Abstract
Objective Identifying patients who are medication nonpersistent (fail to refill in a timely manner) is important for healthcare operations and research. However, consistent methods to detect nonpersistence using electronic pharmacy records are presently lacking. We developed and validated a nonpersistence algorithm for chronically used medications.
Materials and Methods Refill patterns of adult diabetes patients (n = 14,349) prescribed cardiometabolic therapies were studied. We evaluated various grace periods (30-300 days) to identify medication nonpersistence, which is defined as a gap between refills that exceeds a threshold equal to the last days’ supply dispensed plus a grace period plus days of stockpiled medication. Since data on medication stockpiles are typically unavailable for ongoing users, we compared nonpersistence to rates calculated using algorithms that ignored stockpiles.
Results When using grace periods equal to or greater than the number of days’ supply dispensed (i.e., at least 100 days), this novel algorithm for medication nonpersistence gave consistent results whether or not it accounted for days of stockpiled medication. The agreement (Kappa coefficients) between nonpersistence rates using algorithms with versus without stockpiling improved with longer grace periods and ranged from 0.63 (for 30 days) to 0.98 (for a 300-day grace period).
Conclusions Our method has utility for health care operations and research in prevalent (ongoing) and new user cohorts. The algorithm detects a subset of patients with inadequate medication-taking behavior not identified as primary nonadherent or secondary nonadherent. Healthcare systems can most comprehensively identify patients with short- or long-term medication underutilization by identifying primary nonadherence, secondary nonadherence, and nonpersistence.
Keywords: medication adherence, medication nonpersistence, electronic health record, computerized medical record systems, diabetes mellitus
BACKGROUND AND SIGNIFICANCE
Medication nonpersistence in chronic disease management may have negative clinical consequences, including increased mortality1,2 and morbidity.3,4 Therefore, nonpersistence of key preventive medications used to control risk factors for chronic conditions (e.g., blood pressure, lipid, and glucose lowering medications for patients with diabetes) is considered an important clinical metric. Identifying medication nonpersistence is often necessary for health plans, clinicians, and researchers alike, albeit for slightly different reasons. Having an automated algorithm to identify nonpersistence from electronic pharmacy databases in real time would be an effective way to signal healthcare providers that patient outreach and follow-up is needed. Such an algorithm would also have utility for adherence research, which often identifies nonpersistence as a primary outcome or exposure, and as a censoring point for follow-up of medications.1,5,6 In addition, systematic reporting of medication persistence and adherence by health plans is now mandated by the Centers for Medicare & Medicaid Services Five-Star Quality Rating System. To achieve a 5-star rating, health plans will need to show that more than 75% of their patient population with chronic conditions (e.g., diabetes, hypertension, and hypercholesterolemia patients) are ongoing users and taking at least 80% of the preventive medication prescribed to them (e.g., oral diabetes medications, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and statins).
