Abstract
Natriuretic peptides (NPs) were first described as cardiac biomarkers more than two decades ago. Since that time, numerous studies have confirmed NPs’ diagnostic and prognostic utilities as biomarkers of myocardial function. However, we must now admit that despite the NPs’ relatively long period of use in clinical practice, our understanding of the biochemistry and the variety of circulating forms of NPs, as well as of their potential as biomarkers, remains far from being complete and comprehensive. The highly complex nature and wide diversity of circulating forms of NPs make their accurate measurements in plasma far more complex than initially believed. A highly simplistic view of the NPs’ use is that elevated values of NPs indicate the severity of heart failure and thus reflect the prognosis. However, as shown by a variety of studies, deep understanding of how the NP system works will be required for correct interpretation of test results in routine practice of cardiovascular disease. In this review, we summarize the recent advances in understanding of the complexity of the NP system and discuss related analytical issues, which open new horizons, as well as challenges for clinical diagnostics.
Key words: ANP, BNP, glycosylation, immunoassay, natriuretic peptide, NT-proBNP, proBNP, processing
BACKGROUND
Natriuretic peptides (NPs) belong to a family of structurally and functionally related circulating peptides involved in maintaining cardiorenal homeostasis. The finding that the heart not only reacts to the humoral stimulus but actively participates in maintaining the fluid and salt balance by producing physiologically active compounds emerged with the discovery of atrial natriuretic peptide (ANP) and later of B-type natriuretic peptide (BNP) (1, 2). This new concept of the heart as an endocrine organ was introduced in 1981, when de Bold et al. showed that the intravenous injection of atrial extracts into rats led to an increase in sodium chloride and fluid excretion and a decrease in blood pressure, showing involvement in the maintenance of blood pressure, water, and electrolyte balance in organisms (3). The two peptides, ANP and BNP, have been shown to display very similar physiological activities and under normal conditions are mainly produced in the atria. Both peptides are known to reduce vascular resistance and increase both diuresis and sodium excretion, reducing systemic blood pressure (4-6).
The third member of the NP family, C-type natriuretic peptide (CNP), was isolated shortly after the discovery of ANP and BNP (7). However, in contrast to ANP and BNP, which are mainly produced in the myocardium, CNP is predominantly produced in the central nervous system and vascular endothelium and acts as a paracrine factor (8). This member of the NP family was not shown to have much value as biomarker of cardiovascular complications. As a result, CNP did not attract much interest and was not introduced to routine clinical practice.
All 3 members of the NP family share a common structural feature – a ring structure consisting in humans of 17 amino acid residues (aar) and formed by an intramolecular disulfide bond. It is thought to be essential for mediating receptor binding and the biological activity of NPs (Fig. 1).
Figure 1. The primary structures of human ANP, BNP, and CNP.
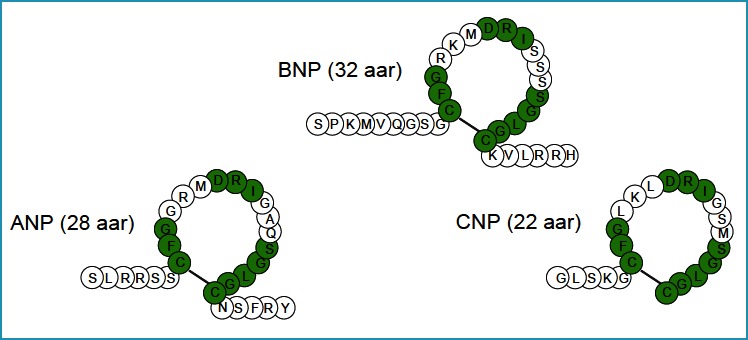
Similar residues are marked by a green background. The ring structure is formed by a disulfide bridge between two cysteine residues.
Two types of natriuretic peptide receptors (NPRs), NPR-A and NPR-B, are responsible for most of the physiological effects of NPs. The binding of NPs to these guanylyl cyclase-coupled receptors leads to an increase in cGMP, resulting in natriuresis, vasorelaxation, diuresis, inhibition of the renin-angiotensin-aldosterone system, enhanced myocardial relaxation, inhibition of fibrosis and hypertrophy, promotion of cell survival, and inhibition of inflammation (reviewed in (9)). Another type of NPR, NPR-C, lacking the guanylyl cyclase domain, is responsible for clearance and possibly involved in regulation of the cell proliferation (reviewed in (10)).
The expression and secretion of ANP and BNP increase significantly in pathological states accompanied by stretching of the heart chambers, volume overload, and ischemic injury, such as heart failure (HF) and myocardial infarction (MI) (11-13). Because the increase in their production and release in circulation is associated with the cardiac pathologies caused by pressure or volume overload, it was suggested that these peptides may be used as biochemical markers of HF. The diagnostic and prognostic utility of ANP and BNP was later confirmed in a large number of studies (reviewed in (14, 15)).
Considering the beneficial physiological activities of NPs in HF, recombinant forms of ANP and BNP were introduced as therapeutic agents for the treatment of this disease (16-18). Recombinant human ANP (carperitide) was approved in Japan in 1995 for intravenous administration in patients with acute heart failure (AHF) and acute decompensated heart failure (ADHF). Later, in 2001, recombinant BNP (nesiritide) was approved by the Food and Drug Administration (FDA) for acute congestive HF (ACHF). However, questions regarding the efficiency and safety of nesiritide diminished its use. Neseritide is currently considered to be safe, but with little beneficial effect (19).
As mentioned above, ANP and BNP share many common features and may be expected to have similar or even equal value as biomarkers. However, BNP was shown to have greater in vitro stability and superior diagnostic performance compared with ANP, and therefore BNP and its related peptides have emerged as the preferred candidates for the diagnosis of HF, as well as other clinical applications. Recent international guidelines recommend its use for the diagnosis, risk stratification, and follow-up of patients with chronic or acute HF (15, 20). As a consequence, the data regarding BNP-related peptides are currently more comprehensive that the data regarding ANP. In this review, we will focus mostly on the analytical issues related to BNP, as this member of the NP family is more interesting and important from a clinical perspective.
SYNTHESIS AND SECRETION OF ANP
ANP is translated from its gene as a 151-amino acid prepropeptide, preproBNP, that is stored in atrial granules (21). It is secreted and cleaved to a mature peptide, ANP, in response to atrial stretching or stimulation by angiotensin II, endothelin, as well as sympathetic stimulation. After cleavage of the signal peptide (a common element of all secretory proteins, which is responsible for addressing the protein to a secretory pathway) from the 151-amino acid preproANP, the precursor pro-ANP is further processed by atrial convertase corin to produce two circulating peptides, ANP 1-28 and the N-terminal fragment NT-proANP 1-98(22). The processing of proANP is considered to occur at the time of secretory granule release.
NT-proANP is also cleaved, forming 3 fragments that exhibit physiological activity: proANP 1-30 (long-acting atriuretic peptide), proANP 31-67 (vessel dilator), and proANP 79-98 (kaliuretic peptide, i.e., potassium excretion). All four fragments are present in the circulation (23).
A longer version of ANP called urodilatin, containing four additional N-terminal residues (ANP 1-32), is primarily found in the kidney. It promotes diuresis by increasing renal blood flow (24).
ANP IMMUNOASSAYS
As was discussed above, ANP was less widely accepted as a HF biomarker than BNP and was thus somewhat overshadowed by its sibling, BNP. As a consequence, there is currently only one commercially available immunoassay specific to an ANP-related peptide, which detects the mid-regional zone of proANP (MR-proANP). This assay is manufactured by Thermo Fisher Scientific and it is not yet FDA cleared yet. It utilizes polyclonal sheep antibodies specific to the 50-72 aar of proANP as coat antibodies along with monoclonal rat antibodies specific to the fragment 73-90 of proANP (25).
