Abstract
Introduction Health information technology (HIT) has the potential to play a significant role in the management of cancer. The purpose of this review is to identify and examine empirical studies that investigate the impact of HIT in cancer care on different levels of the care continuum.
Methods Electronic searches were performed in four academic databases. The authors used a three-step search process to identify 122 studies that met specific inclusion criteria. Next, a coding sheet was used to extract information from each included article to use in an analysis. Logistic regression was used to determine study-specific characteristics that were associated with positive findings.
Results Overall, 72.4% of published analyses reported a beneficial effect of HIT. Multivariate analysis found that the impact of HIT differs across the cancer continuum with studies targeting diagnosis and treatment being, respectively, 77 (P = .001) and 39 (P = .039) percentage points less likely to report a beneficial effect when compared to those targeting prevention. In addition, studies targeting HIT to patients were 31 percentage points less likely to find a beneficial effect than those targeting providers (P = .030). Lastly, studies assessing behavior change as an outcome were 41 percentage points less likely to find a beneficial effect (P = .006), while studies targeting decision making were 27 percentage points more likely to find a beneficial effect (P = .034).
Conclusion Based on current evidence, HIT interventions seem to be more successful when targeting physicians, care in the prevention phase of the cancer continuum, and/or decision making. An agenda for future research is discussed.
Keywords: cancer, health information technology, systematic review, meta-analysis
In 2015, more than 1.6 million new cases of cancer are estimated to be diagnosed while another 589 430 deaths are expected to occur, making it the second most common cause of death in the United States.1–3 Much of this morbidity and mortality can be lessened by efforts targeting cancer prevention,4,5 early detection,4,6 reducing the risk of missed or delayed diagnosis,7,8 and improving quality of care during treatment and survivorship.9 The use of health information technology (HIT) has the potential to transform the health care system10 and play a significant role in the management of chronic diseases.11 HIT has the potential to enhance the management and care given to high-risk individuals, enhance patient safety as a result of interactions and missed opportunities, and improve the coordination of care with better information sharing.11 In addition, HIT has the ability to foster cancer care standardization and improve providers’ ability to be consistent with their recommendations and care protocols.12,13
The levels of the cancer control continuum (i.e., risk assessment, prevention, detection, diagnosis, treatment, survivorship, end-of-life care)14,15 represent many opportunities for the use of HIT to improve cancer care.12,13,16 Several HIT tools such as electronic reminders and electronic access to clinical guidelines aid decision-making by providing physicians with pertinent person-specific information and evidence-based recommendations at the point of care.17,18 This is particularly important given the important roles that physicians play in cancer care19–24 and the documented variability in their recommendation of cancer screening21 and other treatments.9 Despite the theoretical benefits that HIT can play in improving cancer care across the continuum, as well as a growth in the current evidence, to our knowledge very few research studies have empirically examined and synthesized the literature with regards to HIT’s impact on cancer care. One recent systematic review conducted by Koskan and colleagues25 narrowly looks at the use of social media in cancer-related research. Another systematic review conducted by Jimbo and colleagues26 looked only at HIT focusing on cancer detection and reported modest improvements in cancer screening as a result of HIT use. Eadie and colleagues27 reviewed the effectiveness of computer-assisted diagnosis as it pertains to cancer and found a beneficial effect for breast cancer diagnosis only. Despite these findings, less is known about the impact of HIT on other levels of the cancer continuum.
The purpose of the current study is to identify and examine all articles that investigate the impact of HIT on any level of the cancer care continuum. Because of the increasing deployment of HIT as a result of government incentives28 and the rapidly evolving field of HIT, this review will also seek to capture more contemporary HIT and cancer studies that appear in the literature. Therefore, the current review contributes to both the HIT and cancer literature by examining HIT’s impact on a specific disease class and advancing our understanding of the potential of HIT to improve cancer outcomes on all points of care on the cancer continuum. The specific aims of this review are: 1) to assess the overall impact of HIT on cancer outcomes; 2) to understand if and how this impact differs by continuum level; 3) to examine the associations between study characteristics and the likelihood of reporting positive findings; and 4) to propose directions for future research based on the current literature.