Standard definitions for measuring nonadherence and nonpersistence of drug therapy have been proposed by several researchers.7–11 Medication nonadherence is a continuous or categorical construct that measures the extent to which a patient does not take medications as prescribed.8,10 Some of the more well-known adherence metrics include medication possession ratio (MPR), proportion of days covered, and continuous measure of medication gaps (CMG).10 Nonpersistence is the failure to refill a medication within “a time period consistent with use of the drug.”8,10,11 While this definition has also been referred to as “discontinuation” in the literature,10 nonpersistence is actually a broader term that identifies both discontinuers and inconsistent users. Both forms of nonadherence are important to identify in clinical practice. In order to measure nonpersistence, patients must be ongoing users of the therapy. At least 1 dispensing (or sometimes 2) is usually considered evidence of ongoing use. While it is possible to measure persistence as a continuous variable (i.e., length of time with medication on hand or time between refills), it is most often reported dichotomously (i.e., nonpersistent vs persistent).8–11 Some researchers use the terms nonpersistence and nonadherence interchangeably, and define nonpersistence as suboptimal secondary adherence (e.g., MPR <80% or CMG >20%).8,9,12
While methods for evaluating medication nonadherence are well developed and validated for both prevalent and new user prescription cohorts,6,13 no validated algorithm exists for identifying nonpersistence as a dichotomous variable using electronic pharmacy utilization records in these settings. The point at which nonpersistence of a medication becomes clinically significant and meaningful needs to be evaluated in light of the patient population, medications under study, and potential harm of nonpersistence, generating considerable variability in definitions. Since the definition used for nonpersistence impacts the sensitivity and specificity of the measure, this choice should consider how the information is to be used (e.g., to trigger clinical intervention, inform providers, regulatory purposes, or research). Nonetheless, uniformity facilitates cross-study comparisons. In a review of 58 studies of medication nonpersistence, slightly more than half (37) used refill gap algorithms.8 This method presumes medication nonpersistence to begin when the time between contiguous dispensings exceeds a predefined threshold (hereafter referred to as the “allowable gap”). However, the allowable gap was highly variable, ranging from 7 to 180 days, with a median of 30 days.8 Given such wide variation in approaches, caution must be taken when comparing results across studies. Specifying the allowable gap is critical because within-population assessments of rates of nonpersistence vary by 50% or more by changing the gap.14–16 To deal with these challenges, several authors have proposed standard definitions for measuring nonpersistence (and its complement, persistence) of drug therapy, but none have comprehensively assessed the impact of varying the allowable gap.7–11
Moreover, no study to date has assessed the sensitivity of algorithms that include vs exclude the accumulated (“stockpiled”) days of medication supply in the calculation of the allowable gap. Because stockpiling is common and variable, it could strongly leverage nonpersistence estimates. While obtaining an unbiased estimate of stockpiled medications is difficult using electronic pharmacy databases in prevalent medication user cohorts, these stockpiles are generally readily estimated in new prescription cohorts. While the majority of adherence research is conducted in cohorts of prevalent medication users, adherence studies in new prescription cohorts (e.g., randomized clinical trials) are becoming increasingly commonplace. Thus, more refined algorithms that also account for stockpiling are needed. In this study, we evaluate a new user cohort, providing a unique opportunity to examine the performance of a nonpersistence algorithm with versus without knowledge of stockpiled medications.
MATERIALS AND METHODS
Our sampling frame included 163,357 diabetes patients who were 19 years of age or older at baseline; were members of Kaiser Permanente Northern California (KPNC), a large, integrated healthcare delivery system; and had KPNC prescription drug benefits. Consistent with the growing trends in the dispensing of 3-month-supply prescriptions at retail pharmacies across the United States,17 the majority of KPNC patients (>80%) receive 3-month supplies at each refill. We then identified the subjects who were prescribed a new cardiometabolic (e.g., glycemic-lowering, antihypertensive, or lipid-lowering) medication during the first half of 2006. To be considered a new therapy, we required no evidence of pharmacy dispensing of the drug during the previous 24 months. This cohort was the focus of the current study, and also the basis for a previous publication coining the term “new prescription cohort” and describing adherence metrics useful in that type of study design.6 This study was approved by the Kaiser Foundation Research Institute Institutional Review Board.
We identified 18,770 patients who were early persistent users of the index therapy (i.e., dispensed the initial prescription and refilled at least once within 90 days) and excluded patients with gaps (>2 consecutive months) in membership or prescription drug benefits (n = 3987) during the study period (January 1, 2006 through June 30, 2011). We also excluded patients whose index therapy was insulin (n = 323), since our algorithm depends on days’ supply dispensed, which is not defined for insulin.
