Abstract
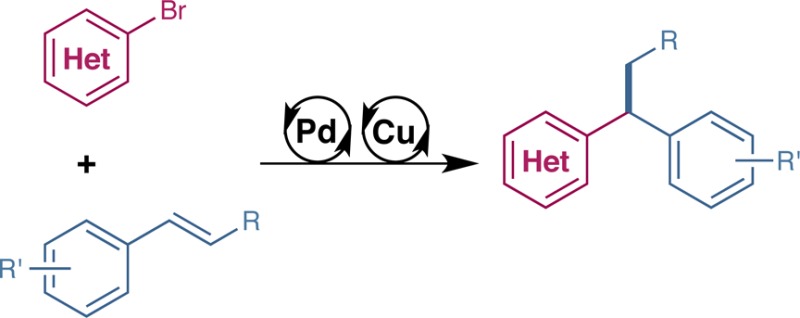
Detailed in this Communication is the enantioselective synthesis of 1,1-diarylalkanes, a structure found in a range of pharmaceutical drug agents and natural products, through the employment of copper(I) hydride and palladium catalysis. Judicious choice of ligand for both Cu and Pd enabled this hydroarylation protocol to work for an extensive array of aryl bromides and styrenes, including β-substituted vinylarenes and six-membered heterocycles, under relatively mild conditions.
Palladium-catalyzed cross coupling has proven to be a successful and reliable method for carbon–carbon bond construction. Among the many substrate classes employed in this field, stoichiometric organometallic reagents (e.g., Mg, Zn, Sn reagents) are traditionally used as coupling partners in Pd chemistry due to their propensity for facile transmetalation, and much successful work has been realized with the use of these reagents to generate functionalized arenes.1 Drawbacks associated with the use of stoichiometric organometallic reagents, including their possible sensitivity toward air/water, promiscuity toward undesired pathways of reactivity, and necessity to preform them before their use in cross coupling, have inspired methods avoiding their intermediacy.2
Recent advances in Cu chemistry have demonstrated that nucleophilic alkylcopper(I) species can be generated catalytically via olefin insertion and successfully intercepted with a range of electrophiles.3,4 As an alternative to stoichiometric organometallic reagents, we questioned whether an Cu(I) alkyl intermediate of this nature could be generated catalytically and exploited in a Pd-catalyzed cross-coupling process to yield the corresponding sp2–sp3 cross-coupled product (Scheme 1). Importantly, we sought a chiral CuH catalyst that would effect an enantioselective olefin hydrocupration to form a stereodefined Cu(I) intermediate, which would undergo transmetalation with Pd with high stereospecificity, ultimately leading to an enantioenriched coupling product. Using this approach, we anticipated that a suitable combination of Cu and Pd catalysts would allow for the enantioselective coupling of styrenes with aryl bromides to form 1,1-diarylalkanes, a biologically active structure common in both pharmaceuticals and natural products.5
Scheme 1. (A) Enantioselective Access to 1,1-Diarylalkanes via Pd and Cu Catalysis; (B) Proposed Catalytic Cycle for Enantioselective Hydroarylation of Styrenes.