METHODS
Search strategy
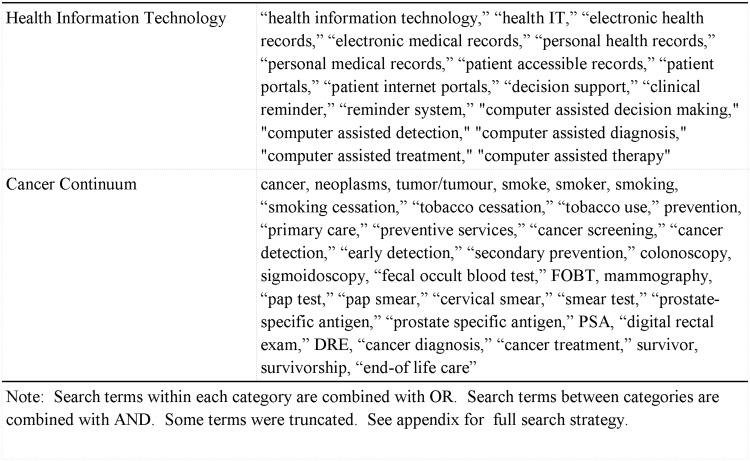
For the current study, we followed all of the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement that were applicable to our study.29 We conducted a systematic review of empirical research on the impact of HIT on cancer outcomes across the continuum. For the purposes of this review, HIT is defined as information systems designed to communicate health information to patients or providers through the use of computers, the Internet, or other technologies. In order to identify relevant articles, the following databases were used: 1) PubMed (Cancer Subset), 2) CINAHL Plus, 3) MEDLINE, and 4) PsycINFO. These databases were searched using a combination of Medical Subject Headings terms and keywords that were developed based on the literature and used in previous systematic reviews of either cancer or HIT. To capture articles addressing HIT, we used search terms such as “health information technology,” “electronic health records,” “electronic medical records,” “personal health records,” “clinical decision support,” and “clinical reminder.” Furthermore, the following search terms were added to capture the use of HIT as it applies to cancer care: “cancer,” “neoplasm,” “tobacco use,” “screening,” “colonoscopy,” “mammography,” “pap test,” and “survivorship.” Figure 1 shows a complete list of keywords and phrases used in our search. The complete search strategy can be found in Supplementary Appendix 1. For the current systematic review, we sought to identify articles that were published in peer reviewed journals in the English language. In order to solely focus on the impact of HIT on different elements of cancer care, we excluded feasibility studies, HIT development and validation articles, and nonempirical studies (e.g., commentaries, published study descriptions, letters-to-the-editor, etc.). Additional studies were identified through the use of a snowball searching technique whereby we examined the reference lists of studies that met our inclusion criteria for additional studies to include.
Figure 1:
Operationalization of the search terms.
Study Selection
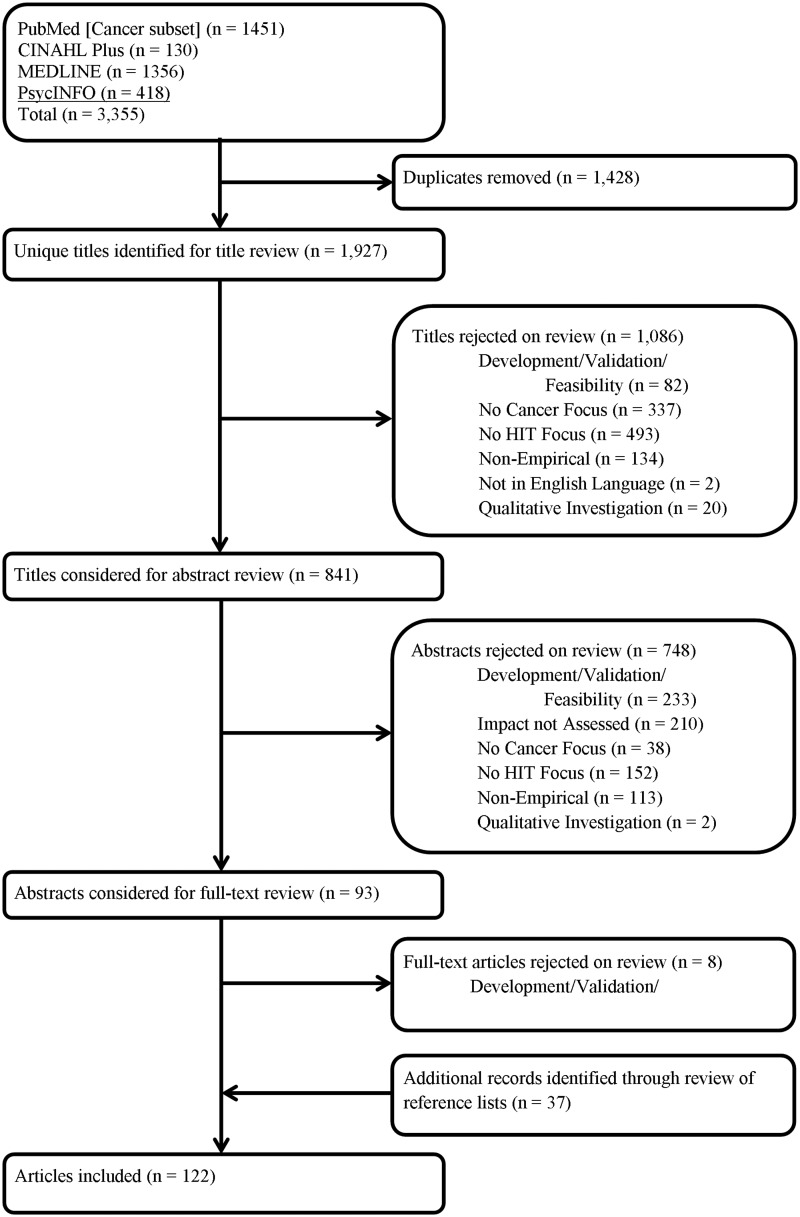
Studies were eligible for inclusion if they presented empirical data from a study aimed at understanding the impact of HIT on an outcome at some point of care on the cancer continuum. We included quantitative research studies published in the English language from the year 2000 to June of 2014. Articles published prior to 2000 were excluded due to the high variability of HIT systems during this period, many of which resulted in design and implementation issues.30 International studies, however, were included in order to capture differences in the impact of HIT across different settings. Two reviewers individually assessed the relevance of each study. Any disagreements between reviewers were reconciled by consensus. We used a three-step inclusion process illustrated in Figure 2. In step 1, we examined article titles and excluded articles that clearly did not have a focus on either HIT or cancer care. However, we erred on the side of inclusion when the study focus was unclear. Due to the nature of systematic reviews and the broad search terms used in this study, our initial search retrieved citations that were clearly not related to our study topic, e.g., articles focusing on user acceptance or satisfaction with HIT. In step 2, the abstracts of citations were retrieved and examined for all studies that were not excluded in phase 1. Similarly, we excluded article abstracts that clearly did not have a focus on either HIT or cancer care, were not empirical, were qualitative, and did not assess the impact of HIT on cancer while again erring on the side of inclusion when any element was unclear. Lastly, the full-text articles of the remaining citations were obtained for independent assessment.