KPNC has a closed pharmacy system that ensures virtually complete capture of prescription drug dispensings. Patients with KPNC prescription drug benefits must purchase their medications at one of ∼120 walk-in pharmacies or, since 1999, via mail order. While it is possible to transfer a prescription to an out-of-plan pharmacy, a recent study in a similar patient population (Kaiser Permanente Colorado) found that the prevalence of out-of-plan pharmacy prescription transfers was still fairly low in 2011 (about 5%) even after the widespread introduction of bargain generic prescription programs beginning in 2006.18 In a 2005–2006 survey of the KPNC diabetes patient population (DISTANCE survey),19 members with prescription drug benefits reported using non-Kaiser pharmacies on average less than 1 time (mean = 0.04) in the past 12 months.20 Thus, misclassification due to incomplete data capture is of minimal concern.
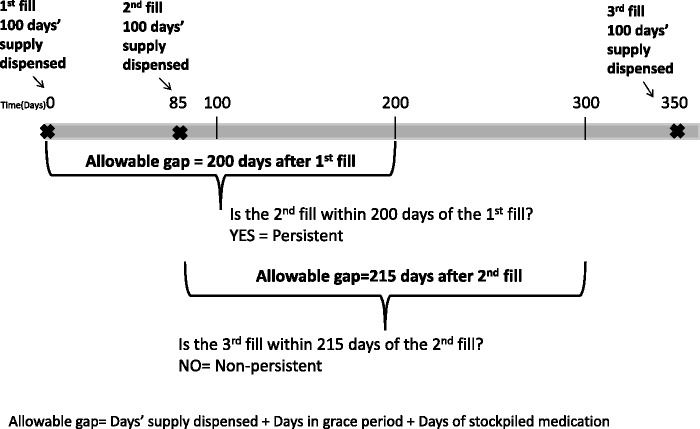
In this study, we defined medication nonpersistence as the failure to refill a drug in a timely manner based on electronic pharmacy dispensing data. This definition was operationalized using a refill gap algorithm to identify subjects whose time between contiguous dispensings exceeded an allowable gap (Figure 1). The allowable gap was defined as the last days’ supply dispensed, plus a grace period of either 30, 75, 100, 180, or 300 days, plus days of stockpiled medication. For each refill dispensed, we calculated the days of stockpiled medication as the difference between the total number of days since first dispensing and the sum of all days’ supply dispensed up until that point. We left censored this metric at zero. We flagged patients (n = 111) who accumulated an unusually large drug stockpile during follow-up (>490 days, the 99th percentile) and ran analyses both with and without these outliers. These two sensitivity analyses produced consistent results and we opted to exclude these patients from the final analyses, leaving a sample of 14,349 patients. We also tested a modified version of this algorithm that ignored the days of stockpiled medication.
Figure 1:
Illustration of refill gap algorithm for identifying medication nonpersistence using a 100-day grace period.
For those identified as nonpersistent for their medication, we estimated the date of nonpersistence as the date when the days’ supply (including stockpiled medications) would have been used up, if medications were taken as prescribed. We followed subjects who we presumed to be nonpersistent for an additional 365 days after the estimated nonpersistence date to determine the number that restarted the index therapy (i.e., had a subsequent refill).
Cohort demographics were reported as means (SD) for continuous variables and frequency counts (%) for categorical variables. Nonpersistence and restart rates were presented as percentages with 95% confidence limits. Cohen’s Kappa coefficient, a measure of inter-rater reliability, was computed to measure the concordance of the nonpersistence measures from algorithms using allowable gaps with versus without stockpiling.
RESULTS
The mean (SD) age in this cohort of 14 349 diabetes patients was 61.6 (11.5) years. Almost half (47%, n = 6752) were women, and the sample was ethnically diverse: Caucasian (46%), Latino (13%), African American (11%), Asian (10%), Filipino (8%), Multiracial/other (7%), and missing (6%). Forty-seven percent of the cohort was newly prescribed an antihypertensive drug, 30% were prescribed a diabetes medication, and 23% were prescribed a dyslipidemia drug (each identified via a new electronic prescription order submitted by the provider). The majority (81%) of dispensings were for a 100 days’ supply of medication. While some patients were initially sold a 30 days’ supply, only 78 subjects (0.5%) were consistently dispensed 1 month’s supply. Patient refill patterns were highly variable; however, 95% of all the refills occurred within 75 days of the run-out date (date we calculated the patient would have run out of medication if taken as prescribed).