Enantioenriched 1,1-diarylalkanes have previously been prepared through the stereospecific cross coupling of enantioenriched benzylic electrophiles.6,7 A nickel-catalyzed stereoconvergent coupling of racemic benzylic alcohols with arylzinc reagents has also been reported.8 Methods that employ prochiral substrates include asymmetric hydrogenation of 1,1-diarylalkenes9 and conjugate addition of arylmetal nucleophiles to cinnamaldehyde derivatives.10 Our approach represents a highly modular alternative for the enantioselective synthesis of this class of compounds. Moreover, aryl bromides and vinylarenes are widely available reagents or feedstock chemicals and are therefore nearly ideal coupling partners.11
Pioneering efforts by several researchers have demonstrated the synergistic potential of Cu and Pd catalysis.12 More recently, Nakao13 and Brown14 have both reported the diastereoselective borylarylation of styrene derivatives using both Cu and Pd in catalytic quantities, while Liao15 demonstrated the enantioselective boroallylation of vinylarenes using a similar system. At the outset of this project, the use of CuH and Pd catalysis in a cooperative manner for hydrofunctionalization was unknown. However, a report detailing the Pd/Cu-catalyzed hydroarylation of styrenes to furnish racemic 1,1-diaryl alkanes was recently published.16
Scheme 1B details our proposed dual catalytic cycle for enabling the described transformation. Formation of active CuH catalyst I would occur through the use of a Cu(I) or Cu(II) salt, chiral ligand, and silane. Enantioselective hydrocupration of olefin II would form stereodefined Cu(I) benzylic intermediate III.17,18 In the second catalytic cycle, ligated Pd(0) IV would oxidatively add to the aryl bromide V to form complex VI. As the key step in this process, the dual catalytic cycle converges via a stereospecific transmetalation of organocopper III with Pd species VI to form chiral Pd(II) alkyl complex VII.19 Stereoretentive reductive elimination furnishes enantioenriched 1,1-diarylalkane VIII and regenerates Pd(0) species IV. Considering previous reports with CuH catalysis, we reasoned that salt metathesis of Cu(I) halide IX with base would be required to regenerate CuH catalyst I.20 We believed that the success of our strategy was predicated on three issues: (i) competitive reduction of aryl halide V via a metal hydride species would need to be suppressed; (ii) choice in ligand would enable productive reactivity of each metal species while not deactivating the other; (iii) the rate of each metal cycle would need to be matched so as to avoid unproductive side reactions.
The optimization of an enantioselective hydroarylation process for styrene and 4-bromoanisole is detailed in Table 1. Examination of several silanes (entries 1–5) indicated that methyldiphenylsilane (MePh2SiH) was the optimal silane tested when combined with sodium trimethylsilanolate (NaOTMS), providing a moderate yield and high level of enantioselectivity for the desired product (entry 4, 58% yield, 83% ee). NaOTMS was a uniquely effective base for this chemistry and little to no product was observed with LiOTMS and KOTMS.21 Testing several biarylphosphine ligands (entries 4, 6, and 7) showed that a modest increase in yield was observed when using BrettPhos as a secondary ligand (entry 6, 66% yield, 85% ee). Employing [Pd(cinnamyl)Cl]2 as the source of Pd for this reaction resulted in improved reactivity while not significantly affecting the enantioselectivity of the process (entry 10, 75% yield, 88% ee). A range of Cu(I) and Cu(II) salts were examined, and a slight improvement was observed using CuOAc as the source of Cu (entry 11, 84% yield, 90% ee). Evaluation of a variety of chiral bisphosphines demonstrated that DTBM-SEGPHOS was an excellent ligand for this transformation with many other ligands giving significantly lower yield and enantioselectivity of the desired product.22
Table 1. Optimization of the Pd/Cu-Catalyzed Enantioselective Hydroarylation of Styrenea.


Yields determined by GC analysis of the crude reaction mixture using tetradecane as an internal standard; enantioselectivity of the purified product was determined by chiral HPLC; ND = not determined.
Cu(OAc)2 used as the Cu source.
CuOAc used as the Cu source.
With the optimized conditions in hand, we turned toward examining the substrate scope of the aryl bromide coupling partners (Table 2).23 Electron-rich aryl bromides (2a and 2n) worked well in this reaction, while 4-bromobenzonitrile and aryl bromides with acidic protons were not compatible with the reactions conditions. A range of functional groups, including ethers (such as 2a, 2h, 2i, 2k, and 2n), an ester (2c), a thioether (2d), an amine (2h), a carbamate (2l), an aryl chloride (2l), and an amide (2l), were all tolerated in this protocol. Employing ortho-substituted aryl bromides as viable substrates required a slightly higher reaction temperature and longer reaction times to provide the product (3n) in good yield and high enantiopurity. Importantly, a variety of brominated heterocycles, such as pyridines (e.g., 2b and 2m), quinolines (2f and 2t), a pyrimidine (2h), a pyridazine (2i), and an azaindole (2j), were competent coupling partners. In addition, a five-membered brominated heterocycle was effective (2o). Certain aryl bromides, such as 4-bromoisoquinoline (2e) and 5-bromo-1-methylindole (2g), resulted in moderate yields but poor to modest enantioselectivities (76% yield, 27% ee and 68% yield, 56% ee, respectively).24 Characterization of 3i via X-ray crystallography revealed the absolute configuration of the stereocenter. This data, combined with the sense of stereoinduction observed with our method for CuH-catalyzed hydroamination of styrenes,3d suggests that the proposed Cu-to-Pd transmetalation step occurs with retention of configuration (see Supporting Information for details).25,26
Table 2. Scope of Aryl Bromide Coupling Partnersa.