Figure 2:
Systematic review flowchart of the impact of health information technology across the cancer care continuum literature.
Data extraction
A coding sheet was developed specifically for this study to systematically extract information from all included studies. Information extracted included the study’s study design; cancer continuum level; study setting; whether the HIT focused on the patient, provider, or both; the cancer type studied; and the type of HIT intervention (e.g., electronic health record (EHR), clinical decision support (CDS) system, etc.). Additionally, the outcome of the included study was extracted (e.g., behavior change, improved decision making, pain management, psychosocial issues, screening rates, etc.). Each included published study represented a unique analysis except when multiple outcomes were assessed within the same article. When multiple outcomes were assessed within an individual study (e.g., the impact of EHRs on documentation of smoking status, receipt of cessation counseling, and prescription of smoking cessation medication),31 each outcome was extracted separately and entered discretely for our analysis. In addition, when studies assessed outcomes for different target populations (e.g., patients and physicians) these analyses would also be considered discrete. For example, one study addressing smoking cessation assessed smoking cessation counseling as provided by primary care physicians, as well as the smoking behaviors of patients. Using this example, both of these study outcomes were considered discrete due to the outcomes being different, as well as targeting different populations. Lastly, for each discrete analysis, we determined whether HIT was found to have a statistically significant beneficial impact on the outcome variable being studied.
Data analysis
Descriptive analyses were used to examine the distribution of all variables extracted from included studies. We then employed the use of the chi-square statistic to investigate differences in study characteristics of articles that found a beneficial effect compared to those that did not. Next, consistent with previous work,32 variables that were found to be associated with a beneficial effect at the P < .20 level in bivariate analyses were entered into the logistic regression models to identify study characteristics associated with reporting beneficial results. When variables that were strongly associated met this cutoff criterion, a single variable was chosen to avoid collinearity. In addition, our regression analysis appropriately takes into consideration the nested nature of discrete analyses within articles by clustering standard errors within each published study. Our findings are reported as odds ratios (ORs); however, ORs are difficult to intuitively understand leading them to be frequently misinterpreted.33 To facilitate interpretation, we also provide absolute risk differences for our significant findings. This method of presenting results is preferable to the use of ORs because it represents the probability of the outcome occurring in a group with respect to the reference category. Lastly, to identify gaps in the current available literature, we cross-tabulated the frequencies of cancer type studied and the level of the cancer care continuum level with the type of HIT intervention used. The data were analyzed using the Stata statistical software (version 13; StataCorp, College Station, TX, USA).
RESULTS
Our keyword search identified an initial yield of 3355 citations (see Figure 2). After removing duplicates, our initial search yield was reduced to 1927 studies. After applying the restrictions for inclusion in the title and abstract review, 1842 studies were excluded. The primary reasons for exclusion are identified in Figure 2 leaving 85 included studies. Then, our snowball search method identified an additional 37 studies, resulting in a total of 122 included articles. As stated previously, when studies identified multiple outcomes, each outcome represented an individual analyses examining the relationship between HIT and a cancer outcome measure. When taking these individual analyses into account, we end up with a total of 156 individual analyses.
Descriptive Analyses
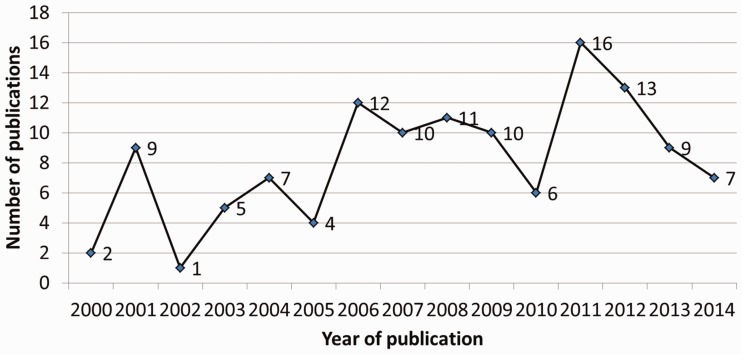
A time trend analysis of included studies can be found in Figure 3. The number of published studies appears to increase over time with fluctuations; and the highest amount of studies on cancer and HIT were published in the year 2011 (n = 16, 13%). Characteristics of the included articles can be found in Table 1. A large proportion of analyses included in our review take place in an academic health center (n = 58, 37.4%) or primary care (n = 31, 20.0%) setting. Nearly half of the analyses followed an experimental design (n = 75, 48.1%). Also, the largest proportion of analyses are focused on breast cancer (n = 65, 41.7%) and smoking cessation (n = 35, 22.4%). By continuum, the largest proportion of analyses fall under the level of diagnosis (n = 49, 31.4%) followed by prevention (n = 39, 25.0%). In addition, the largest proportion of outcomes assessed pertained to decision making (n = 62, 39.7%) followed by 35 (22.4%) analyses that assessed behavior change. Lastly, in most of the analyses the HIT intervention targets the provider or physician (n = 93, 59.6%), as opposed to the patient (n = 55, 35.3%).