SYNTHESIS AND SECRETION OF BNP
The BNP gene encodes a 134-amino acid preproBNP precursor, which is converted to 108-amino acid proBNP by the cleavage of a 26-amino acid signal peptide (26). Interestingly, a fragment of preproBNP signal peptide (17-26 AAR) was shown to be present in the blood of normal individuals and patients with acute MI and was suggested as a circulating biomarker of cardiac ischemia and MI, with some possible advantages over currently used biomarkers such as creatine kinase-MB, myoglobin, and troponins (27). Similarly, a fragment of preproANP signal peptide (16-25 aar) was shown by the same research group to have some potential as an ischemic biomarker (28).
The BNP gene is an early response gene allowing rapid reaction to mechanical, hormonal or sympathetic stimulation: its transcription reaches a maximal level within 1 h after stimulation (29). Synthetized BNP is thought to be stored in limited amount and in acute need is produced de novo. Therefore, the predominant source of circulating BNP appears to be through constitutive secretion from ventricular myocytes. The storage and secretion of ANP are different: ANP is mostly stored in atrial granules and, as a consequence, is available for fast release if needed.
The processing of proBNP gives rise to two fragments: the N-terminal fragment of proBNP (NT-proBNP, 1-76 aar) and the C-terminal region active BNP hormone (77-108 aar) (30). BNP and NT-proBNP appears exclusively as a result of the proteolytic cleavage of proBNP in a stoichiometric ratio of 1:1. For a long time, it was strongly believed and accepted that BNP and NT-proBNP are the principal proBNP-derived molecular forms present in the circulation.
Whether NT-proBNP has any physiological function remains unknown. This fragment is currently considered to be a byproduct formed during maturation of the active BNP hormone. Although NT-proBNP and BNP are produced in an equimolar ratio, the molar plasma concentration of NT-proBNP is several-fold higher than the concentration of BNP. Higher levels of NT-proBNP are thought to be caused by the lower clearance of NT-proBNP from the bloodstream (31). Notably, intact nonprocessed proBNP is also present in the circulation and represents a substantial part of the BNP-immunoreactivity found in the samples of HF patients (discussed below) (32, 33).
POSTTRANSLATIONAL MODIFICATIONS OF proBNP
Both proBNP from the plasma of HF patients and recombinant protein produced in eukaryotic cells were shown to be extensively O-glycosylated at several threonine and serine residues within the N-terminal region (1-76 aar), but not within the BNP-portion of proBNP (77-108 aar) (34-36). In a study by Schellenberger et al., 7 sites of O-glycosylation in recombinant proBNP, expressed in Chinese hamster ovary cells, were identified within the region 1-76 aar of proBNP (i.e., NT-proBNP): Thr36, Ser37, Ser44, Thr48, Ser53, Thr58, and Thr71(34). Notably, no sites of O-glycosylation were identified within the BNP-part of proBNP molecules.
The finding that proBNP undergoes posttranslational modifications during its maturation had a great impact on the understanding of the biochemistry and complexity of circulating proBNP-derived peptides. Although the exact glycosylation sites of endogenous proBNP and NT-proBNP are still not precisely characterized, indirect data indicate the presence of carbohydrate residues in specific parts of the molecules. According to Seferian et al., the central region (28-56 aar) of NT-proBNP is glycosylated, whereas the C-terminal portion of the molecule (61-76 aar) is mostly free of O-glycans (37). However, endogenous proBNP was shown to be glycosylated both in the central region and in the region located close to the cleavage site, in the region 63-76 for proBNP, which was inaccessible to site-specific antibodies because of glycosylation (38).
The level of endogenous NT-proBNP and proBNP glycosylation in humans seems to be characterized by high interindividual variability, which may arise from the site occupancy, structure, and length of oligosaccharide chains (36, 37). The clinical significance of this variability is currently unknown, and it might be interesting to explore whether it is related to the severity of HF or its etiology.
GLYCOSYLATION OF proBNP AND THE EFFICIENCY OF ITS PROCESSING
The diversity of circulating proBNP-derived peptides found in the circulation can be partially explained by the mechanisms of proBNP processing. The processing of proBNP is considered to occur prior to or in the moment of its secretion into the circulation. However, this understanding is primarily based on indirect observations. As for many other precursor polypeptides, the processing of proBNP is mediated by enzyme(s), namely prohormone convertases. Whether there is a unique convertase or several enzymes are responsible for the processing of proBNP remains an open question. Two proprotein convertases, furin and corin, are considered the most likely proBNP-processing enzymes. In vitro experiments have shown both furin and corin to process proBNP, with the formation of distinct BNP forms: BNP 1-32 (furin) and BNP 4-32 (corin) (39). As corin produced a shorter BNP form (i.e., BNP 4-32), this convertase is relatively unlikely to be the main enzyme responsible for the processing of proBNP, which highlights the relevance of furin as a proBNP-processing enzyme (reviewed in (40)).
Glycosylation in the region close to the proBNP cleavage site was shown to play a pivotal role in the regulation of the enzyme-mediated processing of proBNP (38). The presence of glycosidic residues in this region of the proBNP molecule was found to suppress the processing of proBNP. In cell-based assays both furin- and corin-mediated processing of proBNP were shown to be suppressed by O-glycans attached to Thr71. It is currently believed that only proBNP molecules that are not glycosylated at Thr71 can be effectively processed into BNP and NT-proBNP. Whether this suppression of proBNP processing by glycosylation at the Thr71 residue is a physiological regulatory process or a pathophysiological mechanism leading to HF progression remains an open question.
The role of glycosidic residues at other sites of proBNP molecule is currently unclear. Because glycosylation is known to be a highly energy-consuming process, it is very unlikely that it has no specific role in the function of BNP. One possibility is that the glycosylation of proBNP within the central region might protect it from undesirable cleavage at other monobasic or dibasic sites in the human proBNP sequence and thus prevent the formation of longer BNP forms. Additionally, in vitro experiments have shown O-glycosylation to increase the stability of proBNP, which may be essential in light of the presence of proBNP and its potential function in the circulation (discussed below) (41).
MOLECULAR FORMS OF BNP IN PLASMA
Initially, it was believed that there were two circulating fragments present in the circulation, BNP 1-32 and NT-proBNP 1-76, formed by endoproteolytic cleavage of proBNP between the Arg76 and Ser77 residues. However, this concept has recently been greatly modified (42, 43). It was found that only a tiny portion of circulating BNP consists of intact BNP 1-32, which was initially considered a main form of immunoreactive BNP. The absence of BNP 1-32 in plasma samples from patients with advanced HF was reported by Hawkridge et al., challenging the primary simplified concept of BNP 1-32 as a major component of BNP-immunoreactivity in the blood samples of HF patients (44). The work of Niederkofler and coworkers accurately showed that in the plasma of HF patients, BNP 1-32 is present alongside various N- and C-terminal truncated BNP forms, i.e., BNP 3-32, BNP 4-32, BNP 5-32, BNP 5-31, BNP 1-26, and BNP 1-25 (43).