All yields represent the average of isolated yields from two runs performed with 1 mmol of styrene; enantioselectivity determined by chiral SFC.
Average of isolated yields from three runs.
The scope of arylalkene coupling partners was then evaluated with a selection of aryl bromides (Table 3). Ortho-substituted styrenes were well tolerated in this chemistry (1m and 1n). Electron-rich arylalkenes productively coupled with good levels of yield and enantioselectivity (for example entry 1o, 66% yield, 93% ee), while an electron-deficient styrene (entry 1p) produced a much lower level of yield and stereoselectivity (23% yield, 61% ee). This might be indicative of the configurational stability of the RCu intermediate. Finally, β-substituted styrenes can be utilized in this transformation, affording good yields and reasonable levels of enantioselectivity (1s and 1t). Due to a more challenging hydrocupration step, a lower Pd catalyst loading (1 mol% Pd) was required for β-substituted styrenes, presumably to slow down competitive reduction of the aryl bromide and match the rate of the two productive catalytic cycles. In addition, a slightly elevated reaction temperature and use of dimethylphenylsilane (Me2PhSiH) as the reductant was required to achieve good results with this class of olefins.
Table 3. Scope of Styrene Coupling Partners with Various Heteroaryl and Aryl Bromidesa.


All yields represent the average of isolated yields from two runs performed with 1 mmol of alkene; enantioselectivity determined by chiral SFC.
2 equiv of Me2PhSiH used as the silane; reaction run at 45 °C.
Reaction run for 40 h.
0.5 mol% of [Pd(cinnamyl)Cl]2 used with 1.1 mol% BrettPhos L3 and 2 equiv of Me2PhSiH used as the silane; reaction run at 45 °C.
In conclusion, we report the enantioselective Pd/Cu-catalyzed hydroarylation of styrenes to form 1,1-diarylalkanes, a valuable structure in medicinal chemistry. This procedure performs well for a variety of aryl bromides, including six-membered heterocycles, to form the respective products in generally good yields and with high levels of enantioselectivity. A range of vinylarenes, including ortho- and β-substituted styrenes, were also productively coupled in this hydroarylation reaction. Extending this chemistry to other substrates classes is currently being explored in our laboratory.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under award number GM46059. We thank the National Institutes of Health for a supplemental grant for the purchase of supercritical fluid chromatography (SFC) equipment under award number GM058160-17S1. M.T.P. thanks the National Institutes of Health for a postdoctoral fellowship (1F32GM113311). S.D.F. thanks the Danish Council for Independent Research: Natural Sciences and the Carlsberg Foundation for postdoctoral fellowships. We thank Prof. Shaolin Zhu and Yuli He for preliminary investigations, Dr. Peter Mueller for X-ray crystallographic data, and Drs. Yiming Wang and Jeffrey S. Bandar for their advice on the preparation of this manuscript. We thank a reviewer for insightful mechanistic suggestions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b04566.
The authors declare the following competing financial interest(s): MIT has obtained patents for some of the ligands that are described in this Communication from which S.L.B. and former/current co-workers receive royalty payments.