Figure 3:
Time trend analysis of included studies from 2000 to 2014.
Table 1:
Characteristics of studies included in this review
| Total N (%) | |
|---|---|
| Funding | |
| Funded | 100 (64.1) |
| Not Funded | 56 (35.9) |
| Study Design | |
| Experimental | 75 (48.1) |
| Non-experimental | 81 (51.9) |
| Sample Size | |
| 4–93 (Quartile 1) | 39 (25.0) |
| 100–264 (Quartile 2) | 39 (25.0) |
| 278–1295 (Quartile 3) | 39 (25.0) |
| 1508–4 352 082 (Quartile 4) | 39 (25.0) |
| Cancer Typea | |
| Breast Cancer | 65 (41.7) |
| Cervical Cancer | 6 (3.9) |
| Colorectal Cancer | 26 (16.7) |
| Lung | 9 (5.8) |
| Prostate | 6 (3.9) |
| General Cancer | 10 (6.4) |
| Other | 9 (5.8) |
| Smoking Cessationa | 35 (22.4) |
| Continuum Level | |
| Risk Assessment | 15 (9.6) |
| Prevention | 39 (25.0) |
| Detection | 28 (18.0) |
| Diagnosis | 49 (31.4) |
| Treatment | 23 (14.7) |
| Survivorship | 2 (1.3) |
| End-of-life Care | 0 (0.0) |
| HIT Intervention | |
| Clinical Decision Support System | 103 (66.0) |
| Health Record | 19 (12.2) |
| Web-based | 22 (14.1) |
| Other | 12 (7.7) |
| HIT Intervention Focus | |
| Patient | 55 (35.3) |
| Provider | 93 (59.6) |
| Both | 8 (5.1) |
| Study Outcomes | |
| Behavior Change | 35 (22.4) |
| Decision Making | 62 (39.7) |
| Education | 5 (3.2) |
| Pain Management | 1 (0.6) |
| Psychosocial | 8 (5.1) |
| Screening Rates | 20 (12.8) |
| Other | 25 (16.0) |
aNote: Some studies provide results for more than 1 cancer type. As a result, the cancer-type and smoking cessation variables are not mutually exclusive and do not add up to the 156 total observations.
In Table 2, we present the cross-tabulation of different HIT applications (CDS systems, health record interventions, web-based applications, and other HIT interventions) with cancer type and cancer continuum. The greatest proportion of these analyses implement CDS systems (n = 103, 66.0%). Specifically, CDS systems represent the largest proportion of HIT applications used for several cancers including breast cancer (78.5%), colorectal cancer (42.3%), prostate cancer (66.7%), and other cancers (66.7%). The next most common HIT intervention are web-based applications (n = 22, 14.1%) which are relatively common for the detection continuum level. Health record-based interventions were less common (n = 19, 12.2%); but when used were relatively common for cervical cancer (n = 3, 50.0%); detection (n = 9, 32.1%); and, to a lesser degree, treatment (n = 6, 26.1%).
Table 2:
Cross-tabulation of HIT interventions, by cancer type and continuum level
| CDSS | Health Record | Web-based | Other | Total | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Cancer Typea | |||||
| Breast | 51 (78.5) | 6 (9.2) | 4 (6.2) | 4 (6.2) | 65 |
| Cervical | 3 (50.0) | 3 (50.0) | 0 (0.0) | 0 (0.0) | 6 |
| Colorectal | 11 (42.3) | 6 (23.1) | 6 (23.1) | 3 (11.5) | 26 |
| Lung | 8 (88.9) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 9 |
| Prostate | 4 (66.7) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 6 |
| General | 3 (30.0) | 2 (20.0) | 3 (30.0) | 2 (20.0) | 10 |
| Other | 6 (66.7) | 2 (22.2) | 0 (0.0 | 1 (11.1) | 9 |
| Smoking Cessationa | 23 (65.7) | 4 (11.4) | 7 (20.0) | 1 (2.9) | 35 |
| Total (%) | 109 (65.7) | 23 (13.9) | 22 (13.3) | 12 (7.2) | 166 |
| Continuum Level | |||||
| Risk Assessment | 11 (73.3) | 0 (0.0) | 1 (6.7) | 3 (20.0) | 15 |
| Prevention | 26 (66.7) | 4 (10.3) | 7 (18.0) | 2 (5.1) | 39 |
| Detection | 7 (25.0) | 9 (32.1) | 9 (32.1) | 3 (10.7) | 28 |
| Diagnosis | 48 (98.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 49 |
| Treatment | 11 (47.8) | 6 (26.1) | 2 (8.7) | 4 (17.4) | 23 |
| Survivorship | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 |
| End-of-life Care | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 |
| Total (%) | 103 (66.0) | 19 (12.2) | 22 (14.1) | 12 (7.7) | 156 |
aNote: Some studies provide results for more than 1 cancer type. As a result, the cancer-type and smoking cessation variables are not mutually exclusive and do not add up to the 156 total observations.