The proteolytic degradation of BNP 1-32 in the circulation is thought to be responsible for the diversity of BNP-derived forms found in the collected samples. Peptidases such as dipeptidyl peptidase IV (DPP IV) and neutral endopeptidase (neprilysin, NEP) were reported to degrade BNP, giving rise to BNP 3-32 and BNP 5-32, respectively (45, 46). Some studies have suggested that insulin-degrading enzyme (IDE) can also degrade BNP to smaller peptides (47, 48). However, BNP was shown to be a poor substrate for neprilysin and IDE, suggesting that another protease is likely responsible for its cleavage. Additionally, the appearance of BNP 4-32 in the circulation may be due to the specific processing activity of corin, as shown by in vitro experiments with exogenous proBNP (39). According to the study of Belenky et al., peptidyl arginine aldehyde protease can degrade BNP at sites in the peptide chain where arginine is present, as specific inhibitors of this enzyme greatly reduce the degradation of the hormone in vitro (49). In mice, plasma protease meprin was shown to cleave BNP (46); however, its ability to degrade BNP in humans is rather questionable (50). The known sites of BNP proteolytic degradation are summarized in Figure 2.
Figure 2.
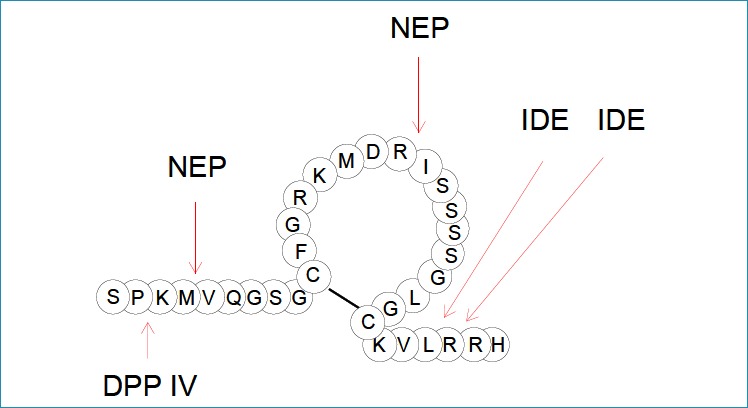
The known main degradation sites of BNP by the action of DPP IV, NEP and IDE
Notably, the instabilityof BNP in EDTA-plasma samples has been reported even at -80 °C. Thus, to protect BNP from degradation during sample storage, high concentrations of protease inhibitors are required (benzamidine up to 10 mmol/L and AEBSF up to 5 mmol/L) (42).
There is still an open question regarding whether all these BNP forms are equally bioactive. The data on this subject are not consistent. In cell-based assays with cardiac fibroblasts and cardiomyocytes, human BNP 3-32 exhibited similar activity to BNP 1-32(51). However, in vivo studies in canine models revealed that human BNP 3-32 exhibited reduced natriuresis and diuresis and a lack of vasodilating actions compared to BNP 1-32(52). Thus, this perspective might suggest that shorter BNP forms do exhibit reduced biological activity compared to the full-length BNP molecule.
ProBNP AS A MAJOR COMPONENT OF BNP-IMMUNOREACTIVITY
A number of studies have convincingly shown that the intact precursor proBNP is the major BNP-immunoreactive form found in collected plasma samples, both in healthy subjects and especially in patients with congestive HF. These findings had a great impact on our understanding of the results of BNP measurements by the routinely used immunoassays, as most of them exhibit cross-reactivity with proBNP due to the presence of BNP-structure within the proBNP sequence. The degree of cross-reactivity was reported to be different for different assays and different forms of proBNP (e.g., glycosylated vs. nonglycosylated) (53).
Because proBNP shares a common 32-amino acid structure with BNP, it is logical to suggest that proBNP might be capable of mediating physiological functions similarly to BNP. However, in cell-based assays, unprocessed proBNP exhibited markedly reduced physiological activity compared with BNP and is currently considered to be insufficient to promote an adequate physiological natriuretic hormone response in HF patients (54). Additionally, proBNP was shown to have significantly lower affinity for NPR-C than BNP and to be more resistant to proteolytic inactivation by human kidney membranes (55).
The role of proBNP in the circulation and why it is released into the circulation in its unprocessed form remain intriguing questions. Whether it is a normal physiological or rather a pathophysiological process still needs to be clarified to better understand its clinical significance.
IS proBNP A CIRCULATING SOURCE OF BNP HORMONE?
High plasma levels of unprocessed proBNP and the potentially reduced bioactivity of proBNP compared to BNP suggest that circulating proBNP may serve as a reserve BNP-containing form to be processed in the circulation for the release of active BNP hormone. The question of whether proBNP might undergo processing in the circulation was addressed in several studies by testing the in vitro production of BNP from proBNP(56, 57). Although the results of these studies indicate that the cleavage of exogenous nonglycosylated proBNP may occur in serum samples, they should be interpreted with caution, as the relevance of serum as an in vitro model to study proBNP processing in the circulation is rather questionable
Following from the inhibitory effect of O-glycans attached to the Thr71 residue of proBNP, the efficiency of proBNP processing depends not only on the activity of convertase(s) but also the glycosylation status of the residue located close to the cleavage site, i.e., Thr71. As we know, proBNP glycosylated at Thr71 is not processed by furin or corin. Thus, it is straightforward to ask which form of proBNP is present in the circulation.
It was found that there are two distinct forms of proBNP in the circulation, which differ in the glycosylation status of Thr71 residue: proBNP glycosylated at Thr71 and proBNP, which lacks glycan residues at this site. Among these two forms, only proBNP which does not bear any glycans attached to Thr71, was shown to be susceptible to proteolytic cleavage and may give rise to active BNP 1-32 hormone. Interestingly, this observed variability in glycosylation status is apparently attributed only to this site and is not the case for other sites of glycosylation within the proBNP molecule.
Our studies in rats have revealed that the processing of human nonglycosylated proBNP in the circulation, resulting in the formation of BNP 1-32, is possible (58). These data, taken together with the findings that there are two distinct forms of proBNP in the plasma of HF patients, one glycosylated (processing-unsusceptible) and the other non-glycosylated (processing-susceptible) in the region close to the cleavage site, suggest the possibility of peripheral proBNP processing in the circulation (39).
DIVERSITY OF proBNP FORMS IN ACUTE AND CHRONIC HF
Recent studies by Vodovar and colleagues shed some light on the interplay between proBNP glycosylation and its processing in the circulation (59). In this study, the degree of plasma proBNP glycosylation was assessed in three groups of HF patients, i.e., patients with ADHF, non-ADHF (dyspnea but no HF) patients and chronic HF patients, by means of mass spectrometry. Among these three groups, the highest percentage of glycosylated proBNP was present in chronic HF. In contrast, the percentages of glycosylated proBNP in ADHF and non-ADHF patients were lower than in chronic HF and similar to each other. These data suggest that proBNP processing is altered more in chronic HF than in ADHF or non-ADHF. Strikingly, furin activity but not its concentration was greater in ADHF than in chronic HF, thus providing a differential mechanism of proBNP processing in disease progression in HF. Considering these findings, one might speculate that the significantly increased production and processing of proBNP in ADHF might represent an attempt by the failing heart to increase the level of circulating BNP and to reduce the overload. In contrast, in chronic HF, with the release of more glycosylated proBNP into the circulation, there will be a defect in the processing of proBNP to mature BNP hormone, as this proBNP form is not susceptible to processing. These findings may reflect the existence of regulatory mechanisms through which plasma BNP rapidly increases in acute conditions by cleavage of the processing-susceptible proBNP form (60).
From a physiological and clinical prospective, there are several important consequences of these new findings. First, it means that the BNP-related peptides are likely different in different HF patients and there is no common BNP status for different forms of HF. Second, there are apparent differences in the processing of NPs between patients with ADHF and patients with chronic HF. From this perspective, one may suggest that immunoassays that can differentiate the glycosylated and nonglycosylated forms of proBNP might have additional value for clinical diagnostics. Such assays are not currently available; however, their development is potentially possible due to the known sensitivity of antibodies to the presence of O-glycans in the recognized epitopes (32).
