purpose of this study was to look at the clinical presentation and treatment outcome of patients with complete molar pregnancy at a tertiary care teaching hospital in Dammam.
MATERIALS AND METHODS
After approval of institutional ethical review committee, the medical records of all molar pregnancy cases among all the deliveries at a tertiary care hospital in Dammam from 2005 to 2014 were reviewed. Data abstracted included patient's age, parity, presenting symptoms, gestational age at diagnosis, uterine size, ultrasonographic findings, BhCG leve at the time of diagnosis and at follow-up after evacuation, and blood loss during evacuation.
All patients were admitted after diagnosis, and 2 units of packed RBC were cross-matched. All procedures were done under general anesthesia; after dilatation of the cervix to 12 mm, suction curettage was performed and simultaneously an infusion of 40 units of syntocinon (Oxytocin® , Novartis Pharmaceuticals Ltd., UK) in one liter of normal saline was started.
BhCG with a radioimmunoassay sensitivity of 5 mIU/mL was used. Following evacuation, BhCG levels for all the patients were obtained. Patients were followed weekly in the clinic until 3 consecutive normal BhCG levels one week apart were achieved. Following the normalization of BhCG, patients were followed by monthly BhCG for 6 months. All patients were counseled to use combined contraceptive pills to prevent pregnancy during the period of follow up.
Data was entered and analyzed using Excel 2000 (Microsoft Corporation, Seattle, WA, USA). Statistical analysis included calculation of mean and standard deviation for continuous variables, and frequency distribution for categorical variables.
RESULTS
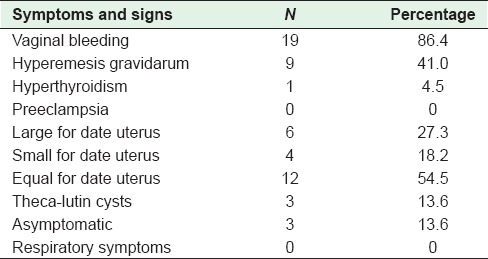
During the ten year period, a total of 25,000 deliveries were done at this hospital. Twenty-two cases of CM were encountered, i.e., 0.9 case per 1000 pregnancies. The age of patients in the study was 32.5 ± 1.2 years [Table 1]. The majority (63.7%) of patients were older than 35 years, and were nulliparous (45.5%) [Table 2]. Only one patient had a history of 2 consecutive molar pregnancies. The commonest symptom was vaginal bleeding (86.4%) followed by hyperemesis gravidarum (41.0%); hyperthyroidism was seen in 1 patient (4.5%) and none had pre-eclampsia [Table 3]. The mean gestational age at the time of diagnosis was 11.4 ± 0.3 weeks. Uterine size was large for dates for 27.3% cases [Table 1], and small for dates in 18.2% cases. Ovarian enlargement by theca-lutin cyst was seen in 3 patients (13.6%) [Table 3].
Table 1.
Mean age, gestational age at diagnosis, and blood loss for patients with molar pregnancy (n=22)
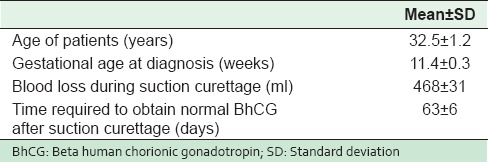
Table 2.
Frequency distribution of age and parity for patients with molar pregnancy (n=22)
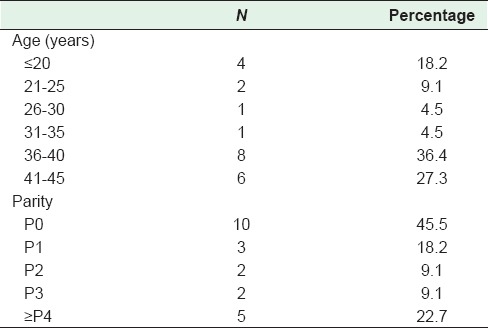
Table 3.