Supplementary Material
References
- For general reviews on Pd-catalyzed cross coupling with organometallic reagents, see:; a Jana R.; Pathak T. P.; Sigman M. S. Chem. Rev. 2011, 111, 1417. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi E., Ed.; John Wiley & Sons, Inc.: New York, 2002; pp 215–994. [Google Scholar]
- For selected examples of reductive Pd-catalyzed cross coupling to form sp2–sp3 products, see:; a Gligorich K. M.; Cummings S. A.; Sigman M. S. J. Am. Chem. Soc. 2007, 129, 14193. 10.1021/ja076746f. [DOI] [PubMed] [Google Scholar]; b Iwai Y.; Gligorich K. M.; Sigman M. S. Angew. Chem., Int. Ed. 2008, 47, 3219. 10.1002/anie.200705317. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Gligorich K. M.; Iwai Y.; Cummings S. A.; Sigman M. S. Tetrahedron 2009, 65, 5074. 10.1016/j.tet.2009.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples that purportedly generate aliphatic Cu(I) species via hydrocupration, see:; a Noh D.; Chea H.; Ju J.; Yun J. Angew. Chem., Int. Ed. 2009, 48, 6062. 10.1002/anie.200902015. [DOI] [PubMed] [Google Scholar]; b Saxena A.; Choi B.; Lam H. W. J. Am. Chem. Soc. 2012, 134, 8428. 10.1021/ja3036916. [DOI] [PubMed] [Google Scholar]; c Miki Y.; Hirano K.; Satoh T.; Miura M. Angew. Chem., Int. Ed. 2013, 52, 10830. 10.1002/anie.201304365. [DOI] [PubMed] [Google Scholar]; d Zhu S.; Niljianskul N.; Buchwald S. L. J. Am. Chem. Soc. 2013, 135, 15746. 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Pirnot M. T.; Wang Y. M.; Buchwald S. L. Angew. Chem., Int. Ed. 2016, 55, 48. 10.1002/anie.201507594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of generating alkyl Cu(I) intermediates via borylcupration, see:; a Ito H.; Kosaka Y.; Nonoyama K.; Sasaki Y.; Sawamura M. Angew. Chem., Int. Ed. 2008, 47, 7424. 10.1002/anie.200802342. [DOI] [PubMed] [Google Scholar]; b Lee Y.; Jang H.; Hoveyda A. H. J. Am. Chem. Soc. 2009, 131, 18234. 10.1021/ja9089928. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kubota K.; Yamamoto E.; Ito H. J. Am. Chem. Soc. 2013, 135, 2635. 10.1021/ja3104582. [DOI] [PubMed] [Google Scholar]; d Sakae R.; Hirano K.; Miura M. J. Am. Chem. Soc. 2015, 137, 6460. 10.1021/jacs.5b02775. [DOI] [PubMed] [Google Scholar]
- For references on various pharmaceuticals and biologically active molecules containing 1,1-diarylalkanes, refer to:; a McRae A. L.; Brady K. T. Expert Opin. Pharmacother. 2001, 2, 883. 10.1517/14656566.2.5.883. [DOI] [PubMed] [Google Scholar]; b Heydorn W. E. Expert Opin. Invest. Drugs 1999, 8, 417. 10.1517/13543784.8.4.417. [DOI] [PubMed] [Google Scholar]; c Hills C. J.; Winter S. A.; Balfour J. A. Drugs 1998, 55, 813. 10.2165/00003495-199855060-00008. [DOI] [PubMed] [Google Scholar]; d Lewis C. A.; Gustafson J. L.; Chiu A.; Balsells J.; Pollard D.; Murry J.; Reamer R. A.; Hansen K. B.; Miller S. J. J. Am. Chem. Soc. 2008, 130, 16358. 10.1021/ja807120z. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Abad A.; López-Pérez J. L.; del Olmo E.; García-Fernández L. F.; Francesch A.; Trigili C.; Barasoain I.; Andreu J. M.; Díaz J. F.; San Feliciano A. J. Med. Chem. 2012, 55, 6724. 10.1021/jm2017573. [DOI] [PubMed] [Google Scholar]