The recent advances in the understanding of the diversity of circulating BNP forms have considerably changed the initial simplistic scheme of proBNP processing, suggesting that only a few forms are present in the circulation, and have led to a new scheme of proBNP maturation and processing (Fig. 3).
Figure 3. The scheme of proBNP maturation and processing with the suggested inhibitory effect of O-glycans bound to the Thr71 on processing efficiency.
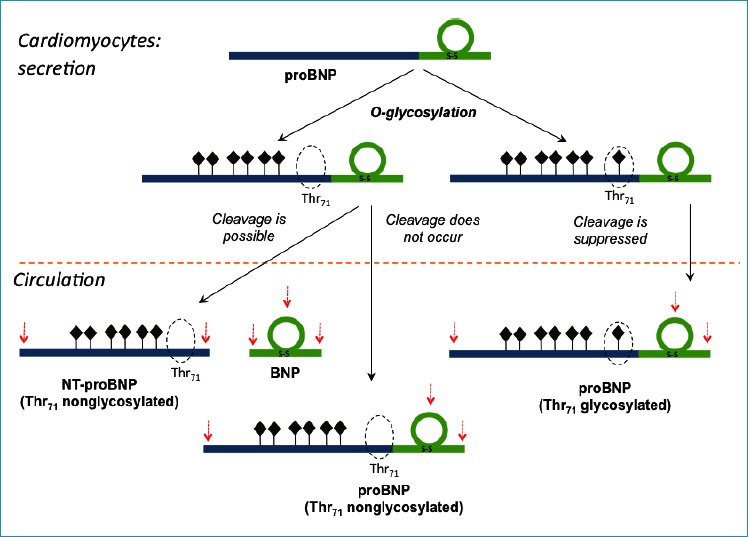
Seven potential sites of O-glycosylation are marked as dark diamonds (34).
The potential N- and C-terminal sites of proteolytic degradation as well as the ones located within the ring structure of BNP (proBNP) are marked by red arrows.
Adapted with modifications from (40).
THE DIVERSITY OF IMMUNOASSAYS FOR proBNP-DERIVED PEPTIDES
More than 20 years after the introduction of NPs as cardiac biomarkers, there are a variety of immunoassays, specific for different forms of the peptide, in use by clinicians. We will briefly discuss the most important and recent findings in this field and the impact of the complex biochemistry of proBNP-derived peptides on the interpretation of the test results.
NT-proBNP ASSAYS
The current situation involving NT-proBNP immunoassays is relatively simple. All approved commercially available NT-proBNP immunoassays are based on the same antibodies and calibrator materials distributed by Roche. As a result of a common source of antibodies and calibrator, only small systematic differences between the available NT-proBNP immunoassays have usually been reported, with the total variation across methods within 10%. However, despite the common source of antibodies and standard materials, assay harmonization remains incomplete (61).
The initially proposed cut-off for NT-proBNP assays is below or above 125 ng/L. A value of 300 ng/L works well with the existing assays for the exclusion of acute HF (62).
The first generation of Roche NT-proBNP assays was based on polyclonal antibodies specific for the regions 1-21 and 39-50; the second generation employs monoclonal antibodies (mAbs) specific for the central region of NT-proBNP: 22-28 (27-31) and 42-46. The epitope specificity of the antibodies used in these assays suggests interference from the glycosylation of NT-proBNP molecule, as these regions of NT-proBNP were shown to be glycosylated. Indeed, the negative effect of glycosylation on NT-proBNP recognition by the antibodies specific to the middle fragment of the molecule has been shown in several studies. Commercial NT-proBNP immunoassays were revealed to show substantial cross-reactivity with non-glycosylated proBNP but can barely recognize glycosylated NT-proBNP and proBNP molecules due to the presence of O-glycans in the epitopes recognized by the antibodies. However, although it has been shown that Roche NT-proBNP assays underestimate the concentration of circulating NT-proBNP (up to 10-fold) (37, 63), their diagnostic and prognostic accuracy is quite good, and they are currently widely used in clinical practice.
However, the recent data suggest that underestimating the NT-proBNP concentration due to the influence of glycosylation of NT-proBNP molecules may have some impact on the clinical significance of this biomarker. This subject has been addressed in the work of Helge Røsjø et al., which showed that the deglycosylation of NT-proBNP by treatment with a mix of specific enzymes (deglycosidases) improved the the diagnostic and prognostic accuracy of the NT-proBNP assay (64). Thus, one may suggest that the epitopes not effected by glycosylation should be preferred for the design of new generations of NT-proBNP assays.
B-TYPE NATRIURETIC PEPTIDE ASSAYS
In contrast to NT-proBNP, the current situation with BNP immunoassay is far more complex. A variety of companies market assays for BNP, which are based on different antibodies and standard materials. Recent studies suggest that there are marked systematic differences among the BNP values obtained using different platforms. The CardioOrmoCheck study reports differences of up to 50% across different BNP immunoassays (65), and such discrepancies occur even for assays using the same antibodies but run on different instruments.
The most common commercial methods for BNP measurement used in the clinical laboratories are sandwich-type immunometric assays. These methods usually employ two antibodies specific for two distantly located epitopes of the BNP peptide chain. One of these antibodies is always specific for the intact cysteine ring, to detect the form, which is considered to be physiologically active, whereas the other is specific either for the C-terminus of the peptide (e.g., in Abbott AxSYM and Architect, Shionogi IRMA) or for the N-terminus (e.g., in Alere Triage and Beckman Access). Obviously, the assays utilizing antibodies specific to the very C-terminus of the BNP molecule should not measure BNP peptides that are degraded at this part of the molecule. Similarly, assays utilizing antibodies specific to the N-terminus of BNP should not measure BNP-related peptides truncated at this part of the molecule.
Among this variety of BNP immunoassays, the “Single Epitope Sandwich” Immunoassay (SES-BNP™) designed by HyTets’s specialists and implemented in a platform by ET healthcare differs from conventional sandwich-type BNP assays (66). This assay utilizes one mAb 24C5 specific to the relatively stable ring fragment of the BNP molecule (epitope 11-17), which is within the biologically active cysteine ring, and the second mAb, Ab-BNP2, which recognizes the immune complex of mAb 24C5 with BNP (proBNP) only. Thus, there is no space between epitopes, and consequently, cleavage between the epitopes does not affect it, as only one epitope is needed for BNP measurements in the SES-BNP™ assay. This assay was shown to be able to recognize BNP as well as the recombinant glycosylated and nonglycosylated forms of proBNP with the same efficiency.
The high sensitivity of SES-BNP™ assay (up to 0.5 pg/mL) is most likely achieved by the “locking” effect of the detection antibody – it stabilizes the immune complex of the capture antibody with the antigen and increases the affinity of the capture antibody for its antigen.
The initially proposed cut-off for BNP is 100 ng/L, which excludes acute HF with high negative predictive value. However, it should be stressed that due to the high substantial differences between different BNP immunoassays, this value should be determined for each assay and standard material used in calibration (67).
The diversity of antibodies and standard materials used in commercially available BNP immunoassays is summarized in Figure 4.
Figure 4. Antibodies and standard materials used in commercial BNP immunoassays.