The classification of nonpersistence was sensitive to the length of the grace period. When accounting for stockpiled medications in the allowable gap, nonpersistence rates were: 66, 52, 46, 36, and 28% using 30-, 75-, 100-, 180-, and 300-day grace periods, respectively (Table 1). Among patients who were nonpersistent, restarting therapy within 1 year was common, and the proportion restarting decreased as the length of the grace period increased. Restart rates were 74, 57, 48, 29, and 9%, using 30-, 75-, 100-, 180-, and 300-day grace periods, respectively (Table 1).
Table 1:
Four-year nonpersistence and restart rates among 14 349 new medication users, using algorithms with vs without medication stockpile in the definition for the allowable gap
| Definition of allowable gap | Nonpersistence % (95% CI) | Restartsa % (95% CI) |
|---|---|---|
| LDS + medication stockpile + grace period of | ||
| 30 days | 66 (65-67) | 74 (73-75) |
| 75 days | 52 (51-52) | 57 (56-59) |
| 100 days | 46 (45-47) | 48 (47-49) |
| 180 days | 36 (35-37) | 29 (27-30) |
| 300 days | 28 (28-29) | 9 (8-10) |
| LDS + grace period of | ||
| 30 days | 81 (80-82) | 81 (81-82) |
| 75 days | 59 (58-60) | 64 (62-65) |
| 100 days | 51 (50-51) | 53 (52-54) |
| 180 days | 37 (36-38) | 30 (29-31) |
| 300 days | 29 (28-30) | 9 (8-9) |
aPercentage of patients who were nonpersistent and had a dispensing of the index therapy within 1 year of the estimated date of nonpersistence.
LDS = last days’ supply dispensed.
Rates of nonpersistence were higher when using the algorithm that ignored stockpiling compared to algorithms that included stockpiling, when shorter grace periods were used but not when using longer grace periods. We calculated a Kappa statistic comparing the concordance of the nonpersistence measures from algorithms using allowable gaps with versus without stockpiling and found that as the length of the grace period increased, so did the degree of concordance between the measures (Kappa coefficients were: 0.63, 0.86, 0.91, 0.97, and 0.98 for the 30-, 75-, 100-, 180-, and 300-day grace periods, respectively). When nonpersistence was identified by algorithms without stockpiling, restart patterns were similar to those reported above (Table 1).
DISCUSSION
Among diabetes patients who were new users of cardiometabolic medications, refill gap algorithms with grace periods greater than or equal to the days’ supply dispensed (i.e., at least 100 days) provided consistent measures of nonpersistence, regardless of whether or not days of stockpiled medication were used in the calculation of the allowable gap. Using this grace period criteria, estimates did not differ substantively when using algorithms with versus without stockpiling. There was <5% absolute difference between estimates and the Kappa coefficient for reliability exceeded 0.90. However, using shorter (e.g., 30-day) grace periods generated less reliable estimates; there was poor agreement based on Kappa coefficients and there was considerable variability in the point estimates and 95% confidence intervals between rates of nonpersistence. This evidence suggests that the absence of medication stockpile data, as is the case in the more commonly used prevalent user cohorts, should not preclude using refill gap algorithms for identifying nonpersistence of chronically used medications when using grace periods equal to or longer than the days’ supply dispensed.
Our findings support previous research that recommends using longer grace periods when identifying nonpersistence or discontinuation using refill gap algorithms. Recently, researchers at the Veteran’s Affair reported an 81% positive predictive value (640 true positives out of 786 patients identified as discontinuing statin treatment) for a refill gap-based algorithm which used a 120-day grace period and self-reported data as the gold standard.21 A 3 months’ supply was the predominant dispensing and their refill patterns were similar to those in our study. In a study of hormone replacement therapy, nonpersistence identified using refill gap algorithms with a 90-day grace period showed acceptable agreement to a time to discontinuation curve produced from a simulation model.22 Raebel and colleagues10 proposed a unifying set of definitions for medication adherence research and reported that a 180-day grace period is commonly used in refill gap algorithms for identifying medication discontinuation.