Bivariate Analyses
Overall, 72.4% of published analyses reported a beneficial effect from HIT (see Table 3). Analyses using experimental study designs (61.3% vs 82.7%, P = .003) in addition to analyses that focus on smoking cessation (57.1% vs 76.9%, P = .022) were less likely to find a beneficial effect. In addition, analyses that target patients were less likely to find a beneficial effect (54.6% vs 82.2%, P < .001). Lastly, individual analyses assessing decision making (82.3% vs 66.0%, P = .026) were more likely to find a beneficial effect. Conversely, analyses looking at behavior change (54.3% vs 77.7%, P = .006) and psychosocial and coping outcomes (37.5% vs 74.3%, P = .023) were less likely to find a beneficial effect.
Table 3:
Bivariate relationships of beneficial and other effects for studies included in this review
| Total | Beneficial Effect | P | |
|---|---|---|---|
| n (%) | n (%) | ||
| Funding | |||
| Funded | 100 (100.0) | 69 (69.0) | .199 |
| Not Funded | 56 (100.0) | 44 (78.6) | |
| Study Design | |||
| Experimental | 75 (100.0) | 46 (61.3) | .003 |
| Non-experimental | 81 (100.0) | 67 (82.7) | |
| Sample Size | |||
| 4–93 (Quartile 1) | 39 (100.0) | 33 (84.6) | .049 |
| 100–264 (Quartile 2) | 39 (100.0) | 29 (74.4) | .756 |
| 278–1295 (Quartile 3) | 39 (100.0) | 24 (61.5) | .079 |
| 1508–4 352 082 (Quartile 4) | 39 (100.0) | 27 (69.2) | .605 |
| Cancer Typea | |||
| Breast Cancer | 65 (100.0) | 48 (73.9) | .739 |
| Cervical Cancer | 6 (100.0) | 6 (100.0) | .123 |
| Colorectal Cancer | 26 (100.0) | 18 (69.2) | .689 |
| Lung | 9 (100.0) | 8 (88.9) | .255 |
| Prostate | 6 (100.0) | 5 (83.3) | .532 |
| General Cancer | 10 (100.0) | 6 (60.0) | .363 |
| Other | 9 (100.0) | 7 (77.8) | .712 |
| Smoking Cessationa | 35 (100.0) | 20 (57.1) | .022 |
| Continuum Level | |||
| Risk Assessment | 15 (100.0) | 11 (73.3) | .935 |
| Prevention | 39 (100.0) | 24 (61.5) | .039 |
| Detection | 28 (100.0) | 23 (82.1) | .204 |
| Diagnosis | 49 (100.0) | 25 (75.5) | .561 |
| Treatment | 23 (100.0) | 16 (69.6) | .739 |
| Survivorship | 2 (100.0) | 2 (100.0) | .380 |
| HIT Intervention | |||
| Clinical Decision Support System | 103 (100.0) | 78 (75.7) | .200 |
| Health Record | 19 (100.0) | 13 (68.4) | .676 |
| Web-based | 22 (100.0) | 15 (68.2) | .630 |
| Other | 12 (100.0) | 7 (58.3) | .255 |
| HIT Intervention Focus | |||
| Patient | 55 (100.0) | 30 (54.6) | <.001 |
| Provider | 93 (100.0) | 76 (81.7) | .002 |
| Both | 8 (100.0) | 7 (87.5) | .318 |
| Study Outcomes | |||
| Behavior Change | 35 (100.0) | 19 (54.3) | .006 |
| Decision Making | 62 (100.0) | 51 (82.3) | .026 |
| Education | 5 (100.0) | 4 (80.0) | .700 |
| Pain Management | 1 (100.0) | 1 (100.0) | .536 |
| Psychosocial | 8 (100.0) | 3 (37.5) | .023 |
| Screening Rates | 20 (100.0) | 16 (80.0) | .417 |
| Other | 25 (100.0) | 19 (76.0) | .663 |
| Total | 156 (100.0) | 113 (72.4) |
aNote: Some studies provide results for more than 1 cancer type. As a result, the cancer-type and smoking cessation variables are not mutually exclusive and do not add up to the 156 total observations.
Multivariate Analyses
Table 4 presents the results of multivariate analyses controlling for various study characteristics including sample size, study design (experimental or other), the type of HIT application, HIT target population (i.e., patient, provider, or both), continuum level, and the study outcome assessed by the individual analyses. We found that studies focusing on diagnosis and treatment were, respectively, 77 (P = .001) and 39 (P = .039) percentage points less likely to find a beneficial effect than those focused on prevention. Also, studies that target patients (as opposed to physicians or other health care providers) were 31 percentage points less likely to find a beneficial effect (P = .030). Lastly, there were differences in the likelihood of studies finding a beneficial effect when assessing specific outcomes. Studies assessing behavior change (e.g., abstinence from smoking, changes in guideline recommendations, etc.) were 41 percentage points less likely to find a beneficial effect than studies assessing other outcomes (P = .006). Conversely, studies assessing decision making were 27 percentage points more likely to find a beneficial effect (P = .034).
