Abstract
About one third of all patients recovering from stroke will experience some degree of post stroke depression (PSD). PSD lengthens recovery times and reduces overall quality of life for these individuals. While demographic, environmental, and clinical factors may explain some of the variability seen, these factors cannot fully predict the development of PSD. Furthermore, the precise mechanism of action is largely unknown. Recent work has begun to shed light on the influence of the monoamine neurotransmitter dopamine. This article summarizes the current evidence for the involvement of the dopaminergic system for PSD, using both preclinical and clinical models. Finally, a conceptual model is proposed that addresses the contributions of dopamine to the development of PSD.
Background
Stroke is the fifth leading cause of death in the United States, and is a major contributor to functional disability in adults (Mozaffarian et al., 2016). Regardless of the level of disability, those who survive may at times experience some short periods of grief and sadness during the recovery process. But about one third of patients experience symptoms of longer duration and greater severity, and are diagnosed with post stroke depression (PSD) (Alajbegovic, Djelilovic-Vranic, Nakicevic, Todorovic, & Tiric-Campara, 2014). These statistics make the risk of depression after stroke nearly double that of the general population (Ayerbe, Ayis, Rudd, Heuschmann, & Wolfe, 2011). Even so, it is likely that the estimated prevalence of PSD is underreported due to varied (or absent) screening procedures for patients and varied inclusion/exclusion criteria and instrumentation for clinical studies (Dwyer Hollender, 2014). Despite being possibly underreported, it has been shown that PSD can result in increased mortality, longer recovery times, and reductions in the patient's overall quality of life (Ayerbe et al., 2011). Thus, it is important for nurse and other health care providers to be able to correctly identify individuals at risk for PSD and to intervene before the development of severe symptoms.
The purpose of this review is to summarize the current evidence for demographic and clinical factors contributing to PSD, and then the role of, and a conceptual model for, dopaminergic contributions to PSD. The evidence base is given for both clinical and preclinical models, amassed from a standard literature search using PubMed and Google Scholar databases. Articles were published in English prior to October 2015, and keywords included terms such as “stroke”, “depression”, “post stroke depression”, “dopamine”, “antidepressant”, and “inflammation”. From this evidence, a conceptual model is proposed that addresses demographic, clinical, and dopaminergic influences on the development of PSD.
Relationship between post stroke depression and major depressive disorder
PSD shares many similarities to the more common major depressive disorder (MDD) in symptomology. The hallmark feature of both MDD and PSD is a depressed mood, accompanied by a loss of pleasure in activities that were previously enjoyed. More minor symptoms (such as changes in appetite and/or weight, sleep disturbances, and others) may or may not be present in each disorder and vary between individuals.
The monoamine theory of major depressive disorder states that the symptoms of depression are due to reductions in the monoamine neurotransmitters in the brain (Delgado, 2000). This includes serotonin, dopamine, as well as norepinephrine, and epinephrine. This theory is widely accepted, with the majority of emphasis placed on the serotonergic system. But since the symptoms for PSD must be directly following a stroke, it is reasonable to conclude that the physiologic mechanisms triggering PSD may be different than the mechanisms that create MDD. One of these physiological processes unique to stroke could be the inflammatory disruption in the monoamine neurotransmitter pathways described by Narushima (Narushima, Kosier, & Robinson, 2003).
Demographic, environmental, and clinical factors contributing to post stroke depression
Furthermore, although they do not explain all of the variance seen, certain demographic, environmental, and clinical factors are unique to the development of PSD. Younger female patients were found to have higher rates of PSD (Alajbegovic et al., 2014; El Husseini et al., 2012), as do those that do not have a strong social support system (Ayerbe et al., 2011). A prior medical history of MDD and/or history of previous stroke or other vascular risk factors also increases the likelihood of PSD (Allan et al., 2013; Andersen, Vestergaard, Ingemann-Nielsen, & Lauritzen, 1995). Other clinical variables have also been associated with PSD. For example, a greater burden of physical impairment after the recovery process is complete increases the likelihood of symptoms (El Husseini et al., 2012).
Etiology of post stroke depression
It has been hypothesized that the location of the stroke could be the most important variable for prediction of PSD. This is a reasonable, as strokes in certain locations could potentially disrupt monoamine pathways (Narushima et al., 2003). For instance, left basal ganglia strokes are highly likely to result in PSD (Robinson, 2003). The basal ganglia is a major center for the production of dopamine, a monoamine neurotransmitter. The association between left frontal lobe/left hemisphere strokes and PSD is compelling, although these associations could be mediated by prevalence differences between various stroke locations by sex (Alajbegovic et al., 2014). These locations are also rich in monoamine projections. While the consensus is that location alone does not predict whether an individual will or will not develop PSD, disruption in monoamine pathways does seem to play some role (Esparrago Llorca, Castilla-Guerra, Fernandez Moreno, Ruiz Doblado, & Jimenez Hernandez, 2015).
