Abstract
Introduction:
The enzyme cyclooxygenase (COX) is an enzyme that catalyzes the formation of one of the mediators of inflammation, the prostaglandins. Inhibition of COX allegedly can improve inflammation-induced pathological conditions.
Aim:
The purpose of the present study was to evaluate the potential of Sargassum sp. components, Fucoidan and alginate, as COX inhibitors.
Material and methods:
The study was conducted by means of a computational (in silico) method. It was performed in two main stages, the docking between COX-1 and COX-2 with Fucoidan, alginate and aspirin (for comparison) and the analysis of the amount of interactions formed and the residues directly involved in the process of interaction.
Results:
Our results showed that both Fucoidan and alginate had an excellent potential as inhibitors of COX-1 and COX-2. Fucoidan had a better potential as an inhibitor of COX than alginate. COX inhibition was expected to provide a more favorable effect on inflammation-related pathological conditions.
Conclusion:
The active compounds Fucoidan and alginate derived from Sargassum sp. were suspected to possess a good potential as inhibitors of COX-1 and COX-2.
Keywords: alginate, COX-1, COX-2, Fucoidan, Sargassum sp
1. INTRODUCTION
Seaweeds are classified into red algae (Rhodophyta), brown algae (Ochrophyta, Phaephyceae) and green algae (Chlorophyta) (1, 2). It constitutes natural, renewable resources distributed throughout the Pacific oceans. Seaweeds have been used primarily for human consumption either as food or medicine to cope with stomach diseases, eczema, cancer, kidney disorders, asthma, arteriosclerosis, heart disease, and lung disease (3-5). It is also being studied in biomedicine due to containing abundant bioactive components, including sulfated polysaccharides, carotenoids, proteins, essential fatty acids, vitamins, minerals, terpenoids, phlorotannin, oxylipins and steroids (6, 7). For example, alginates derived from brown algae are often used as an additive to improve food textures (5).
Non-steroidal anti-inflammatory drugs (NSAIDs) are drugs most commonly used to reduce inflammation (8). Many studies reported that the therapeutic effects and the side effects of NSAIDs were targeted at inhibition of cyclooxygenase (COX) (9). An isoform of COX, the COX-1, is used to catalyze the formation of prostaglandins (PG) on platelets, vascular endothelium, mucosa of the stomach, kidney, pancreas, islets of Langerhans, seminal vesicles and brain (10, 11). The isoform COX-2 can be induced by various growth factors, proinflammatory agents, endotoxins, mitogens and agents of the tumor, indicating that the isoform has a role in pathological processes (12). The product of COX-1, prostaglandins (PGI2 and PGE2), maintains the integrity of the gastrointestinal tract by reducing gastric acid secretion, increasing the thickness of the mucous layer, stimulating bicarbonate secretion and increasing blood flow in the mucosa (11, 13, 14).
In addition to preventing the synthesis of COX products, another mechanism of NSAID compounds is through inhibition of leukotriene, prevention of the release of the compound of oxygen radicals and lysosomal enzymes and prevention of aggregation, adhesion and chemotaxis of neutrophils (15, 16). In addition, the stimulation of peroxisome proliferator-activated receptor (PPAR) and inhibition of nuclear factor-kappa B (NF-κB) and other transcription factors are also involved in the action of NSAIDs (17).
Figure 1.