Signs and symptoms of patients with molar pregnancy (n=22)
BhCG level was variable in all patients but not less than 100,000 mIU/ml. Blood loss during evacuation was 468 ± 31 ml [Table 1], and 4 patients (18.2%) required blood transfusion because of low hemoglobin and symptoms of anemia. Time required to obtain normal BhCG values following evacuation was 63 ± 6 days [Table 1]. One patient, who had high BhCG titer (189,000 mIU/ml) at diagnosis, had an invasive mole and was treated with Methotrexate. None of the cases developed choriocarcinoma.
DISCUSSION
In the present study, 22 cases of CM were encountered out of 25,000 deliveries done at our hospital; 0.9 cases per 1000 pregnancies. This is similar to what has been reported previously.[10] The major risk factors for developing molar pregnancy are history of previous molar pregnancy and maternal age.[12] Generally, HM is more common in extreme maternal age. In the present study, 63.7% cases of HM occurred in women older than 35 years, and 18.2% in women less than 20 years of age, a finding consistent with previous studies.
The study by Gockley et al., indicated that compared to average age women, adolescents had seven times higher risk of developing CM, and older women had two times higher risk.[6] Similarly, studies have shown a higher risk of CM among women who had a history of previous CM.[13,14] The families with intermarriages have shown familial history of molar pregnancy.[15] In the present study, one 20 year old patient had two previous consecutive molar pregnancies at the age of 16, and 18 years; there was no familial history of molar pregnancy or intermarriages.
In the last two decades, due to early diagnosis, there has been a change in the clinical picture of HM. Sun et al. reported fewer number of patients with CM presenting with vaginal bleeding. This was attributed to the implementation of early routine ultrasound for pregnant women in the region.[7] In contrast, a majority of cases in our study presented with vaginal bleeding. The reason for this may be the labeling of any spotting and brownish vaginal discharge by a patient as vaginal bleeding.
In the recent years, patients rarely present with a compound theca-lutin cyst in the ovaries.[12] In this study, only 3 patients (13.6%) had ovarian enlargement This low number is due to a policy to refer any patient with bleeding or hyperemesis in early pregnancy for ultrasound to exclude twins or molar pregnancy.
Pre-eclampsia in the second trimester, a typical feature of CM, is now rarely seen as the majority of cases are diagnosed early in the first trimester. In our study, the uterine size was large for dates in 27.3% of patients which is similar to what is reported in other studies.[16,17]
In our institution, the standard care for women with the diagnosis of complete mole is suction curettage irrespective of uterine size. Medical methods were not used for any patients because of increased need for subsequent chemotherapy.[18]
The American College of Obstetricians and Gynecologists recommends that for patients with HM, BhCG levels should be measured 48 hours after evacuation and every 1 to 2 weeks until levels are undetectable. After attaining undetectable levels, follow-up measurements are made at monthly intervals for an additional 6 months.[19] This short protocol has led to fewer patients lost to follow up, and an initiation of chemotherapy after early diagnosis for patients with potentially malignant changes. This protocol has also resulted in the reduction of the cost of BhCG assays and relief from the anxiety and fear from anticipated GTD.