There are some practical reasons why using longer grace periods might be preferable. Because 30% to 50% of US adults with chronic disease are not adherent to their medication regimen,23 a generous grace period in nonpersistence algorithms is required to distinguish ongoing users with poor adherence from patients who have discontinued treatment. If the goal is identifying subjects who likely discontinued permanently, a very liberal grace period is needed. For example, only 9% of those identified as nonpersistent using a 300-day grace period restarted therapy within 1 year, compared to 48% restarting when using a 100-day grace period. The finding that patient use of cardiometabolic therapies is characterized by alternating periods of persistence and nonpersistence was also observed in a cohort of statin users by Brookhart et al.24
Nonpersistence and suboptimal adherence are both consistent with longer gaps between refills in the dispensing data. The algorithm we propose is correlated with secondary measures of nonadherence (e.g., MPR, CMG); however, while nonpersistence and nonadherence overlap substantially, not all nonpersistent patients are captured by identifying secondary nonadherence (Figure 2). Secondary adherence methods (e.g., MPR <80%) are commonly the only metric used by health systems to identify at risk patients, but these metrics have known limitations. For example, MPR does not quantify the timeliness of refilling and potentially overestimates adherence due to early fills.9 Additionally, since secondary adherence metrics calculate adherence using 2 or more dispensings up to the last refill, they fail to identify patients who were adherent (e.g., MPR ≥-80%) while refilling and then discontinue (Figure 2, Box C). Those patients, however, are identified by this nonpersistence algorithm. Because nonpersistence and nonadherence are complementary measures, identifying both (in addition to primary nonadherence for new user cohorts) offers the most comprehensive identification of patients with inadequate medication-taking behavior.
Figure 2:
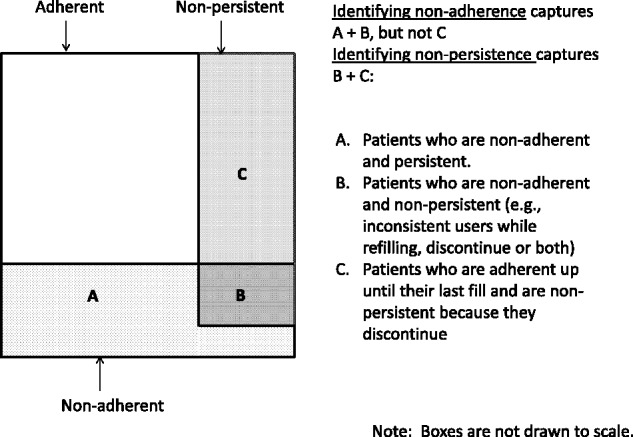
Venn diagram illustrating the overlap of measures of secondary adherence (e.g., continuous measure of medication gap [CMG] ≤20%), nonadherence (e.g., CMG >20%), and nonpersistence.