Table 4:
Relationship between study characteristics and beneficial outcomes
| Absolute Risk Difference (%) | Odds Ratio (95% CI) | |
|---|---|---|
| Funding | ||
| Funded | −0.02 | 0.91 (0.28–2.99) |
| Not Funded | Ref | Ref |
| Study Design | ||
| Experimental | −0.09 | 0.60 (0.16–2.21) |
| Non-experimental | Ref | Ref |
| Sample Size | ||
| 4–93 (Quartile 1) | Ref | Ref |
| 100–264 (Quartile 2) | −0.07 | 0.66 (0.19–2.34) |
| 278–1295 (Quartile 3) | −0.13 | 0.49 (0.15–1.55) |
| 1508–4 352 082 (Quartile 4) | 0.12 | 0.53 (0.15–1.91) |
| Continuum Level | ||
| Risk Assessment | −0.14 | 0.49 (0.08–2.94) |
| Prevention | Ref | Ref |
| Detection | −0.12 | 0.52 (0.12–2.20) |
| Diagnosis | −77** | 0.01 (0.001–0.17) |
| Treatment | −39* | 0.16 (0.03–0.91) |
| HIT Intervention Focus | ||
| Patient | −31* | 0.19 (0.04–0.86) |
| Provider | Ref | Ref |
| Both | −0.07 | 0.70 (0.05–9.62) |
| Study Outcomes | ||
| Behavior Change | −41** | 0.14 (0.03–0.57) |
| Decision Making | +27* | 5.80 (1.15–29.38) |
| Psychosocial | −30 | 0.25 (0.03–2.47) |
*P < .05.
**P < .01.
DISCUSSION
The main finding of this review is that the beneficial impact of HIT differs across the cancer continuum. More specifically, analyses targeting diagnosis and treatment were less likely to find a beneficial effect when compared to analyses targeting prevention. Due to the nature of applications designed for the areas of diagnosis and treatment, a possible challenge lies in the fact that these systems are more complex. In addition, these technologies require more information to come to a decision or recommendation. For example, an HIT application within the continuum levels of prevention and detection may only require a patient’s current smoking status or date of a patient’s last cancer screening. However, when diagnosing cancer, systems use computer analysis of digital images to differentiate between normal and abnormal images and more detailed patient data and characteristics (e.g., comorbidities, family history, etc.). A similar finding is reported by Eadie and colleagues27 who found that there was less evidence of a beneficial effect on cancer as a result of computer-assisted diagnosis systems.
The second major finding of the current review is that analyses that examined HIT interventions targeted to patients were less likely to find a beneficial outcome than articles that use HIT interventions targeted to physicians. This finding may indicate that current information technologies may not impact patients in the cancer care setting. However, significant hurdles exist when it comes to tailoring these technologies to this population. Several factors may be related to patients’ acceptance of HIT interventions such as education, prior computer experience, and computer anxiety34 making it difficult to discern the true effect of the technology as opposed to other factors.35 Studies that may not take these factors into consideration may be less likely to find a beneficial effect. Additionally, the lower likelihood of these studies to report positive findings may also be a result of the ineffective use of these technologies. A recent review of personal health records (PHRs) found that PHRs in cancer care were accepted by patients, however, were under- and ineffectively utilized.36
Another important finding of our systematic review is that studies that assessed the impact of HIT on behavioral change were less likely to find a beneficial effect. Conversely, studies that assessed the impact of HIT on improved decision making were more likely to find a beneficial effect. Behavior change may be an outcome that is more difficult to change with HIT applications because behavior change is a process that is dependent on other factors. Examples of other factors that can influence the behavior change process may include an individual’s readiness to change, the presence of reinforcing or protective factors, and an individual’s level of self-efficacy in performing or abstaining from a behavior. Decision making may be more likely to provide a beneficial effect as it may be accepted in the clinical setting by providers as a tool to aid them in making clinical decisions. Similarly, cancer patients may be more accepting of decision aids as they may have little knowledge about their cancer and are faced with difficult decisions. This finding is generally consistent with the initial goals of the development of HIT to improve decision making for chronic diseases.37
We also find that various applications of HIT are used differently throughout the different continuum levels. A vast majority of studies using CDS systems found a beneficial effect in terms of prevention, detection, diagnosis, and treatment. Lindholm and colleagues38 used clinical reminders to prompt clinicians to deliver treatment for tobacco dependence in the primary care setting and resulted in the increased delivery of treatment interventions for smokers and improvements in the clinical workflow. In addition, Nease and colleagues39 found that a reminder system led to increases in colorectal cancer screening in primary care practices. In the context of diagnosis, CDS systems are widely used in the form of computer-assisted diagnosis and computer-assisted detection systems. With respect to treatment, examples of CDS systems targeting providers are used to modify physician prescribing behavior40,41 and aiding in the pain management of cancer patients42 while those targeting patients are designed to serve as decision aids for treatments.43
More than half of studies using web-based applications found a beneficial effect; however, this finding varied by the continuum level. Rubenstein and colleagues44 used an internet-based familial risk assessment tool to collect family history for several cancers and provide tailored prevention messages which led to an increase in screening adherence, but no statistical difference existed between the intervention and control group. When it comes to prevention, Woolf and colleagues45 used a web-based approach to encourage patients to pursue health behaviors, such as smoking cessation, but did not provide significant results. When it comes to providers, Atlas and colleagues46 provided physicians with a web page listing that connected patients with providers and allowed for the ordering of mammograms, computer generated letters, and follow-up phone calls to patients which significantly increased screening rates.
Results also varied for interventions implementing a health record-based intervention (e.g., a PHR or enhancements to EHRs). Linder and colleagues31 enhanced their EHR to include smoking status icons for patients, provide tobacco treatment reminders, and included the addition of a Tobacco Smart Form to facilitate medication ordering and counseling referrals. This intervention improved clinical outcomes such as smoking status documentation, as well as improved counseling to smokers; however, no change was found in the prescription of cessation medications. Sequist and colleagues47 used a personal health record to deliver electronic messages to patients regarding colorectal cancer screening which led to an initial increase in screening rates; however, this difference was not sustained over the course of the study. A PHR-based intervention implemented by Krist and colleagues48 generated reminders to patients and led to an increase in screening for colorectal, breast, and cervical cancer. EHR interventions are also used in the detection continuum for quality improvement by providing feedback to physicians via audit and feedback. Ornstein and colleagues49 used this method to improve the proportion of patients who are up-to-date on colorectal cancer screening as well as increase screening recommendations in intervention sites.