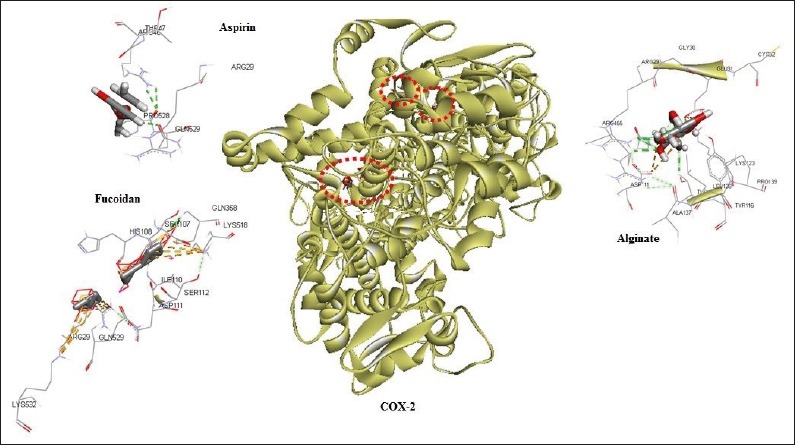
Possible interactions of aspirin, fucoidan and alginate with COX-1.
Aspirin is one of the COX inhibitory compounds. Administration of aspirin in low doses (£100 mg/day) was reported to inhibit the activity of COX-1 by acetylating SER529 residue, leading to inhibition of the production of thromboxane A2(TXA2) and inhibiting TXA2-mediated platelet aggregation. Aspirin is also found to inhibit COX-1 on gastric and duodenal mucosa, causing a reduction in PGE2-mediated cytoprotection against acidic environments (18). Studies of NSAIDs-induced gastric damage gave rise to a notion that inhibition of both COX-1 and COX-2 may occur, given that COX-2 can replace COX-1 in producing prostaglandins (19). The purpose of the present study was to investigate the potential of the active compounds Fucoidan and alginate derived from Sargassum sp. as COX inhibitors.
Figure 2.
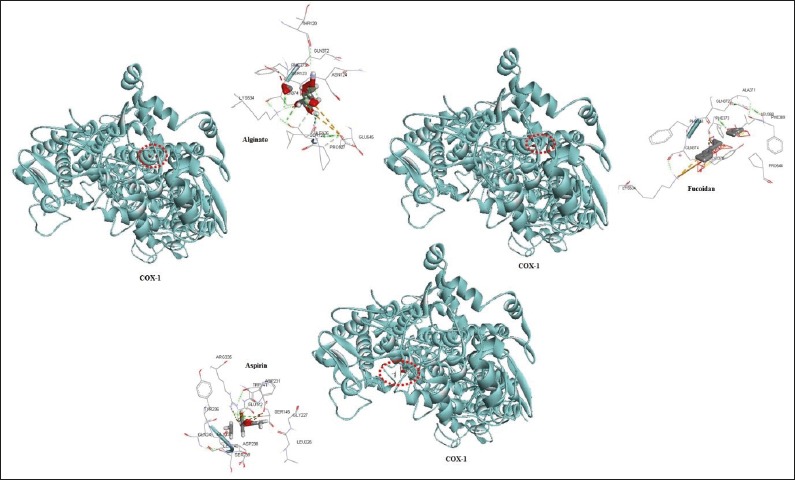
Possible interactions of aspirin, fucoidan and alginate with COX-2.
2. MATERIAL AND METHODS
2.1. Searching for Protein Sequences
The component structure of aspirin (CID: 2244), alginate (CID: 91666324) and Fucoidan (CID: 10452) was obtained from PubChem Open Chemistry Database, whereas the protein sequence of COX-1 (GI: 3914292) and COX-2 (GI: 2970564) was obtained from sequence database of the National Center for Biotechnology Information (NCBI), the United States National Library of Medicine (NLM) and the National Institute of Health (NIH) (http://www.ncbi.nlm.nih.gov).
2.2. 3D- Structure Modeling of DNA, Proteins, and Bioactive Components
The 3D-structure model of COX-1 and COX-2 was predicted using the SWISS-MODEL web-server (20, 21) by the homology modeling method. The 3D structure of proteins was then validated using the Ramachandran plot analysis. Conversion *.sdf files into *.pdb files of aspirin, Fucoidan and alginate was performed using the software OpenBabel (22).