Several strengths and limitations of this study should be considered. Any methodology that depends on gaps in medication supply is limited in its ability to discriminate between an intentional discontinuation and inconsistent or greatly delayed refilling. From a practical perspective, any poor adherence warrants clinical follow-up. A strength of the study is that all subjects received care at a single health plan (KPNC), a nonprofit, fully-integrated healthcare delivery system that provides comprehensive medical services to over 3.2 million members. While members resemble the general population except for the extreme tails of the income distribution,25–27 there are ways that they differ from the general population. KPNC typically dispenses 3 months’ supply of medication. Whether our findings apply to populations with different dispensing patterns (e.g., exclusively 30 days’ supply) needs further study. However, our results are relevant for many large healthcare systems that dispense 3-month prescriptions for chronically used medications, including the Veteran’s Affair and Medicaid systems in at least 14 states.17,28 Beginning in 2008, there has been a steady increase in 3-month prescriptions dispensed through mail order and retail pharmacies in the United States. This trend is likely to continue, especially for maintenance medications as insurers adjust their drug benefit plans to remain competitive in the market17 amidst findings which suggest that patients with 3-month refills have improved medication adherence and persistence, nominal wastage, and greater savings as compared to patients on 30-day refill plans.28 This study’s ability to capture the vast majority of pharmacy utilization is an advantage over settings with partial capture of dispensing data. Missed pharmacy utilization can be misinterpreted as nonadherence, a directional bias. A major, unverifiable assumption of any refill gap algorithm is that patients are taking the medication as prescribed and that the dosing instructions in the electronic data are current. However, studies using refill gap algorithms have shown significant relationships with, or been directly validated against, health outcomes and self-reported adherence.6,29–34 While imperfect, pharmacy utilization based measures are a useful alternative when gold standards such as laboratory assessments or pill counters are not feasible, and much more practical for assessing adherence in large populations in usual care settings.
In conclusion, nonpersistence algorithms with grace periods equal to or greater than the number of days’ supply dispensed gave similar results regardless of whether or not days of stockpiled medication were used in calculating the allowable gap. To improve comparability of results across studies and in different settings (i.e., prevalent vs new user cohorts), algorithms with longer grace periods should be considered. Identifying nonpersistence solely on refill patterns will always be vulnerable to uncertainty. Nonetheless, this data-driven approach for identifying nonpersistence is a reasonable complement to be used in addition to primary nonadherence and secondary nonadherence measures (e.g., MPR <80% or CMG >20%) to provide the most comprehensive capture of inadequate medication-taking behavior. This is because nonpersistent patients are missed by secondary adherence measures if they were adherent up to their last fill (i.e., the censoring point for secondary adherence measures), and then discontinue or become inconsistent users. The proposed nonpersistence algorithm is an efficient tool to flag patients with inadequate medication-taking behavior who would benefit from clinician follow-up or, on a larger scale, to identify nonpersistence as an exposure (e.g., for evaluating its health economic consequences), effect modifier (e.g., for per protocol analyses of clinical trials of new medications), or outcome (e.g., for interventions that aim to improve adherence) for epidemiologic or health services research.
FUNDING
This work was supported by the National Institutes of Health grant numbers: R01 DK080726, R01 DK081796, RC1 DK086178, R01 DK65664, P30 DK092924, and R01 AG032249. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
COMPETING INTERESTS
The authors have no competing interests to declare.
CONTRIBUTORS
The study was conceptualized by MMP, HHM, AA, and AJK and supervised by AJK. AJK, HHM, and AA were responsible for acquiring funding. MMP collected the data and performed data analysis. MMP, HHM, AA, and AJK participated in the interpretation of the results. The manuscript was drafted by MMP and was critically reviewed for important intellectual content by HHM, AA, and AJK. All authors read and approved the final manuscript and agree to be accountable for the accuracy and integrity of the work presented.
