Abstract
Background
The efficacy and safety of once-daily tiotropium + olodaterol (T+O) maintenance treatment was demonstrated in the large, multinational, replicate, randomized, Phase III, Tonado® 1 (NCT01431274) and 2 (NCT01431287) studies in patients with moderate to very severe COPD. However, there may be racial differences in the effects of T+O on lung function in patients with COPD.
Methods
In this Tonado® subgroup analysis, we assessed efficacy and safety of T+O in Japanese participants.
Results
Versus the overall population, the 413 Japanese patients randomized and treated were slightly older, with more men, lower body mass index, lower baseline St George’s Respiratory Questionnaire (SGRQ) scores, fewer current smokers, but with higher pack-year smoking history. A lower proportion of Japanese patients used inhaled corticosteroids, short-acting muscarinic antagonists, or short- or long-acting β-adrenergic agonists at baseline, but use of long-acting muscarinic antagonists was higher. At Week 24, mean improvements with T+O 5/5 μg in forced expiratory volume in 1 second area under the curve from 0–3 hours response were 151 mL versus olodaterol and 134 mL versus tiotropium 5 μg; mean improvements with T+O 2.5/5 μg were 87 mL versus olodaterol and 70 mL versus tiotropium 2.5 μg. Mean improvements with T+O 5/5 μg in trough forced expiratory volume in 1 second were 131 mL versus olodaterol and 108 mL versus tiotropium 5 μg; mean improvements with T+O 2.5/5 μg were 60 mL versus olodaterol and 47 mL versus tiotropium 2.5 μg. SGRQ scores improved from baseline to a greater extent with both doses of T+O versus monotherapies. Responses were similar in the overall population. Adverse-event incidence was generally balanced across treatment groups.
Conclusion
Consistent with results from the overall population, T+O 5/5 μg was superior to each monotherapy for lung function and SGRQ in the Japanese sub-population of patients with COPD in Tonado®.
Keywords: COPD, bronchodilators, maintenance treatment
Introduction
The incidence of COPD is predicted to increase in Japan and the People’s Republic of China over the next 30 years due to factors such as greater tobacco use and an ageing population.1,2 Furthermore, an epidemiology study of airflow limitation in Japanese adults aged ≥40 years showed that COPD is under-recognized, with 10.9% of those assessed having airflow limitation, yet only 9.4% had received a previous diagnosis of COPD.2 In maintenance treatment of COPD, the combination of a long-acting muscarinic antagonist (LAMA) with a long-acting β2-agonist (LABA) is recommended for patients who have persistent symptoms with single-agent therapy.3,4 The LAMA tiotropium is well established as an effective maintenance treatment for COPD, with improvements in lung function, health-related quality of life, and dyspnea, reduced hospitalizations compared to placebo or ipratropium, and decreased risk of exacerbations compared to salmeterol or ipratropium.5–7 The LABA olodaterol has a different mode of action to tiotropium while exhibiting preferable pharmacokinetic and pharmacodynamic profiles,8 and has been shown to be effective when administered once daily in combination with tiotropium in patients with COPD.9–11 In Japan, tiotropium has been a well-established first-line treatment in COPD with documented efficacy and safety profiles, while the efficacy and safety of olodaterol monotherapy have been assessed over 4 weeks in Japanese patients with COPD.12
Combined tiotropium + olodaterol has been shown to be effective, with an acceptable safety profile when administered once daily for 6 weeks in the VIVACITO® study,9 and for 52 weeks in Tonado® 1 and 2, two large, replicate, randomized, double-blind, Phase III studies, in patients with moderate to very severe COPD.13 A considerable number of Japanese patients with COPD participated in these large Phase III studies and there are known to be physiologic differences between Japanese and overall (global) populations. These differences include lower average body weight and alternative background medication preferences, ie, a higher frequency of LAMA use and a lower frequency of inhaled corticosteroid (ICS) use. These differences may originate from the treatment guidelines issued in 2004 and 2009 by the Japanese Respiratory Society in which a LAMA is recommended as first-line treatment for COPD and a LABA/ICS combination is recommended for patients with severe disease (Global initiative for chronic Obstructive Lung Disease [GOLD] 4). Consequently, there is a question as to whether the combination of tiotropium + olodaterol has similar efficacy and safety in the Japanese population.
In this sub-analysis of the Japanese patients included in the Tonado® studies, we assessed whether there were any differences in the efficacy and safety of tiotropium + olodaterol between Japanese and overall populations.
Methods
Study design
We present the results from the Japanese patients included in the combined data set from the multinational, replicate, Phase III, multicenter, randomized, double-blind, active-controlled, five-arm, parallel-group studies Tonado® 1 and 2 (NCT01431274 and NCT01431287). The study design has been described previously;13 briefly, patients were randomized to receive once-daily olodaterol 5 μg, tiotropium 2.5 μg, tiotropium 5 μg, tiotropium + olodaterol 2.5/5 μg, or tiotropium + olodaterol 5/5 μg for 52 weeks (Figure 1). Primary end points in the Tonado® studies were assessed at Week 24 of treatment; safety was assessed throughout the study. Ethical approval for this study was obtained from the respective institutions (Supplementary materials). Written, informed consent was obtained from all patients.