2.3. Docking Computation
Docking simulation among aspirin, Fucoidan and alginate on COX-1 and COX-2 was performed using the software HEX 8.0 (23). The docking protocol consists of three stages of visualization: rigid-body energy minimization, semi-flexible repair and finishing refinement in explicit solvents. Upon completion of each stage, docking conformations were then scored and sorted based the scoring function to facilitate the selection of the best conformation to be used at later stages.
2.4. Analysis of Protein-Protein Interactions
Docking analysis results were subsequently visualized using the software Discovery Studio 4.1, LigPlot+ (24) and Chimera 1.6.2. Analysis of protein-protein interactions was carried out to determine the formed bonds, including hydrogen bonding, hydrophobic bonding and van der Waals bonding. Additionally, Pharmacophore analysis was also conducted to determine residues directly involved in the process of interaction, as well as the analysis of energy minimization to improve molecular structure and shape during the interaction.
3. RESULTS
3.1. Aspirin had a higher effectiveness as an inhibitor of COX-1
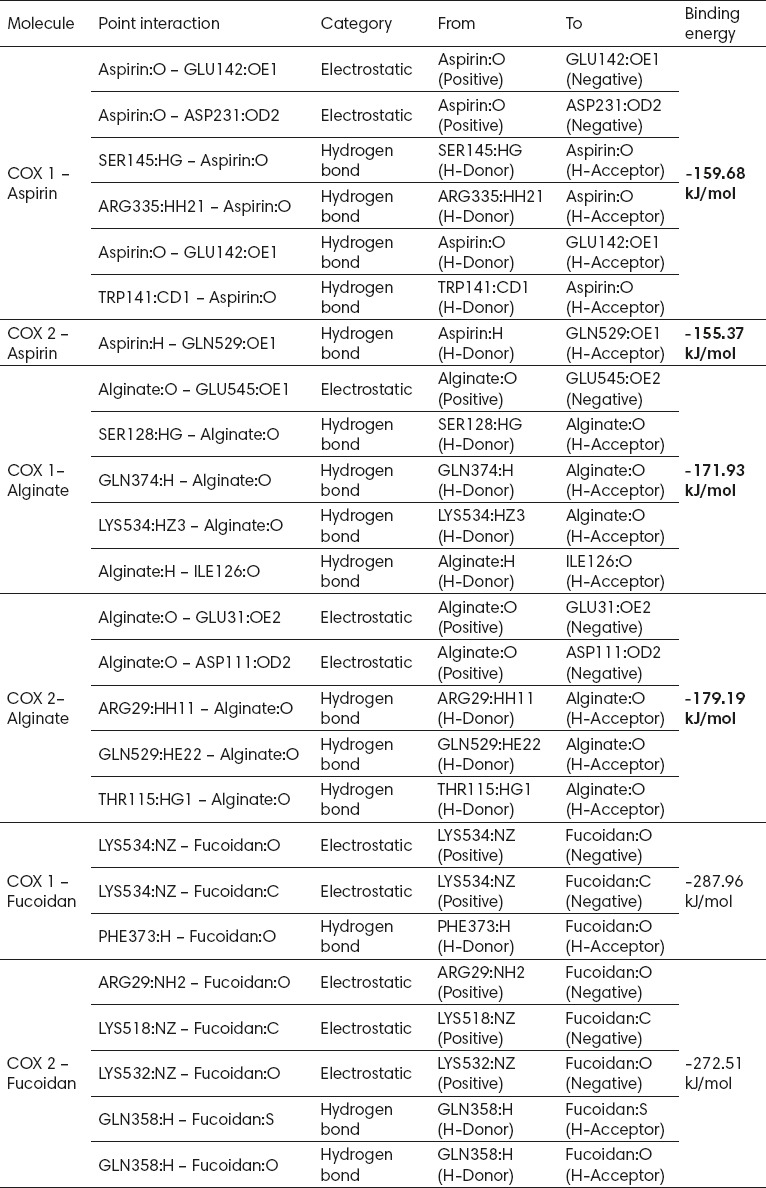
Aspirin, being one of anti-inflammatory non-steroidal drugs (NSAIDs), was used as the standard in the present study. Aspirin had a higher effectiveness as ab inhibitor for COX-1 than for COX-2, as shown by the binding energy and the types of bond formed from the interaction. Aspirin was known to require a lower binding energy to COX-1 (159.68 kJ/mol) than to COX-2 (-155.37 kJ/mol). In addition, the number of the bond formed also supported these results. Aspirin interaction with COX-1 formed two electrostatic interactions (GLU142, ASP231) and three hydrogen bonds (SER145, ARG335, GLU142, TRP141), while its interaction with COX-2 formed only one hydrogen bond (GLN529).