Future research on HIT’s impact on cancer care should focus on specific types of cancer such as such as prostate cancer, ovarian cancer, and skin cancer which are underrepresented in the literature. In addition, while our systematic review indicates that HIT has been empirically assessed for most levels of the cancer continuum; we identified only one study in the continuum of survivorship and no studies assessing HIT’s impact on end-of-life care in the context of cancer care. Future research should assess the impact of HIT and technology-based alternatives to different elements of follow-up care for cancer survivors. While no current studies were uncovered pertaining to palliative and hospice care, this is consistent with another article reporting the underutilization of HIT in this area.50 In addition, more research should be devoted to the impact of internet-based survivorship care plans. Lastly, more research should be dedicated to patient-centered cancer care applications, including identifying success factors as well as studying factors leading to the acceptance of PHRs by cancer patients, though this may vary by population.
The main benefit of this study is that, to our knowledge, it is the first study to examine and compare the impact of HIT on all levels of the cancer continuum. While previous reviews have examined the impact of HIT, they were limited in scope or were confined to one category of cancer care on the continuum. In addition, our study has revealed some gaps in our current understanding of the impact of HIT on cancer care and proposes directions for future research. However, despite these benefits there are several limitations to our study worth noting. We recognize the possibility that our search strategy may not have captured all potential articles meeting our inclusion criteria. In order to minimize this, we retrieved the references of all included studies and reviewed them for additional citations. Our inclusion criteria may have also imposed an additional limitation given all studies published prior to 2000 were excluded. While our intentions were to exclude evaluation studies of HIT which may have suffered from design and implementation issues, this criteria may have also excluded useful studies assessing the impact of HIT on cancer care. Also, the relatively small number of published studies resulted in an overall small sample size limiting our ability to perform more complex statistical analysis. In addition, it was unclear in some situations what category of HIT a study should be categorized as (e.g., an EHR-based intervention that also included electronic reminders). In these cases, we chose to categorize the study as identified by the author in their title or abstract. Lastly, the included articles may be subject to publication bias as studies that report null or negative findings may be less likely to be published; however, this review did find a high quantity of studies reporting negative outcomes suggesting this limitation may be minor.
There is a growing body of literature examining the impact of HIT on cancer care which includes both experimental and observational data. In this review, we identify studies across the levels of the cancer continuum and find that differences do exist between the continuum levels with respect to reporting positive outcomes. HIT interventions seem to be more successful when targeting physicians, care in the prevention phase of the cancer continuum, and/or decision making as an outcome.
FUNDING
This work was supported by the National Cancer Institute (NCI) Cancer Prevention and Control Training Program Grant R25 CA04788.
COMPETING INTERESTS
None.
CONTRIBUTORS
W.T. and N.M. conceived and designed the study, performed the systematic review, and conducted the analysis. W.T. wrote the manuscript. N.M. critically revised the manuscript and provided insights on the review discussion. Both authors approved the final manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available online at http://jamia.oxfordjournals.org/.
REFERENCES
*A list of the included references for this systematic review can be found in the Supplementary Appendix 2 for this article.
- 1.Centers for Disease Control and Prevention. Leading causes of death. http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed April 22, 2015.
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer facts and figures 2015. Atlanta: Author; 2015. [Google Scholar]
- 4.American Cancer Society. Cancer prevention and early detection facts and figures 2013. Atlanta: Author; 2013. [Google Scholar]
- 5.Elk R, Landrine H. Cancer disparities: causes and evidence-based solutions. New York: Springer; 2011. [Google Scholar]
- 6.Clarke TC, Soler-Vila H, Fleming LE, et al. Trends in adherence to recommended cancer screening: the US population and working cancer survivors. Front Oncol. 2012;2:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh H, Daci K, Petersen LA, et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104:2543–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh H, Hirani K, Kadiyala H, et al. Characteristics and predictors of missed opportunities in lung cancer diagnosis: an electronic health record–based study. J Clin Oncol. 2010;28:3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer treatment and survivorship facts and figures 2012–2013. Atlanta: Author; 2012. [Google Scholar]
- 10.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 11.Rao S, Brammer C, McKethan A, et al. Health information technology: Transforming chronic disease management and care transitions. Prim Care. 2012;39:327–344. [DOI] [PubMed] [Google Scholar]
- 12.Wallace PJ. Reshaping cancer learning through the use of health information technology. Health Aff. 2007;26:w169–w177. [DOI] [PubMed] [Google Scholar]
- 13.Taplin SH, Clauser S, Rodgers AB, et al. Interfaces across the cancer continuum offer opportunities to improve the process of care. JNCI Monographs. 2010;2010:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Cancer control continuum. http://cancercontrol.cancer.gov/od/continuum.html. Accessed April 22, 2015. [Google Scholar]