During BhCG follow up, patients are advised not to get pregnant for at least 6 months after BhCG levels have normalized in case of CM, and for 12 months in GTD. The development of a new generation of reliable OCP has enabled women to avoid pregnancy.[20] Combined oral contraceptive pills (OCP) are the best choice; all patients in the study used OCP to prevent pregnancy and allowed post-evacuation BhCG monitoring. However, a study showed that the use of OCP before BhCG remission may increase risk of persistent GTD after a molar pregnancy;[21] other studies showed no increase in the risk of GTD with the use of OCP after molar pregnancy.[19,22,23]
The incidence of persistent trophoblastic disease with CM is 8%, and the risk of malignant complications with CM is 0.5%. One patient in the present study developed an invasive mole during follow up and required chemotherapy. Prophylactic chemotherapy after molar evacuation is not standard care in our institution. This patient was 18 weeks pregnant and uterine size corresponded to a 30 weeks pregnant uterus, and BhCG at diagnosis was 189,000 mIU/ml. A study showed that a single course of folinic acid and methotrexate decreased the incidence of post molar GTD from 47.4% to 14.3% (p < 0.05) in patients with high-risk moles.[23] Prophylactic chemotherapy may decrease the risk of GTN in women with CM who are at a higher risk of malignant transformation. However, currently, there is limited evidence in support of this practice which is currently not recommended because of the risk of drug resistance, delay in the treatment of GTN, and unnecessary exposure of women to toxic side effects.[24]
CONCLUSION
Molar pregnancy is an uncommon condition in our region. Women aged older than 35 years and nulliparous are at a higher risk of developing molar pregnancy, with vaginal bleeding as the commonest presenting symptom. Patients with high BhCG levels (>100,000 mIU/ml) and large for date uteri at diagnosis, are at high risk of developing GTD, and need a careful follow-up thereafter. Early booking of pregnant women to antenatal care clinics and routine first trimester ultrasound has made diagnosis easier and earlier before any complications arise. Histological review for all miscarriages is mandatory for the detection and diagnosis of HM. The majority of cases followed a benign course. The weakness of this study is retrospective review of the data and low number of patients. The hospital-based data from other regions are necessary for the calculation of the real incidence of molar pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Petts G, Fisher RA, Short D, Lindsay I, Seckl MJ, Sebire NJ. Histopathological and immunohistochemical features of early hydatidiform mole in relation to subsequent development of persistent gestational trophoblastic disease. J Reprod Med. 2014;59:213–20. [PubMed] [Google Scholar]
- 2.Kolomietz E, Maire G, Nanji S, Chang MC, Vlasschaert M, Dodge J, et al. Placental molar disease: What are the benefits and barriers to the adoption of a comprehensive diagnostic service? Int J Gynecol Pathol. 2015;34:411–8. doi: 10.1097/PGP.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 3.Eysbouts YK, Bulten J, Ottevanger PB, Thomas CM, Ten Kate-Booij MJ, van Herwaarden AE, et al. Trends in incidence for gestational trophoblastic disease over the last 20 years in a population-based study. Gynecol Oncol. 2016;140:70–5. doi: 10.1016/j.ygyno.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Levine DA, De Los Santos JF, Fleming GF. Handbook for Principles and Practice of Gynecologic Oncology. 2nd ed. Philadelphia: Wolters Kluwer; 2015. Molar pregnancy and gestational trophoblastic neoplasms. Ch. 12. [Google Scholar]
- 5.Martin BH, Kim JH. Changes in gestational trophoblastic tumors over four decades. A Korean experience. J Reprod Med. 1998;43:60–8. [PubMed] [Google Scholar]