- For general reviews on stereoselective transition-metal cross coupling, see:; a Swift E. C.; Jarvo E. R. Tetrahedron 2013, 69, 5799. 10.1016/j.tet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cherney A. H.; Kadunce N. T.; Reisman S. E. Chem. Rev. 2015, 115, 9587. 10.1021/acs.chemrev.5b00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For representative examples of transition-metal-catalyzed stereospecific cross coupling to synthesize enantioenriched 1,1-diarylalkanes, see:; a Imao D.; Glasspoole B. W.; Laberge V. S.; Crudden C. M. J. Am. Chem. Soc. 2009, 131, 5024. 10.1021/ja8094075. [DOI] [PubMed] [Google Scholar]; b Taylor B. L. H.; Swift E. C.; Waetzig J. D.; Jarvo E. R. J. Am. Chem. Soc. 2011, 133, 389. 10.1021/ja108547u. [DOI] [PubMed] [Google Scholar]; c Zhou Q.; Srinivas H. D.; Dasgupta S.; Watson M. P. J. Am. Chem. Soc. 2013, 135, 3307. 10.1021/ja312087x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yonova I. M.; Johnson A. G.; Osborne C. A.; Moore C. E.; Morrissette N. S.; Jarvo E. R. Angew. Chem., Int. Ed. 2014, 53, 2422. 10.1002/anie.201308666. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Konev M. O.; Hanna L. E.; Jarvo E. R. Angew. Chem., Int. Ed. 2016, 55, 6730. 10.1002/anie.201601206. [DOI] [PubMed] [Google Scholar]
- Do H.-Q.; Chandrashekar E. R. R.; Fu G. C. J. Am. Chem. Soc. 2013, 135, 16288. 10.1021/ja408561b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Woodmansee D. H.; Pfaltz A. Chem. Commun. 2011, 47, 7912. 10.1039/c1cc11430a. [DOI] [PubMed] [Google Scholar]; b Tolstoy P.; Engman M.; Paptchikhine A.; Bergquist J.; Church T. L.; Leung A. W.-M.; Andersson P. G. J. Am. Chem. Soc. 2009, 131, 8855. 10.1021/ja9013375. [DOI] [PubMed] [Google Scholar]
- Paquin J.-F.; Defieber C.; Stephenson C. R. J.; Carreira E. M. J. Am. Chem. Soc. 2005, 127, 10850. 10.1021/ja053270w. [DOI] [PubMed] [Google Scholar]
- For a report that includes computational studies on the proclivity of various alkene substrates to undergo hydrocupration with a SEGPHOS-based CuH catalyst, see:Yang Y.; Shi S.-L.; Niu D.; Liu P.; Buchwald S. L. Science 2015, 349, 62. 10.1126/science.aab3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sonogashira K.; Tohda Y.; Hagihara N. Tetrahedron Lett. 1975, 16, 4467. 10.1016/S0040-4039(00)91094-3. [DOI] [Google Scholar]; b Jabri N.; Alexakis A.; Normant J. F. Tetrahedron Lett. 1981, 22, 959. 10.1016/0040-4039(81)89020-X. [DOI] [Google Scholar]; c Liebeskind L. S.; Fengl R. W. J. Org. Chem. 1990, 55, 5359. 10.1021/jo00306a012. [DOI] [Google Scholar]; d Huang J.; Chan J.; Chen Y.; Borths C. J.; Baucom K. D.; Larsen R. D.; Faul M. M. J. Am. Chem. Soc. 2010, 132, 3674. 10.1021/ja100354j. [DOI] [PubMed] [Google Scholar]; e Chinchilla R.; Nájera C. Chem. Soc. Rev. 2011, 40, 5084. 10.1039/c1cs15071e. [DOI] [PubMed] [Google Scholar]; f Nahra F.; Macé Y.; Lambin D.; Riant O. Angew. Chem., Int. Ed. 2013, 52, 3208. 10.1002/anie.201208612. [DOI] [PubMed] [Google Scholar]; g Vercruysse S.; Cornelissen L.; Nahra F.; Collard L.; Riant O. Chem. - Eur. J. 2014, 20, 1834. 10.1002/chem.201304284. [DOI] [PubMed] [Google Scholar]; h Nahra F.; Macé Y.; Boreux A.; Billard F.; Riant O. Chem. - Eur. J. 2014, 20, 10970. 10.1002/chem.201404015. [DOI] [PubMed] [Google Scholar]; i Ratniyom J.; Dechnarong N.; Yotphan S.; Kiatisevi S. Eur. J. Org. Chem. 2014, 2014, 1381. 10.1002/ejoc.201301634. [DOI] [Google Scholar]
- Semba K.; Nakao Y. J. Am. Chem. Soc. 2014, 136, 7567. 10.1021/ja5029556. [DOI] [PubMed] [Google Scholar]