3.2. Active compounds of Sargassum sp. had potential as inhibitors of COX-1 and COX-2
The active compounds of Sargassum sp. analyzed in this study were alginate and Fucoidan. Analysis showed that both alginate and Fucoidan could act as inhibitors of COX-1 and COX-2. Relative to aspirin, alginate and Fucoidan were thought to have a better potential as inhibitors of COX-1 and COX-2. It was shown by the fact that the energy required for the interaction between the two compounds on COX was smaller than that required by aspirin to bind to COX (Table 1).
Table 1.
Comparison of possible interactions of the active compounds of Sargassum sp. with aspirin on COX-1 and COX-2
3.3. Fucoidan was thought to have a better potential as an inhibitor of COX-1 and COX-2 than alginate and aspirin
Analysis of bonding energy indicated that aspirin required energy of -159 kJ/mol and -155.37 kJ/mol to bind to COX-1 and COX-2, respectively. Alginate required less bonding energy of -171.93 kJ/mol and -179.19 kJ/mol to bind to COX-1 and COX-2, respectively. Fucoidan required much smaller energy to interact with COX-1 and COX-2 (-287.96 kJ/mol and -272.51 kJ/mol) than aspirin or alginate. The bonding energy required by Fucoidan was almost half of that required by aspirin to bind to COX-1 and COX-2; Fucoidan was thought to have a good potential as an inhibitor of COX-1 and COX-2.
3.4. Alginate had a better potential as an inhibitor of COX-2 than aspirin
Analysis of residues directly involved in the process of interaction showed that aspirin and alginate competitively bound COX-2, in which the two compounds would bind to the amino acid residue GLN529 in their interactions. In the process of competitive binding it was thought that alginate more easily bound than aspirin to COX-2 due to its smaller binding energy; thus, alginate had an excellent potential as an inhibitor of COX-2. In addition, on the basis of binding energy, the interaction of COX-2/aspirin only formed one hydrogen bond (GLN529), while the interaction of COX-2/alginate formed two electrostatic bonds (GLU31, ASP111) and three hydrogen bonds (ARG29, GLN529, THR115). The number of alginate-COX-2 bonds formed showed that the bonds between them were strong and stable.
4. DISCUSSION
Of various inflammatory mediators, prostaglandins (PG) are among the most important mediators. Prostaglandins are released due to various chemical and mechanical stimuli. A key enzyme of the synthesis of prostaglandin is prostaglandin endoperoxide synthase (PGHS) or cyclooxygenase (COX), which has two catalytic sites. The first is the active site of cyclooxygenase that serves to convert arachidonic acid into endoperoxide PGG2. The other was the active site of peroxidase that serves to convert PGG2 into another endoperoxide, PGH2. Furthermore, PGH2 will be processed by a specific enzyme to form PG, prostacyclin and thromboxane A2. Of all types of PG, PGE2 and prostacyclin are major mediators of inflammation (25).