Antidepressant therapy and dopamine
One way that we know that the monoamine dopamine is involved in depression is through the response to medication therapy. Dopamine agonists and dopamine reuptake inhibitor medications are commonly used to treat other disorders, such as Parkinson's disease and attention deficit hyperactivity disorder (ADHD), but they may also have usefulness in the treatment of PSD. It has been shown that methylphenidate and levodopa (along with physical therapy) improves mood after stroke (Delbari, Salman-Roghani, & Lokk, 2011). Methylphenidate is a dopamine reuptake inhibitor, commonly used for ADHD. L-DOPA is a precursor to the catecholamine neurotransmitters and is one of the most common treatments for the dopamine deficit that occurs in Parkinson's disease. These medications increase the amount of dopamine available in the brain, which may help to increase mood after stroke.
It has been shown that selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine (Prozac), do reduce the rates of PSD, but do not reduce the symptom burden for those that develop PSD (Yi, Liu, & Zhai, 2010). Some have suggested that the extension of serotonergic action by the medication also causes a dampening of other neurotransmitter systems, including dopamine (Blier & El Mansari, 2013). Many of these refractory symptoms are dopaminergically controlled, such as loss of motivation and pleasure (Blier & El Mansari, 2013).
Antidepressant therapies could also have an anti-inflammatory effect on dopaminergic pathways that might help to alleviate PSD. One hypothesis of PSD is that the stroke injury itself causes a pro-inflammatory environment in the brain that then results in depression (Kim et al., 2012). There is evidence that antidepressants given post stroke improve motor outcomes through modulation of dopamine degeneration that results from that inflammation (Siepmann et al., 2015). At the same time, the antidepressants also help to improve symptoms of depression. For instance, treatment with the SSRI paroxetine (Paxil) reduces pro-inflammatory cytokines from stroke, which is thought to prevent degeneration of nigrostrial (dopaminergic) neurons (Esparrago Llorca et al., 2015). To further support this supposition, genetic changes that reduce the anti-inflammatory effects of specific cytokines are also associated with increased rates of PSD (Kim et al., 2012).
Even with some successes, antidepressant medications often times still fall short. Time to effectiveness varies by class, but can be on the order of weeks to months before therapeutic effect. Individualized genetic and biochemical differences cause antidepressants to be more or less effective for each person, and finding the right combination for each patient requires time consuming trial and error. Furthermore, the side effects of antidepressant medications are common and can include: nausea, increased appetite and weight gain, loss of sexual desire and other sexual problems, fatigue and drowsiness, insomnia, dry mouth, blurred vision, and constipation. Many of these side effects are only compounded by the physiological effects of the stroke, and so prove to be too bothersome for many patients. In addition, drug-drug interactions can be a problem as many of these patients have multiple other comorbidities and thus polypharmacy prior to their stroke (Esparrago Llorca et al., 2015). If antidepressant therapy is used, it is important to start early in the acute phase, although even then it may not be as effective for PSD as many would hope (Hackett, Anderson, House, & Halteh, 2008). The current inability to correctly identify those most at risk for PSD may then lead to medicating all stroke patients, when not all will need PSD prophylaxis.
Alternatively, some have suggested psychotherapy for those most at risk. A large systematic review found this to only have small to modest effects on PSD outcomes (Hackett et al., 2008). But another small pilot intervention using psychotherapeutic techniques to promote self care behaviors found no difference in PSD at 3 months post stroke (Hoffmann, Ownsworth, Eames, & Shum, 2015). However, strong social support networks are protective against PSD development (Ayerbe et al., 2011).
Animal models of dopamine in PSD
Given that the evidence base from clinical observation is somewhat mixed, we then look to animal models to gain insight on post stroke depression and the role of the dopaminergic system. Animals undergo a targeted lesioning procedure that mimics the effects of stroke and are then exposed to some sort of inescapable chronic stress to create PSD. Most often, the forced swim test or tail suspension is used. These procedures are meant to simulate the chronic stress of a patient adapting to life changes post stroke. Some of these key features of depression that have been recreated are anxiety and reduced feeding behavior, which is attributed to anhedonia. Interestingly, some of these symptoms are reversed after administration of a monoamine oxidase inhibitor-an antidepressant medication that reduces the degredation of dopamine (Kato, Iwata, Okamoto, Ishii, & Narita, 2000). These changes are supported by other animal studies on dopaminergic genetic changes that may contribute to one's likelihood to develop depression after stroke. For instance, the dopaminergic receptor genes DRD1a and DRD2 were differentially expressed 2 days after reperfusion following stroke in mice (Sieber et al., 2014).