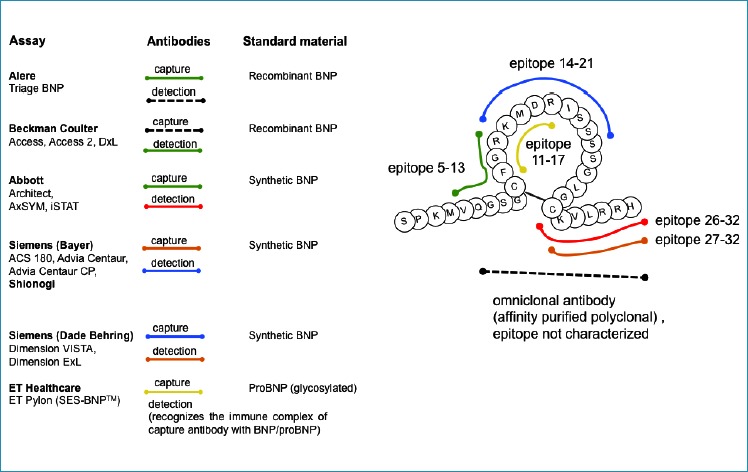
Adapted with modifications from: http://www.ifcc.org/media/102208/NP%20Assay%20Table%20C%20SMCD%20vJuly_2011.pdf.
The great heterogeneity of proBNP-derived peptides circulating in human blood can partially explain the systematic differences among the results provided by immunoassay methods considered specific for BNP. Another important cause of non-harmonized BNP assays may be the lack of a suitable reference material for the calibration of BNP assays by manufacturers. As a consequence of the non-harmonized assays, the obtained results are often unique to a certain method or instrument, so that different results from different assays and platforms are poorly comparable. Thus, a common calibrator used in all these BNP assays might help to reduce the variability of the obtained values. Currently, no such common calibrator has yet been suggested. Considering the prevalence of proBNP as a major BNP-immunoreactive form, one might suggest that proBNP could become a common calibrator and stable standard for BNP immunoassays. The introduction of a common reference material should reduce the systematic differences among current methods and result in better harmonization among results.
Additionally, it was revealed that all BNP immunoassays share some cross-reactivity with proBNP, as proBNP has the same structure (BNP-part) as the BNP molecule (53). Considering that proBNP is the major BNP-immunoreactive form in the circulation of HF patients, this cross-reactivity is clinically relevant. Some assays may hardly recognize proBNP at all, especially its glycosylated form, due to the steric hindrance of the glycosidic residues.
Therefore, due to the high complexity of BNP-related peptides and the prevalence of proBNP in the circulation, much of the BNP measured by contemporary assays is either nonprocessed proBNP or degradation products of BNP 1-32 rather than intact mature BNP 1-32.
BNP ASSAYS AND THE RECENT ADVANCES IN THE TREATMENT OF HF
The recent data regarding the use of neprilysin inhibitor and angiotensin receptor blocker LCZ696 as a therapeutic agent developed by Novartis in patients exhibiting HF with a reduced ejection fraction have greatly stimulated the interest to the use of BNP and NT-proBNP in HF diagnostics and the monitoring of therapy and also raised some important questions.
The PARADIGM-HF trial (Prospective Comparison of ARNI (angiotensin receptor neprisylin inhibitor) With ACEI (angiotensin-converting enzyme inhibitor) to Determine Impact on Global Mortality and Morbidity in HF) demonstrated a marked improvement in outcomes with LCZ696 compared with enalapril (inhibitor of angiotensin-converting enzyme) alone in patients with predominantly New York Heart Association functional class II HF (68).
Neprilysin is a widely expressed membrane-bound protease, particularly abundant in kidney, that cleaves substrates on the amino side of hydrophobic residues. It has been shown to cleave and inactivate a number of peptides, including glucagon, enkephalins, substance P, neurotensin, oxytocin, bradykinin and amyloid beta (reviewed in (69)). Both ANP and CNP are known to be substrates of neprilysin (70). However, BNP was shown to be a poor substrate for neprilysin, as its specific inhibitors failed to block BNP degradation by human kidney membranes, suggesting that it is unlikely to be a significant regulator of BNP concentration in the kidney (50).
As neprilysin is thought to be responsible for degrading NPs, it is possible that the beneficial effect of this new drug is achieved by inhibiting NP degradation, increasing the level of NPs, ANP and BNP and, as a consequence, improving HF.
However, the suggestion that inhibition of neprilysin should lead to a prompt and prominent increase in BNP level is rather debatable. The effect of neprilysin inhibition should be more prominent for ANP than for BNP, as ANP is known to be a much better substrate for neprilysin than BNP (71). Given the complexity and diversity of proBNP-derived peptides, it is hardly possible that the effect of LCZ696 on the BNP level can be so simple. As the major form of BNP-immunoreactivity is proBNP, we should rather consider its degradation and the effect of the drug on the level of proBNP rather than BNP. Unfortunately, there are currently no data on the degradation of proBNP by the action of neprilysin.
Whether treatment with neprilysin inhibitor will interfere with the use of BNP measurement for HF diagnosis and prognosis or treatment monitoring remains an open question. Considering the complex biochemistry of proBNP-derived peptides, it is definitely not obvious how the BNP and NT-proBNP levels are affected by treatment with LCZ696 in different disease states (72).
On the one hand, if treatment with LCZ696 indeed affects the BNP levels measured by BNP immunoassays, then it seems that BNP measurements may be ambiguous in this case. For this purpose, NT-proBNP measurements seem to be preferred, although it should be considered that the increase in circulating BNP might decrease proBNP production and thus decrease the NT-proBNP level, which would then fail to reflect the improvement of cardiac function. On the other hand, measurements of BNP may be very important to understand at what level of BNP increase the drug therapy works and reflect the action of the drug, whereas NT-proBNP levels may reflect the effects of the drug on the heart.
The complexity of the NP system and the diversity of HF states suggest that the measurements of either BNP or NT-proBNP alone might not be sufficient to fully understand the HF status of patients, but rather both biomarkers (or their ratio) should be used to fully utilize the diagnostic and prognostic value of these biomarkers.
Thus, the use of this new and seemingly promising HF drug generates a number of serious questions for clinical diagnostics that must be answered before this drug becomes routinely used in clinical practice along with NP measurements.
ProBNP IMMUNOASSAYS
By its nature, the proBNP molecule shares a common structure with both BNP (BNP part within proBNP sequence) and NT-proBNP (N-terminal part within proBNP sequence). Thus, a proBNP-specific assay should be based on one antibody specific to the N-terminal part and the second one to the C-terminal part. An assay based on a capture mAb specific for the region 26-32 of the BNP molecule and a detection antibody specific for the fragment 13-20 of proBNP was designed by HyTest specialists (32). It was shown that this highly sensitive immunoassay is not affected by the glycosylation of proBNP molecules, as the epitopes of the utilized antibodies are free of O-glycans.
Giuliani et al. developed a specific mAb that recognizes the hinge region of the proBNP molecule (75-80 aar) (73). A sandwich immunoassay for the measurement of proBNP was designed by combining this mAb with a polyclonal antibody directed against the BNP part of the proBNP molecule (Fig. 5). An automated version of this method was performed on the BioPlex 2200 Analyzer Multiplex System (Bio-Rad), and its analytical characteristics were evaluated. Notably, the presence of O-glycans at Thr71 may affect the interaction of the hinge-specific antibodies with endogenous proBNP due to the close proximity to the recognized epitope and, as a consequence, underestimate the amount of intact proBNP detected by this assay in plasma samples of HF patients.
Figure 5.