The active components of algae have been known to have pharmacological actions as antiviral compounds to treat a variety of diseases, including eczema, cancer, kidney disorders, asthma, arteriosclerosis, heart disease and lung disease (3-5, 26). But, the present study was the first to report the potential of the components (alginate and Fucoidan) of Sargassum sp. as COX-inhibiting compounds. Results of our study showed that alginate and Fucoidan were potential inhibitory compounds, either to COX-1 or COX-2, in which alginate was a more potent inhibitor of COX-2 than aspirin. COX-2 is expressed in normal endothelial cells in response to shear stress, in which inhibition of COX-2 is significantly associated with suppression of the synthesis of prostacyclin (27, 28).
Both COX-1 and COX-2 have been detected in atherosclerotic lesions in humans (29); however, the specific effects of COX inhibition in the progression of the lesion remain a matter of controversy. Administration of aspirin in low doses and COX-2 inhibitors has been known to improve or otherwise worsen endothelial dysfunction, hypercholesterolemia and hypertension (30, 31). COX-2 has been implicated in plaque destabilization via its increased expression and co-localization with microsomal PGE synthase-1 and metalloproteinase-2 (MMP-2) and MMP-9 (32).
Aspirin is among the COX-inhibiting compounds acting by acetylating the COX binding site, thus preventing the formation of prostaglandins. Aspirin bonding to COX-1 can inhibit the production of prostaglandins that are responsible for the formation of platelets, preventing blood from clotting. Its bonding to COX-2 has been known to reduce the inflammatory response. The present study found that Fucoidan and alginate were highly potential as COX-2 inhibitors; thus, consumption of Sargassum sp. is thought to provide an aspirin-like effect.
The 3-dimensional structure of COX-1 consists of 3 independent folding units, namely an epidermal growth factor-like domain, a membrane-bound motif and an enzymatic domain. The activity sites of peroxidase and COX is adjacent, but spatially different. The conformation of the membrane-bound motif strongly suggested the enzyme was integrated to only a layer of the lipid bilayer, thus belonging to the monotopic membrane proteins. The S(-) stereoisomer of flurbiprofen interacts by means of its carboxylic group with ARG120, thus putting the second phenyl ring in the Van der Waal’s interaction of TYR385 (25). It was thought that there were other sub-sites for drug compounds to bind to the slanting channel. Results of our study indicated that alginate interacted with COX-1 at residues GLU545, SER128, GLN374, LYS534 and ILE126, while Fucoidan interacted via LYS534 and PHE373.
5. CONCLUSION
The active compounds of Sargassum sp., including Fucoidan and alginate, had good potential as inhibitors of COX-1 and COX-2.
Footnotes
• Conflict of interest: none declared
REFERENCES
- 1.Gupta S, Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011;22:315–26. [Google Scholar]
- 2.Pereira L. In: A review of the nutrient composition of selected edible seaweeds ecology in seaweed:nutrient composition and medicinal uses. Pomin V.H, editor. Now York, NY, USA: Nova Science Publishers Inc; 2011. pp. 15–47. [Google Scholar]
- 3.Besada V, Andrade JM, Schultze F, González JJ. Heavy metals in edible seaweeds commercialized for human consumption. J Mar Syst. 2009;75:303–15. [Google Scholar]
- 4.Cruz-Suárez LE, León A, Peña-Rodríguez A, Rodríguez-Peña G, Moll B, Ricque-Marie D. Shrimp/Ulva co-culture:A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture. 2010;301:64–8. [Google Scholar]
- 5.Lee JB, Takeshita A, Hayashi K, Hayashi T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011;86:995–9. [Google Scholar]
- 6.Bhaskar N, Miyashita K. Lipid composition of Padina tetratomatica (Dictyotales, Pheophyta), a brown seaweed of the west coast of India. Indian J Fish. 2005;52:263–8. [Google Scholar]
- 7.Zhang CY, Wu WH, Wang J, Lan MB. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.) and its effects on calcium oxalate crystallization. Mar Drugs. 2012;10:119–30. doi: 10.3390/md10010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira SH. Peripheral analgesic sites of action of antiinflammatory drugs. Int J Clin Pract Suppl. 2002;128:2–10. [PubMed] [Google Scholar]