Conceptual model
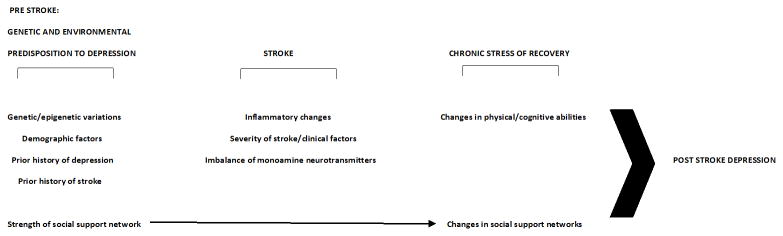
Given the evidence towards a multifactorial model of PSD, we hypothesize a conceptual model of changes leading to PSD in Figure 1. This conceptual model represents a time line of four phases: from a prestroke predisposition to depression, through the acute phase of the stroke, to the chronic stress of recovery and eventual post stroke depression.
Figure 1. Conceptual model of post stroke depression.
In the prestroke period, genetic and epigenetic factors (such as genotype for DRD1a and DRD2 (Sieber et al., 2014)), demographic factors (such as younger age or female gender (Alajbegovic et al., 2014; El Husseini et al., 2012)), prior medical history of depression, stroke, or vascular disease (Allan et al., 2013; Andersen et al., 1995), and the relative strength of a patient's social support network (Ayerbe et al., 2011) interact. All of these things may have already primed depressive patterns of behavior, but the interactions and relative strengths and weaknesses between them may alter the appearance of symptoms prior to the stroke event. During the stroke, inflammatory changes in the brain can disrupt the balance of the monoamine neurotransmitters (dopamine, and others). The level of these inflammatory changes is dependent on the severity of the stroke and other clinical factors. During this time period, there may be changes to the patient's social support network due to changes in living environment and employment status. After the acute period, the lasting changes to the patient's physical and cognitive capabilities, and continuing changes to social support networks during the recovery process all serve to increase or decrease the likelihood of the development of PSD.
Implications for nursing practice
This conceptual model could serve as a guide for clinical practice; namely that nurses should aim for early identification of demographic and clinical factors that might predispose an individual stroke patient to the development of PSD. This model is applicable to all stroke patients early in the acute care period. At a minimum, nurses should be alerted to the demographic characteristics, prior medical history, and social support risk factors mentioned in this article. Early identification of these factors can help to target those individuals most at risk for severe symptoms. This may include more intensive patient education on recognizing the beginning symptoms of PSD, or providing resources for stroke support groups. Furthermore, nurses involved in the rehabilitation period should be alert to changes in social support networks during the recovery process. For example, social support may have come from co-workers prior to the stroke, but these patients may no longer be able to maintain employment post-stroke.
In the future, health care providers may be able to more accurately identify those most at risk for PSD with genetic testing. While this testing is still in the discovery phase of research, such options are a part of a push towards precision medicine, and thus will change nursing practice in the coming years. Nurses will then need to educate their patients about the risks and benefits of genetic testing for the risk of PSD.
Future research directions
While this review and conceptual model addresses many of the known factors associated with PSD, more research still remains to be done. Most of the existing work on PSD relates to ischemic stroke (Dwyer Hollender, 2014). This does not include hemorrhagic stroke subtypes (aneurysmal subarachnoid hemorrhage and intracerebral hemorrhage) and traumatic brain injury, and so it is unknown whether they have similar associations to PSD. Furthermore, future prospective studies on genetic and epigenetic changes related to PSD could potentially give us the ability to identify those individuals that are most likely to be affected by PSD. If identified early in the recovery period, these individuals could be targeted with medical and behavioral therapy interventions to reduce the appearance and severity of symptoms.
Acknowledgments
Source of funding: Funding support for this work was given by the NIH/NINR grant 5R01NR013610 (PI: Conley) and by the postdoctoral training support of Dr. Stanfill by the T32 NR009759 (PI: Conley).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to report.
Contributor Information
Ansley Stanfill, Email: stanfill@pitt.edu, 440 Victoria Building, 3500 Victoria St, Pittsburgh, PA 15261, Phone: (404)434-0511, Fax: (412)624-8521.
Lucas Elijovich, Email: lelijovich@semmes-murphey.com, Semmes Murphey Clinic, 6325 Humphreys Blvd, Memphis, TN 38120, Phone: (901)522-2637, Fax: (901)259-2021.
Brandon Baughman, Email: bbaughman@semmes-murphey.com, Semmes Murphey Clinic, 6325 Humphreys Blvd, Memphis, TN 38120, Phone: (901)522-2637, Fax: (901)259-2021.