- a Smith K. B.; Logan K. M.; You W.; Brown M. K. Chem. - Eur. J. 2014, 20, 12032. 10.1002/chem.201404310. [DOI] [PubMed] [Google Scholar]; b Logan K. M.; Smith K. B.; Brown M. K. Angew. Chem., Int. Ed. 2015, 54, 5228. 10.1002/anie.201500396. [DOI] [PubMed] [Google Scholar]
- Jia T.; Cao P.; Wang B.; Lou Y.; Yin X.; Wang M.; Liao J. J. Am. Chem. Soc. 2015, 137, 13760. 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]
- Semba K.; Ariyama K.; Zheng H.; Kameyama R.; Sakaki S.; Nakao Y. Angew. Chem., Int. Ed. 2016, 55, 6275. 10.1002/anie.201511975. [DOI] [PubMed] [Google Scholar]
- For computational studies exploring the regioselectivity of alkene insertion with analogous Cu(I) boryl complexes, see:Dang L.; Zhao H.; Lin Z.; Marder T. B. Organometallics 2007, 26, 2824. 10.1021/om070103r. [DOI] [Google Scholar]
- For a study regarding alkene insertion of stoichiometric Cu(I) boryl complexes, see:Laitar D. S.; Tsui E. Y.; Sadighi J. P. Organometallics 2006, 25, 2405. 10.1021/om060131u. [DOI] [Google Scholar]
- For evidence of the transmetalation between an alkylcopper species and Pd to be stereospecific, see refs (14) and (16).
- a Tsuda T.; Hashimoto T.; Saegusa T. J. Am. Chem. Soc. 1972, 94, 658. 10.1021/ja00757a069. [DOI] [Google Scholar]; b Wang Y.-M.; Bruno N. C.; Placeres Á. L.; Zhu S.; Buchwald S. L. J. Am. Chem. Soc. 2015, 137, 10524. 10.1021/jacs.5b07061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimethylsilanoate bases have previously been found to be efficient bases in Pd-catalyzed cross-coupling chemistry. For a representative example, see:Denmark S. E.; Tymonko S. J. Org. Chem. 2003, 68, 9151. 10.1021/jo0351771. [DOI] [PubMed] [Google Scholar]
- Refer to the Supporting Information for an extensive list of ligands, bases, and Cu sources examined.
- Running the reaction in an oil bath heated to 35 °C appeared to give a slight increase in enantioselectivity and yield, and this was done for subsequent hydroarylation reactions reported in this Communication.
- These two entries gave variable yield and enantioselectivity: 3e, 69–81% yield, 19–34% ee; 3g, 52–82% yield, 49–65% ee. Studies with 4-bromoisoquinoline (1e) have indicated that increasing the Pd catalyst loading increases the enantioselectivity of product 3e, a phenomenon we generally observed with other substrates and that is consistent with a unimolecular epimerization event competing with bimolecular transmetalation. The hydroarylation of 1e and 3-bromoquinoline (1f) with styrene in one reaction vessel also resulted in diminished enantioselectivity of quinoline product 3f (see Supporting Information for details and additional experiments).
- Recent reports on the Pd/Cu-catalyzed boryl- and hydroarylation of styrene derivatives have indicated that retention or inversion of configuration can occur during the purported transmetalation step; see refs (14) and (16).
- Deuterium labeling and computational studies for the CuH-catalyzed hydroboration of styrene derivatives indicate that hydrocupration occurs through cis-addition, and boryl transmetalation is stereoretentive. The sense of stereoinduction with (R)-DTBM-SEGPHOS for CuH-catalyzed hydroboration, hydroamination, and hydroarylation is the same; see ref (3d) and the following:Noh D.; Yoon S. K.; Won J.; Lee J. Y.; Yun J. Chem. - Asian J. 2011, 6, 1967. 10.1002/asia.201100146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