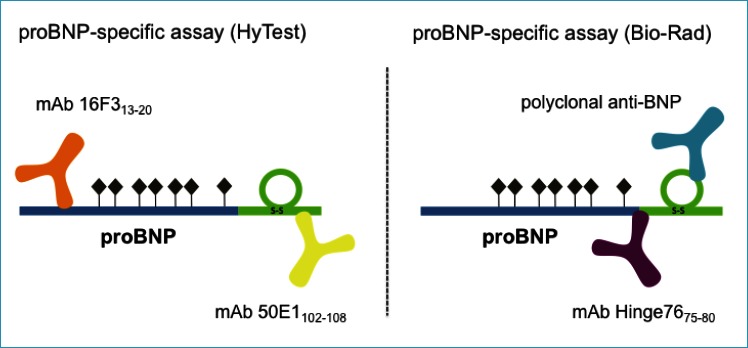
Schematic representation of proBNP-specific assays designed by HyTest and Bio-Rad. Seven potential O-glycosylation sites within the proBNP sequence are marked with dark diamonds (34)
Thus far, proBNP-specific assays have been shown to be equivalent to but no better than BNP or NT-proBNP assays. (74). However, one may speculate that considering the potentially reduced bioactivity of unprocessed proBNP, measurements of the concentration of bioactive BNP or, alternatively, the ratio of BNP to unprocessed proBNP might be clinically relevant.
It should be stressed, however, that there are currently no assays that are specific to BNP with no cross-reactivity to proBNP. The development of such assays is rather challenging due to the presence of the common structure in the proBNP molecule. Whether such an assay would have additional clinical significance over conventional BNP assays, which detect both BNP and proBNP, remains a question to be answered in future clinical studies.
CONCLUSIONS
NPs are widely accepted to be useful and cost-effective biomarkers for HF. Both BNP and NT-proBNP testing are currently routinely used in clinical practice and have been incorporated into most national and international cardiovascular guidelines. Despite this wide acceptance, the complex biochemistry of NPs requires deep insight into analytical issues for the accurate interpretation of test results in clinical practice. Moreover, the constant implementation of new therapeutic agents (e.g., LCZ696) for HF treatment generates new challenges for their use in diagnostics and the monitoring of therapy and requires comprehensive understanding of how this complex system works. Thus, new immunoassays based on the improved understanding of the complex biochemistry of proBNP-derived peptides should perhaps be considered for future development.
The large systematic differences among methods when comparing the results obtained from different laboratories using different assays represents another important issue to be solved. Novel approaches such as the introduction of a common reference material for BNP immunoassays should be considered to improve this situation.
Additionally, the diversity of circulating forms of BNP-related peptides with different physiological activities suggests that a new generation of immunoassays specific to the distinct forms of BNP, NT-proBNP and proBNP or able to measure the ratio between different BNP forms might offer potential clinical significance over existing assays that do not distinguish different circulating forms of proBNP-derived peptides.
To summarize, we may conclude that recent advances in understanding the complexity of the NP system have both improved the comprehension of the clinical meaning of test results and generated a number of new challenges to be addressed in future studies to improve the diagnostics and treatment of cardiovascular complications.
Acknowledgments
We are grateful to Dr. Alexander B. Postnikov for the constructive criticism and helpful comments in the preparation of this manuscript.
Abbreviations (in alphabetical order)
- aar
amino acid residues
- ADHF
acute decompensated heart failure
- AHF
acute heart failure
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- cGMP
cyclic GMP
- CNP
C-type natriuretic peptide
- DPP IV
dipeptidyl peptidase IV
- FDA
Food and Drug Administration
- IDE
insulin-degrading enzyme
- HF
heart failure
- mAb
monoclonal antibody
- Ml
myocardial infarction
- NEP
neutral endopeptidase (neprilysin)
- NPR-A
natriuretic peptide receptor A
- NPR-B
natriuretic peptide receptor B
- NPR-C
natriuretic peptide receptor C
- NT-proANP
N-terminal fragment of proANP
- NT-proBNP
N-terminal fragment of proBNP
- proANP
ANP precursor
- proBNP
BNP precursor
- SES-BNP
Single Epitope Sandwich BNP immunoassay.
REFERENCES
- 1.Flynn T.G., de Bold M.L., de Bold A.J. 1983. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun 117:859-865. [DOI] [PubMed] [Google Scholar]
- 2.Sudoh T., Kangawa K., Minamino N., Matsuo H. 1988. A new natriuretic peptide in porcine brain. Nature 332:78-81. [DOI] [PubMed] [Google Scholar]
- 3.de Bold A.J., Borenstein H.B., Veress A.T., Sonnenberg H., 1981. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28:89-94. [DOI] [PubMed] [Google Scholar]
- 4.Marin-Grez M., Fleming J.T., Steinhausen M. 1986. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 324:473-476. [DOI] [PubMed] [Google Scholar]
- 5.Harris P.J., Thomas D., Morgan T.O., 1987. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 326:697-698. [DOI] [PubMed] [Google Scholar]
- 6.Gunning M., Ballermann B.J., Silva P., Brenner B.M., Zeidel M.L. 1990. Brain natriuretic peptide: interaction with renal ANP system. Am J Physiol 258:F467-F472. [DOI] [PubMed] [Google Scholar]
- 7.Sudoh T., Minamino N., Kangawa K., Matsuo H. 1990. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun 168:863-870. [DOI] [PubMed] [Google Scholar]
- 8.Vollmar A.M., Gerbes A.L., Nemer M., Schulz R. 1993. Detection of C-type natriuretic peptide (CNP) transcript in the rat heart and immune organs. Endocrinology 132:1872-1874. [DOI] [PubMed] [Google Scholar]
- 9.Potter L.R., Abbey-Hosch S., Dickey D.M. 2006. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27:47-72. [DOI] [PubMed] [Google Scholar]
- 10.Potter L.R., Hunter T. 2001. Guanylyl cyclase-linked natriuretic peptide receptors: structure and regulation. J Biol Chem 276:6057-6060. [DOI] [PubMed] [Google Scholar]
- 11.Mukoyama M., Nakao K., Saito Y., Ogawa Y., Hosoda K., Suga S., Shirakami G., Jougasaki M., Imura H. 1990. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med 323:757-758. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa Y., Nakao K., Mukoyama M., Hosoda K., Shirakami G., Arai H., Saito Y., Suga S., Jougasaki M., Imura H. 1991. Natriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats. The ventricle is a major site of synthesis and secretion of brain natriuretic peptide. Circ Res 69:491-500. [DOI] [PubMed] [Google Scholar]
- 13.Hosoda K., Nakao K., Mukoyama M., Saito Y., Jougasaki M., Shirakami G., Suga S., Ogawa Y., Yasue H., Imura H. 1991. Expression of brain natriuretic peptide gene in human heart. Production in the ventricle. Hypertension 17:1152-1155. [DOI] [PubMed] [Google Scholar]
- 14.Rubattu S., Sciarretta S., Valenti V., Stanzione R., Volpe M. 2008. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens 21:733-741. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K., Mair J., Mueller C., Huber K., Weber M., Plebani M., Hasin Y., Biasucci L.M., Giannitsis E., Lindahl B., et al. 2012. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J 33:2001-2006. [DOI] [PubMed] [Google Scholar]
- 16.Colucci W.S., Elkayam U., Horton D.P., Abraham W.T., Bourge R.C., Johnson A.D., Wagoner L.E., Givertz M.M., Liang C.S., Neibaur M., et al. 2000. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med 343:246-253. [DOI] [PubMed] [Google Scholar]