Yvette Conley, Email: yconley@pitt.edu, 440 Victoria Building, 3500 Victoria St, Pittsburgh, PA 15261, Phone: (412)383-7641, Fax: (412)624-8521.
References
- Alajbegovic A, Djelilovic-Vranic J, Nakicevic A, Todorovic L, Tiric-Campara M. Post stroke depression. Med Arch. 2014;68(1):47–50. doi: 10.5455/medarh.2014.68.47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LM, Rowan EN, Thomas AJ, Polvikoski TM, O'Brien JT, Kalaria RN. Long-term incidence of depression and predictors of depressive symptoms in older stroke survivors. Br J Psychiatry. 2013;203(6):453–460. doi: 10.1192/bjp.bp.113.128355. [DOI] [PubMed] [Google Scholar]
- Andersen G, Vestergaard K, Ingemann-Nielsen M, Lauritzen L. Risk factors for post-stroke depression. Acta Psychiatr Scand. 1995;92(3):193–198. doi: 10.1111/j.1600-0447.1995.tb09567.x. [DOI] [PubMed] [Google Scholar]
- Ayerbe L, Ayis S, Rudd AG, Heuschmann PU, Wolfe CD. Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke. 2011;42(7):1907–1911. doi: 10.1161/STROKEAHA.110.605808. [DOI] [PubMed] [Google Scholar]
- Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368(1615):20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbari A, Salman-Roghani R, Lokk J. Effect of methylphenidate and/or levodopa combined with physiotherapy on mood and cognition after stroke: a randomized, double-blind, placebo-controlled trial. Eur Neurol. 2011;66(1):7–13. doi: 10.1159/000329275. [DOI] [PubMed] [Google Scholar]
- Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- Dwyer Hollender K. Screening, diagnosis, and treatment of post-stroke depression. J Neurosci Nurs. 2014;46(3):135–141. doi: 10.1097/JNN.0000000000000047. [DOI] [PubMed] [Google Scholar]
- El Husseini N, Goldstein LB, Peterson ED, Zhao X, Pan W, Olson DM, Laskowitz DT. Depression and antidepressant use after stroke and transient ischemic attack. Stroke. 2012;43(6):1609–1616. doi: 10.1161/STROKEAHA.111.643130. [DOI] [PubMed] [Google Scholar]
- Esparrago Llorca G, Castilla-Guerra L, Fernandez Moreno MC, Ruiz Doblado S, Jimenez Hernandez MD. Post-stroke depression: an update. Neurologia. 2015;30(1):23–31. doi: 10.1016/j.nrl.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Hackett ML, Anderson CS, House A, Halteh C. Interventions for preventing depression after stroke. Cochrane Database Syst Rev. 2008;(3):CD003689. doi: 10.1002/14651858.CD003689.pub3. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Ownsworth T, Eames S, Shum D. Evaluation of brief interventions for managing depression and anxiety symptoms during early discharge period after stroke: a pilot randomized controlled trial. Top Stroke Rehabil. 2015;22(2):116–126. doi: 10.1179/1074935714Z.0000000030. [DOI] [PubMed] [Google Scholar]
- Kato M, Iwata H, Okamoto M, Ishii T, Narita H. Focal cerebral ischemia-induced escape deficit in rats is ameliorated by a reversible inhibitor of monoamine oxidase-a: implications for a novel animal model of post-stroke depression. Biol Pharm Bull. 2000;23(4):406–410. doi: 10.1248/bpb.23.406. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Shin IS, Kim JT, Park MS, Yoon JS. Associations of cytokine gene polymorphisms with post-stroke depression. World J Biol Psychiatry. 2012;13(8):579–587. doi: 10.3109/15622975.2011.588247. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci. 2003;15(4):422–430. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- Robinson RG. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54(3):376–387. doi: 10.1016/s0006-3223(03)00423-2. [DOI] [PubMed] [Google Scholar]
- Sieber MW, Guenther M, Jaenisch N, Albrecht-Eckardt D, Kohl M, Witte OW, Frahm C. Age-specific transcriptional response to stroke. Neurobiol Aging. 2014;35(7):1744–1754. doi: 10.1016/j.neurobiolaging.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Siepmann T, Kepplinger J, Zerna C, Schatz U, Penzlin AI, Pallesen LP, Barlinn K. The Effects of Pretreatment versus De Novo Treatment with Selective Serotonin Reuptake Inhibitors on Short-term Outcome after Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2015 doi: 10.1016/j.jstrokecerebrovasdis.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Yi ZM, Liu F, Zhai SD. Fluoxetine for the prophylaxis of poststroke depression in patients with stroke: a meta-analysis. Int J Clin Pract. 2010;64(9):1310–1317. doi: 10.1111/j.1742-1241.2010.02437.x. [DOI] [PubMed] [Google Scholar]