- 15.Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesse BW, Hanna C, Massett HA, et al. Outside the box: Will information technology be a viable intervention to improve the quality of cancer care? JNCI Monographs. 2010;2010:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berner ES. Clinical decision support systems: state of the art. AHRQ Publication No. 09-0069-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- 19.Rippe JM. The case for medical management of obesity: a call for increased physician involvement. Obes Res. 1998;6:23S–33S. [DOI] [PubMed] [Google Scholar]
- 20.Hunt JR, Kristal AR, White E, et al. Physician recommendations for dietary change: their prevalence and impact in a population-based sample. Am J Public Health. 1995;85:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170–2178. [DOI] [PubMed] [Google Scholar]
- 22.Taylor V, Lessler D, Mertens K, et al. Colorectal cancer screening among African Americans: the importance of physician recommendation. J Natl Med Assoc. 2003;95:806–812. [PMC free article] [PubMed] [Google Scholar]
- 23.May DS, Kiefe CI, Funkhouser E, et al. Compliance with mammography guidelines: physician recommendation and patient adherence. Prev Med. 1999;28:386–394. [DOI] [PubMed] [Google Scholar]
- 24.Bazargan M, Bazargan SH, Calderón JL, et al. Mammography screening and breast self-examination among minority women in public housing projects: the impact of physician recommendation. Cell Mol Biol. 2003;49:1213–1218. [PubMed] [Google Scholar]
- 25.Koskan A, Klasko L, Davis SN, et al. Use and taxonomy of social media in cancer-related research: a systematic review. Am J Public Health. 2014;104:e20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimbo M, Nease DE, Ruffin MT, et al. Information technology and cancer prevention. CA Cancer J Clin. 2006;56:26–36. [DOI] [PubMed] [Google Scholar]
- 27.Eadie LH, Taylor P, Gibson AP. A systematic review of computer-assisted diagnosis in diagnostic cancer imaging. Eur J Radiol. 2012;81:e70–e76. [DOI] [PubMed] [Google Scholar]
- 28.Blumenthal D. Launching HITECH. N Engl J Med. 2010;362:382–385. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 30.Joyce P, Green R, Winch G. Healthcare delivery systems: designing quality into health information systems. Stud Health Technol Inform. 2007;129:43–47. [PubMed] [Google Scholar]
- 31.Linder JA, Rigotti NA, Schneider LI, et al. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med. 2009;169:781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. [DOI] [PubMed] [Google Scholar]
- 33.Tajeu GS, Sen B, Allison DB, et al. Misuse of odds ratios in obesity literature: an empirical analysis of published studies. Obesity. 2012;20:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Or CKL, Karsh B-T. A systematic review of patient acceptance of consumer health information technology. J Am Med Inform Assoc. 2009;16:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demiris G, Afrin LB, Speedie S, et al. Patient-centered applications: Use of information technology to promote disease management and wellness. A white paper by the AMIA knowledge in motion working group. J Am Med Inform Assoc. 2008;15:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaelber DC, Jha AK, Johnston D, et al. A research agenda for personal health records (PHRs). J Am Med Inform Assoc. 2008;15:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Aff. 2010;29:614–621. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm C, Adsit R, Bain P, et al. A demonstration project for using the electronic health record to identify and treat tobacco users. WMJ. 2010;109:335–340. [PMC free article] [PubMed] [Google Scholar]
- 39.Nease DE, Jr, Ruffin MTt, Klinkman MS, et al. Impact of a generalizable reminder system on colorectal cancer screening in diverse primary care practices: a report from the prompting and reminding at encounters for prevention project. Med Care. 2008;46:S68–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouaud J, Seroussi B, Antoine EC, et al. A before-after study using OncoDoc, a guideline-based decision support-system on breast cancer management: impact upon physician prescribing behaviour. Stud Health Technol Inform. 2001;84:420–424. [PubMed] [Google Scholar]
- 41.Kralj B, Iverson D, Hotz K, et al. The impact of computerized clinical reminders on physician prescribing behavior: evidence from community oncology practice. Am J Med Qual. 2003;18:197–203. [DOI] [PubMed] [Google Scholar]
- 42.Bertsche T, Askoxylakis V, Habl G, et al. Multidisciplinary pain management based on a computerized clinical decision support system in cancer pain patients. Pain. 2009;147:20–28. [DOI] [PubMed] [Google Scholar]
- 43.Siminoff LA, Gordon NH, Silverman P, et al. A decision aid to assist in adjuvant therapy choices for breast cancer. Psychooncology. 2006;15:1001–1013. [DOI] [PubMed] [Google Scholar]
- 44.Rubinstein WS, Acheson LS, O'Neill SM, et al. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial. Genet Med. 2011;13:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woolf SH, Krist AH, Johnson RE, et al. A practice-sponsored web site to help patients pursue healthy behaviors: An ACORN study. Ann Fam Med. 2006;4:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atlas SJ, Grant RW, Lester WT, et al. A cluster-randomized trial of a primary care informatics-based system for breast cancer screening. J Gen Intern Med. 2011;26:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sequist TD, Zaslavsky AM, Colditz GA, et al. Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2011;171:636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krist AH, Woolf SH, Rothemich SF, et al. Interactive preventive health record to enhance delivery of recommended care: a randomized trial. Ann Fam Med. 2012;10:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ornstein S, Nemeth LS, Jenkins RG, et al. Colorectal cancer screening in primary care: translating research into practice. Med Care. 2010;48:900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abernethy AP, Wheeler JL, Bull J. Development of a health information technology–based data system in community-based hospice and palliative care. Am J Prev Med. 2011;40:S217–S224. [DOI] [PubMed] [Google Scholar]