- 17.Mills R.M., Hobbs R.E., Young J.B. 2002. “BNP” for heart failure: role of nesiritide in cardiovascular therapeutics. Congest Heart Fail 8:270-273. [DOI] [PubMed] [Google Scholar]
- 18.Crozier I.G., Nicholls M.G., Ikram H., Espiner E.A., Gomez H.J., Warner N.J. 1986. Haemodynamic effects of atrial peptide infusion in heart failure. Lancet 2:1242-1245. [DOI] [PubMed] [Google Scholar]
- 19.Pleister A.P., Baliga R.R., Haas G.J. 2011. Acute study of clinical effectiveness of nesiritide in decompensated heart failure: nesiritide redux. Curr Heart Fail Rep 8:226-232. [DOI] [PubMed] [Google Scholar]
- 20.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., et al. 2013. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62:e147-e239. [DOI] [PubMed] [Google Scholar]
- 21.Oikawa S., Imai M., Ueno A., Tanaka S., Noguchi T., Nakazato H., Kangawa K., Fukuda A., Matsuo H. 1984. Cloning and sequence analysis of cDNA encoding a precursor for human atrial natriuretic polypeptide. Nature 309:724-726. [DOI] [PubMed] [Google Scholar]
- 22.Yan W., Wu F., Morser J., Wu Q. 2000. Corin, a transmembrane cardiac serine protease, acts as a proatrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A 97:8525-8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vesely D.L. 1995. Atrial natriuretic hormones originating from the N-terminus of the atrial natriuretic factor prohormone. Clin Exp Pharmacol Physiol 22:108-114. [DOI] [PubMed] [Google Scholar]
- 24.Schulz-Knappe P., Forssmann K., Herbst F., Hock D., Pipkorn R., Forssmann W.G. 1988. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr 66:752-759. [DOI] [PubMed] [Google Scholar]
- 25.Kristen A.V., Biener M., Hegenbart U., Hardt S., Schnabel P.A., Rocken C., Schonland S.O., Katus H.A., Giannitsis E. 2014. Evaluation of the clinical use of midregional pro-atrial natriuretic peptide (MR-proANP) in comparison to N-terminal pro-B-type natriuretic peptide (NT-proBNP) for risk stratification in patients with light-chain amyloidosis. Int J Cardiol 176:1113-1115. [DOI] [PubMed] [Google Scholar]
- 26.Sudoh T., Maekawa K., Kojima M., Minamino N., Kangawa K., Matsuo H. 1989. Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun 159:1427-1434. [DOI] [PubMed] [Google Scholar]
- 27.Siriwardena M., Kleffmann T., Ruygrok P., Cameron V.A., Yandle T.G., Nicholls M.G., Richards A.M., Pemberton C.J. B-type natriuretic peptide signal peptide circulates in human blood: evaluation as a potential biomarker of cardiac ischemia. Circulation 122:255-264. [DOI] [PubMed] [Google Scholar]
- 28.Pemberton C.J., Siriwardena M., Kleffmann T., Ruygrok P., Palmer S.C., Yandle T.G., Richards A.M. 2012. First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: initial assessment as cardiovascular biomarkers. Clin Chem 58:757-767. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa O., Ogawa Y., Itoh H., Suga S., Komatsu Y., Kishimoto I., Nishino K., Yoshimasa T., Nakao K. 1995. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest 96:1280-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kambayashi Y., Nakao K., Mukoyama M., Saito Y., Ogawa Y., Shiono S., Inouye K., Yoshida N., Imura H. 1990. Isolation and sequence determination of human brain natriuretic peptide in human atrium. FEBS Lett 259:341-345. [DOI] [PubMed] [Google Scholar]
- 31.Pemberton C.J., Johnson M.L., Yandle T.G., Espiner E.A. 2000. Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Hypertension 36:355-359. [DOI] [PubMed] [Google Scholar]
- 32.Seferian K.R., Tamm N.N., Semenov A.G., Mukharyamova K.S., Tolstaya A.A., Koshkina E.V., Kara A.N., Krasnoselsky M.I., Apple F.S., Esakova T.V., et al. 2007. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin Chem 53:866-873. [DOI] [PubMed] [Google Scholar]
- 33.Yandle T.G., Richards A.M., Gilbert A., Fisher S., Holmes S., Espiner E.A. 1993. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab 76:832-838. [DOI] [PubMed] [Google Scholar]
- 34.Schellenberger U., O’Rear J., Guzzetta A., Jue R.A., Protter A.A., Pollitt N.S. 2006. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch Biochem Biophys 451:160-166. [DOI] [PubMed] [Google Scholar]
- 35.Crimmins D.L., Kao J.L. 2008. A glycosylated form of the human cardiac hormone pro B-type natriuretic peptide is an intrinsically unstructured monomeric protein. Arch Biochem Biophys 475:36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammerer-Lercher A., Halfinger B., Sarg B., Mair J., Puschendorf B., Griesmacher A., Guzman N.A., Lindner H.H. 2008. Analysis of circulating forms of proBNP and NT-proBNP in patients with severe heart failure. Clin Chem 54:858-865. [DOI] [PubMed] [Google Scholar]
- 37.Seferian K.R., Tamm N.N., Semenov A.G., Tolstaya A.A., Koshkina E.V., Krasnoselsky M.I., Postnikov A.B., Serebryanaya D.V., Apple F.S., Murakami M.M., et al. 2008. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin Chem 54:866-873. [DOI] [PubMed] [Google Scholar]
- 38.Semenov A.G., Postnikov A.B., Tamm N.N., Seferian K.R., Karpova N.S., Bloshchitsyna M.N., Koshkina E.V., Krasnoselsky M.I., Serebryanaya D.V., Katrukha A.G. 2009. Processing of pro-brain natriuretic Peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem 55:489-498. [DOI] [PubMed] [Google Scholar]
- 39.Semenov A.G., Tamm N.N., Seferian K.R., Postnikov A.B., Karpova N.S., Serebryanaya D.V., Koshkina E.V., Krasnoselsky M.I., Katrukha A.G. 2010. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin Chem 56:1166-1176. [DOI] [PubMed] [Google Scholar]
- 40.Semenov A.G., Seferian K.R. 2011. Biochemistry of the human B-type natriuretic peptide precursor and molecular aspects of its processing. Clin Chim Acta 412:850-860. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J., Pristera N., Wang W., Zhang X., Wu Q. Effect of sialylated O-glycans in pro-brain natriuretic peptide stability. Clin Chem 56:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller W.L., Phelps M.A., Wood C.M., Schellenberger U., Van Le A., Perichon R., Jaffe A.S. 2011. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail 4:355-360. [DOI] [PubMed] [Google Scholar]
- 43.Niederkofler E.E., Kiernan U.A., O’Rear J., Menon S., Saghir S., Protter A.A., Nelson R.W., Schellenberger U. 2008. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail 1:258-264. [DOI] [PubMed] [Google Scholar]
- 44.Hawkridge A.M., Heublein D.M., Bergen H.R., 3rd, Cataliotti A., Burnett J.C., Jr., Muddiman D.C. 2005. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A 102:17442-17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandt I., Lambeir A.M., Ketelslegers J.M., Vanderheyden M., Scharpe S., De Meester I. 2006. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem 52:82-87. [DOI] [PubMed] [Google Scholar]
- 46.Pankow K., Wang Y., Gembardt F., Krause E., Sun X., Krause G., Schultheiss H.P., Siems W.E., Walther T. 2007. Successive action of meprin A and neprilysin catabolizes B-type natriuretic peptide. Circ Res 101:875-882. [DOI] [PubMed] [Google Scholar]