- 6.Gockley AA, Melamed A, Joseph NT, Clapp M, Sun SY, Goldstein DP, et al. The effect of adolescence and advanced maternal age on the incidence of complete and partial molar pregnancy. Gynecol Oncol. 2016;140:470–3. doi: 10.1016/j.ygyno.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Sun SY, Melamed A, Goldstein DP, Bernstein MR, Horowitz NS, Moron AF, et al. Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: Does early diagnosis alter risk for gestational trophoblastic neoplasia? Gynecol Oncol. 2015;138:46–9. doi: 10.1016/j.ygyno.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay SK, Sengupta BS, al-Ghreimil M, Edrees YB, Lambourne A. Epidemiologic study of gestational trophoblastic diseases in Saudi Arabia. Surg Gynecol Obstet. 1988;167:393–8. [PubMed] [Google Scholar]
- 9.Felemban AA, Bakri YN, Alkharif HA, Altuwaijri SM, Shalhoub J, Berkowitz RS. Complete molar pregnancy. Clinical trends at King Fahad Hospital, Riyadh, Kingdom of Saudi Arabia. J Reprod Med. 1998;43:11–3. [PubMed] [Google Scholar]
- 10.Khashoggi TY. Prevalence of gestational trophoblastic disease. A single institution experience. Saudi Med J. 2003;24:1329–33. [PubMed] [Google Scholar]
- 11.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–8. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Joneborg U, Marions L. Current clinical features of complete and partial hydatidiform mole in Sweden. J Reprod Med. 2014;59:51–5. [PubMed] [Google Scholar]
- 13.Sebire NJ, Fisher RA, Foskett M, Rees H, Seckl MJ, Newlands ES. Risk of recurrent hydatidiform mole and subsequent pregnancy outcome following complete or partial hydatidiform molar pregnancy. BJOG. 2003;110:22–6. [PubMed] [Google Scholar]
- 14.Eagles N, Sebire NJ, Short D, Savage PM, Seckl MJ, Fisher RA. Risk of recurrent molar pregnancies following complete and partial hydatidiform moles. Hum Reprod. 2015;30:2055–63. doi: 10.1093/humrep/dev169. [DOI] [PubMed] [Google Scholar]
- 15.Fallahian M, Foroughi F, Vasei M, Tavana S, Ghanbary M, Monajemzadeh M, et al. Outcome of subsequent pregnancies in familial molar pregnancy. Int J Fertil Steril. 2013;7:63–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Lindholm H, Flam F. The diagnosis of molar pregnancy by sonography and gross morphology. Acta Obstet Gynecol Scand. 1999;78:6–9. [PubMed] [Google Scholar]
- 17.Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86:775–9. doi: 10.1016/0029-7844(95)00268-V. [DOI] [PubMed] [Google Scholar]
- 18.Tidy JA, Gillespie AM, Bright N, Radstone CR, Coleman RE, Hancock BW. Gestational trophoblastic disease: A study of mode of evacuation and subsequent need for treatment with chemotherapy. Gynecol Oncol. 2000;78(3 Pt 1):309–12. doi: 10.1006/gyno.2000.5839. [DOI] [PubMed] [Google Scholar]
- 19.Committee on Practice Bulletins. Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #53. Diagnosis and treatment of gestational trophoblastic disease. Obstet Gynecol. 2004;103:1365–77. doi: 10.1097/00006250-200406000-00051. [DOI] [PubMed] [Google Scholar]
- 20.Braga A, Maestá I, Short D, Savage P, Harvey R, Seckl MJ. Hormonal contraceptive use before hCG remission does not increase the riskof gestational trophoblastic neoplasia following complete hydatidiform mole: A historical database review. BJOG. 2016;123:1330–5. doi: 10.1111/1471-0528.13617. [DOI] [PubMed] [Google Scholar]
- 21.Gaffield ME, Kapp N, Curtis KM. Combined oral contraceptive and intrauterine device use among women with gestational trophoblastic disease. Contraception. 2009;80:363–71. doi: 10.1016/j.contraception.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Bakhtiyari M, Mirzamoradi M, Kimyaiee P, Aghaie A, Mansournia MA, Ashrafi-Vand S, et al. Postmolar gestational trophoblastic neoplasia: Beyond the traditional risk factors. Fertil Steril. 2015;104:649–54. doi: 10.1016/j.fertnstert.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Savage PM, Sita-Lumsden A, Dickson S, Iyer R, Everard J, Coleman R, et al. The relationship of maternal age to molar pregnancy incidence, risks for chemotherapy and subsequent pregnancy outcome. J Obstet Gynaecol. 2013;33:406–11. doi: 10.3109/01443615.2013.771159. [DOI] [PubMed] [Google Scholar]
- 24.Fu J, Fang F, Xie L, Chen H, He F, Wu T, et al. Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2012;10:CD007289. doi: 10.1002/14651858.CD007289.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