- 9.Warner TD, Giluliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroidal drug selectivities for cyclooxygenase-1 rather than cyclooxygenase-2 are associated with human gastrointestinal toxicity:a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–8. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JA, Warner TD. Cyclooxygenase-2:pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol. 1999;128:1121–32. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tegeder I, Neupert W, Guhring H, Geisslinger G. Effects of selective and unselective cyclooxygenase inhibitors on prostanoid release from various rat organs. J Pharmacol Exp Ther. 2000;292:1161–8. [PubMed] [Google Scholar]
- 12.Siegle I, Klein T, Backman JT, Saal JG, Nusing RM, Fritz P. Expression of cyclooxygenase-2 in human synovial tissue. Arthritis Rheum. 1998;41:122–9. doi: 10.1002/1529-0131(199801)41:1<122::AID-ART15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119:521–35. doi: 10.1053/gast.2000.9561. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi K, Kagawa S, Mimaki H, Aoi M, Kawauchi S. COX and NOS isoforms involved in acid-induced duodenal bicarbonate secretion in rats. Dig Dis Sci. 2002;47:2116–24. doi: 10.1023/a:1019601702559. [DOI] [PubMed] [Google Scholar]
- 15.Celotti F, Laufer S. Anti-inflammatory drugs:new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res. 2001;43:419–36. doi: 10.1006/phrs.2000.0784. [DOI] [PubMed] [Google Scholar]
- 16.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacological Reports. 2007;59:247–58. [PubMed] [Google Scholar]
- 17.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and inflammation:from basic science to clinical applications. Int J Obes. 2003;27:S41–S45. doi: 10.1038/sj.ijo.0802499. [DOI] [PubMed] [Google Scholar]
- 18.Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–94. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 19.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID induced gastric damage in rats:requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–14. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 20.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace:a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 21.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:387–92. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Boyle N, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel:An open chemical toolbox. Journal of Cheminformatics. 2011;3:33. doi: 10.1186/1758-2946-3-33. doi:10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macindoe G, Mavridis L, Venkatraman V, Devignes MD, Ritchie DW. HexServer:an FFT-based protein docking server powered by graphics processors. Nucleic Acids Res. 2010;38:445–9. doi: 10.1093/nar/gkq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laskowski RA, Swindells MB. LigPlot+:multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;24(51):2778–86. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 25.Botting RM. Inhibitors of cyclooxygenases:mechanisms, selectivity and uses. Journal of Physiology and Pharmacology. 2006;5:113–24. [PubMed] [Google Scholar]
- 26.Peng Y, Xie E, Fredimoses M, Yang X, Zhou X, Wang Y, Yang B, Liu X, Liu J, Liu Y. Nutritional and Chemical Composition and Antiviral Activity of Cultivated Seaweed Sargassum naozhouense Tseng et Lu. Mar Drugs. 2013;11:20–32. doi: 10.3390/md11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimbrone MA, Jr, Topper JN, Nagel T, et al. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 28.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 29.Belton O, Byrne D, Kearney D, et al. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 2000;102:840–5. doi: 10.1161/01.cir.102.8.840. [DOI] [PubMed] [Google Scholar]
- 30.Widlansky ME, Price DT, Gokce N, et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension. 2003;42:310–5. doi: 10.1161/01.HYP.0000084603.93510.28. [DOI] [PubMed] [Google Scholar]
- 31.Bulut D, Liaghat S, Hanefeld C, et al. Selective cyclo-oxygenase-2 inhibition with parecoxib acutely impairs endothelium-dependent vasodilatation in patients with essential hypertension. J Hypertens. 2003;21:1663–7. doi: 10.1097/00004872-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Cipollone F, Prontera C, Pini B, et al. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation. 2001;104:921–7. doi: 10.1161/hc3401.093152. [DOI] [PubMed] [Google Scholar]