- 47.Muller D., Schulze C., Baumeister H., Buck F., Richter D. 1992. Rat insulin-degrading enzyme: cleavage pattern of the natriuretic peptide hormones ANP, BNP, and CNP revealed by HPLC and mass spectrometry. Biochemistry 31:11138-11143. [DOI] [PubMed] [Google Scholar]
- 48.Ralat L.A., Guo Q., Ren M., Funke T., Dickey D.M., Potter L.R., Tang W.J. 2011. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J Biol Chem 286:4670-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belenky A., Smith A., Zhang B., Lin S., Despres N., Wu A.H., Bluestein B.I. 2004. The effect of class-specific protease inhibitors on the stabilization of B-type natriuretic peptide in human plasma. Clin Chim Acta 340:163-172. [DOI] [PubMed] [Google Scholar]
- 50.Dickey D.M., Potter L.R. 2010. Human B-type natriuretic peptide is not degraded by meprin A. Biochem Pharmacol 80:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heublein D.M., Huntley B.K., Boerrigter G., Cataliotti A., Sandberg S.M., Redfield M.M., Burnett J.C., Jr. 2007. Immunoreactivity and guanosine 3′,5′-cyclic monophosphate activating actions of various molecular forms of human B-type natriuretic peptide. Hypertension 49:1114-1119. [DOI] [PubMed] [Google Scholar]
- 52.Boerrigter G., Costello-Boerrigter L.C., Harty G.J., Lapp H., Burnett J.C., Jr. 2007. Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol 292:R897-R901. [DOI] [PubMed] [Google Scholar]
- 53.Luckenbill K.N., Christenson R.H., Jaffe A.S., Mair J., Ordonez-Llanos J., Pagani F., Tate J., Wu A.H., Ler R., Apple F.S. 2008. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem 54:619-621. [DOI] [PubMed] [Google Scholar]
- 54.Liang F., O’Rear J., Schellenberger U., Tai L., Lasecki M., Schreiner G.F., Apple F.S., Maisel A.S., Pollitt N.S., Protter A.A. 2007. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol 49:1071-1078. [DOI] [PubMed] [Google Scholar]
- 55.Dickey D.M., Potter L.R. 2011. ProBNP(1-108) is resistant to degradation and activates guanylyl cyclase-A with reduced potency. Clin Chem 57:1272-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huntley B.K., Sandberg S.M., Heublein D.M., Sangaralingham S.J., Burnett J.C., Jr., Ichiki T. 2015. Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure. Circ Heart Fail 8:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ichiki T., Huntley B.K., Heublein D.M., Sandberg S.M., McKie P.M., Martin F.L., Jougasaki M., Burnett J.C., Jr. 2011. Corin Is Present in the Normal Human Heart, Kidney, and Blood, with Pro-B-Type Natriuretic Peptide Processing in the Circulation. Clin Chem. [DOI] [PubMed] [Google Scholar]
- 58.Semenov A.G., Seferian K.R., Tamm N.N., Artem’eva M.M., Postnikov A.B., Bereznikova A.V., Kara A.N., Medvedeva N.A., Katrukha A.G. 2011. Human Pro-B-Type Natriuretic Peptide Is Processed in the Circulation in a Rat Model. Clinical Chemistry 57:883-890. [DOI] [PubMed] [Google Scholar]
- 59.Vodovar N., Seronde M.F., Laribi S., Gayat E., Lassus J., Boukef R., Nouira S., Manivet P., Samuel J.L., Logeart D., et al. 2014. Post-translational modifications enhance NT-proBNP and BNP production in acute decompensated heart failure. Eur Heart J 35:3434-3441. [DOI] [PubMed] [Google Scholar]
- 60.Ichiki T., and Burnett J.C. Jr.. 2014. Post-transcriptional modification of pro-BNP in heart failure: is glycosylation and circulating furin key for cardiovascular homeostasis? Eur Heart J 35:3001-3003. [DOI] [PubMed] [Google Scholar]
- 61.Prontera C., Zaninotto M., Giovannini S., Zucchelli G.C., Pilo A., Sciacovelli L., Plebani M., Clerico A. 2009. Proficiency testing project for brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP (NT-proBNP) immunoassays: the CardioOrmocheck study. Clin Chem Lab Med 47:762-768. [DOI] [PubMed] [Google Scholar]
- 62.Januzzi J.L., van Kimmenade R., Lainchbury J., Bayes-Genis A., Ordonez-Llanos J., Santalo-Bel M., Pinto Y.M., Richards M. 2006. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 27:330-337. [DOI] [PubMed] [Google Scholar]
- 63.Nishikimi T., Ikeda M., Takeda Y., Ishimitsu T., Shibasaki I., Fukuda H., Kinoshita H., Nakagawa Y., Kuwahara K., Nakao K. 2012. The effect of glycosylation on plasma N-terminal proBNP-76 levels in patients with heart or renal failure. Heart 98:152-161. [DOI] [PubMed] [Google Scholar]
- 64.Rosjo H., Dahl M.B., Jorgensen M., Roysland R., Brynildsen J., Cataliotti A., Christensen G., Hoiseth A.D., Hagve T.A., Omland T. 2015. Influence of Glycosylation on Diagnostic and Prognostic Accuracy of N-Terminal Pro-B-Type Natriuretic Peptide in Acute Dyspnea: Data from the Akershus Cardiac Examination 2 Study. Clin Chem 61:1087-1097. [DOI] [PubMed] [Google Scholar]
- 65.Clerico A., Zaninotto M., Prontera C., Giovannini S., Ndreu R., Franzini M., Zucchelli G.C., Plebani M.Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical, B. 2012. State of the art of BNP and NT-proBNP immunoassays: the CardioOrmoCheck study. Clin Chim Acta 414:112-119. [DOI] [PubMed] [Google Scholar]
- 66.Tamm N.N., Seferian K.R., Semenov A.G., Mukharyamova K.S., Koshkina E.V., Krasnoselsky M.I., Postnikov A.B., Serebryanaya D.V., Apple F.S., Murakami M.M., et al. 2008. Novel immunoassay for quantification of brain natriuretic peptide and its precursor in human blood. Clin Chem 54:1511-1518. [DOI] [PubMed] [Google Scholar]
- 67.McCullough P.A., Duc P., Omland T., McCord J., Nowak R.M., Hollander J.E., Herrmann H.C., Steg P.G., Westheim A., Knudsen C.W., et al. 2003. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis 41:571-579. [DOI] [PubMed] [Google Scholar]
- 68.McMurray J.J., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S.D., Swedberg K., et al. 2014. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371:993-1004. [DOI] [PubMed] [Google Scholar]
- 69.Nalivaeva N.N., Belyaev N.D., Zhuravin I.A., Turner A.J. 2012. The Alzheimer’s amyloid-degrading peptidase, neprilysin: can we control it? Int J Alzheimers Dis 2012:383796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kenny A.J., Bourne A., Ingram J. 1993. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J 291 (Pt 1):83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walther T., Stepan H., Pankow K., Becker M., Schultheiss H.P., Siems W.E. 2004. Biochemical analysis of neutral endopeptidase activity reveals independent catabolism of atrial and brain natriuretic peptide. Biol Chem 385:179-184. [DOI] [PubMed] [Google Scholar]
- 72.Jaffe A.S., Apple F.S., Mebazaa A., Vodovar N. 2015. Unraveling N-Terminal Pro-B-Type Natriuretic Peptide: Another Piece to a Very Complex Puzzle in Heart Failure Patients. Clin Chem 61:1016-1018. [DOI] [PubMed] [Google Scholar]
- 73.Giuliani I., Rieunier F., Larue C., Delagneau J.F., Granier C., Pau B., Ferriere M., Saussine M., Cristol J.P., Dupuy A.M., et al. 2006. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clin Chem 52:1054-1061. [DOI] [PubMed] [Google Scholar]
- 74.Waldo S.W., Beede J., Isakson S., Villard-Saussine S., Fareh J., Clopton P., Fitzgerald R.L., Maisel A.S. 2008. Pro-B-type natriuretic peptide levels in acute decompensated heart failure. J Am Coll Cardiol 51:1874-1882. [DOI] [PubMed] [Google Scholar]