REFERENCES
- 1.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166(17):1842–1847. [DOI] [PubMed] [Google Scholar]
- 2.Lipska KJ, Wang Y, Kosiborod M, et al. Discontinuation of antihyperglycemic therapy and clinical outcomes after acute myocardial infarction in older patients with diabetes. Circ Cardiovasc Qual Outcomes. 2010;3(3):236–242. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez LA, Cea-Soriano L, Martin-Merino E, Johansson S. Discontinuation of low dose aspirin and risk of myocardial infarction: case-control study in UK primary care. BMJ. 2011;343:d4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vera MA, Choi H, Abrahamowicz M, Kopec J, Goycochea-Robles MV, Lacaille D. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2011;70(6):1020–1024. [DOI] [PubMed] [Google Scholar]
- 5.Bauer AM, Parker MM, Schillinger D, et al. Associations between antidepressant adherence and shared decision-making, patient-provider trust, and communication among adults with diabetes: diabetes study of Northern California (DISTANCE). J Gen Intern Med. 2014;29(8):1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44(5):1640–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28(9):1411–1424; discussion 1410. [DOI] [PubMed] [Google Scholar]
- 8.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574; discussion 575–567. [DOI] [PubMed] [Google Scholar]
- 9.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449–457. [PubMed] [Google Scholar]
- 10.Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(8 Suppl 3):S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. [DOI] [PubMed] [Google Scholar]
- 12.Grey C, Jackson R, Wells S, et al. Maintenance of statin use over 3 years following acute coronary syndromes: a national data linkage study (ANZACS-QI-2). Heart. 2014;100(10):770–774. [DOI] [PubMed] [Google Scholar]
- 13.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814–823. [DOI] [PubMed] [Google Scholar]
- 14.Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Refill persistence with chronic medication assessed from a pharmacy database was influenced by method of calculation. J Clin Epidemiol. 2006;59(1):11–17. [DOI] [PubMed] [Google Scholar]
- 15.Hudson M, Rahme E, Richard H, Pilote L. Comparison of measures of medication persistency using a prescription drug database. Am Heart J. 2007;153(1):59–65. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen LH, Lokkegaard E, Andreasen AH, Hundrup YA, Keiding N. Estimating the effect of current, previous and never use of drugs in studies based on prescription registries. Pharmacoepidemiol Drug Saf. 2009;18(2):147–153. [DOI] [PubMed] [Google Scholar]
- 17.Liberman JN, Girdish C. Recent trends in the dispensing of 90-day-supply prescriptions at retail pharmacies: implications for improved convenience and access. Am Health Drug Benefits. 2011;4(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 18.Delate T, Albrecht G, Olson KL. Out-of-plan pharmacy use by members of a managed care organization. Perm J. 2012;16(2):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffet HH, Adler N, Schillinger D, et al. Cohort Profile: The Diabetes Study of Northern California (DISTANCE)–objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol. 2008;38(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karter AJ, Moffet HH, Liu J, et al. Glycemic response to newly initiated diabetes therapies. Am J Manag Care. 2007;13(11):598–606. [PMC free article] [PubMed] [Google Scholar]
- 21.Rector TS, Nugent S, Spoont M, Noorbaloochi S, Bloomfield HE. Screening electronic veterans' health records for medication discontinuation. Am J Manag Care. 2012;18(7):352–358. [PubMed] [Google Scholar]
- 22.Nielsen LH. Validation of methods for identifying discontinuation of treatment from prescription data. Appl Statist. 2010;59(Part 4):707–722. [Google Scholar]
- 23.Marcum ZA, Sevick MA, Handler SM. Medication nonadherence: a diagnosable and treatable medical condition. JAMA. 2013;309(20):2105–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167(8):847–852. [DOI] [PubMed] [Google Scholar]
- 25.Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry*. Am J Med. 2001;111:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiatt RA, Friedman GD. Characteristics of patients referred for treatment of end-stage renal disease in a defined population. Am J Public Health. 1982;72:829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taitel M, Fensterheim L, Kirkham H, Sekula R, Duncan I. Medication days' supply, adherence, wastage, and cost among chronic patients in Medicaid. Medicare Medicaid Res Rev. 2012;2(3):E1–E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004;42(7):649–652. [DOI] [PubMed] [Google Scholar]
- 30.Ho PM, Magid DJ, Masoudi FA, McClure DL, Rumsfeld JS. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc Disord. 2006;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. [DOI] [PubMed] [Google Scholar]
- 32.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. [DOI] [PubMed] [Google Scholar]
- 33.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846–857. [DOI] [PubMed] [Google Scholar]
- 34.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. [DOI] [PubMed] [Google Scholar]