Figure 1.
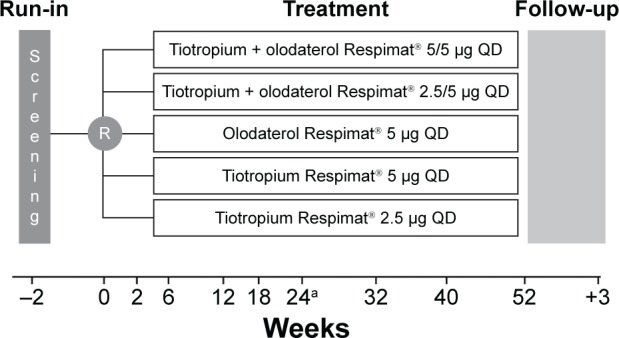
Tonado® study design.
Notes: aPrimary end point assessment. Reproduced with permission of the content. Reproduced with permission of the European Respiratory Society ©: European Respiratory Journal. 2015;45(4):969–979; DOI: 10.1183/09031936.00136014.13
Abbreviations: R, randomization; QD, once daily.
Patients
Patients were randomized if they met the following main inclusion criteria: outpatients aged ≥40 years with a history of moderate to very severe COPD (GOLD 2–4); post-bronchodilator forced expiratory volume in 1 second (FEV1) <80% of predicted normal; post-bronchodilator FEV1/forced vital capacity (FVC) <70%; current or ex-smokers with a smoking history of >10 pack-years. Exclusion criteria included significant disease other than COPD, clinically relevant abnormal baseline laboratory parameters, or a history of asthma.
End points and assessments
In the Tonado® studies, three primary end points were evaluated at Week 24: change from baseline in FEV1 area under the curve from 0–3 hours (AUC0–3) divided in hours; trough FEV1 (mean of the values measured at 23 hours and 23 hours and 50 minutes following drug administration at the clinic visit of the previous day for the week 24 visit); and St George’s Respiratory Questionnaire (SGRQ) total score. Secondary end points were Mahler Transition Dyspnea Index (TDI) at Week 24 (key secondary end point) and change from baseline in trough FVC. Further end points included peak FEV1 from 0–3 hours and peak FVC from 0–3 hours; FEV1 and FVC at 5, 15, and 30 minutes and 1, 2, and 3 hours following inhalation, peak expiratory flow rate, rescue medication use, and Patients’ Global Rating assessments.13 Safety was assessed via adverse events (AEs), recorded throughout the study. Vital signs, blood chemistry, and electrocardiogram data were also collected.
Statistical analysis
This subgroup analysis for the Japanese sub-population from the Tonado® studies was performed using the same statistical analyses for the same key end points at the same time points as for the overall population, ie, analysis of the primary end points at Week 24 was performed for all randomized patients with non-missing baseline and at least one post-baseline measurement at or before Week 24 for any primary efficacy end point and who received at least one dose of study medication (full analysis set). Tiotropium + olodaterol therapy was compared to the component monotherapies at 5% level of significance in which the mean changes from baseline in FEV1 AUC0–3 and trough FEV1 and the mean SGRQ total score and TDI focal score were analyzed using a mixed-effects model for repeated measurements approach including the fixed, categorical effects of treatment, planned test day, and treatment-by-test-day interaction, as well as the continuous, fixed covariates of baseline and baseline-by-test-day interaction. A spatial power covariance structure was used to model within-patient errors. Kenward–Roger approximation was used to estimate denominator degrees of freedom.
Analyses for the Japanese patient sub-population are descriptive. All P-values from treatment comparisons are nominal (ie, not alpha protected), as the studies were not powered to show significant differences. All analyses were performed using pooled data from Tonado® 1 and 2.
Safety end points
All treated patients were included in the safety analysis, which was descriptive only. AEs were included if they occurred in the period between the first dose of study medication and ≤21 days after the last study medication administration. AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 16.1 and unblinded data were reviewed regularly by an independent Data Monitoring Committee.
Results
Patient population
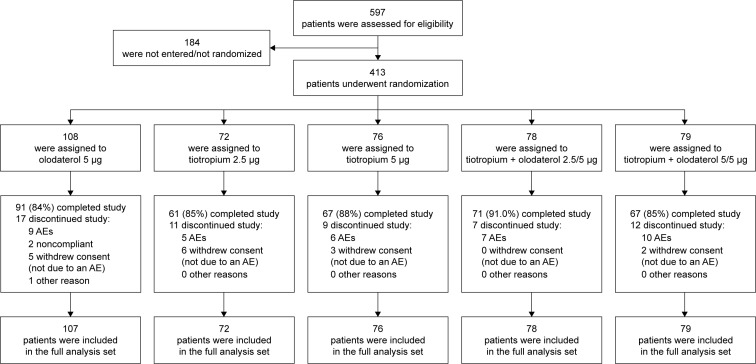
In total, 413 Japanese patients were randomized and treated in the Tonado® studies (Figure 2). Lung-function characteristics were similar at baseline for the Japanese sub-population compared to the overall population (Table 1). The mean age of Japanese study participants was slightly higher (69 versus 64 years), with a greater proportion of men (94% versus 73%), and a lower body mass index score (22.4 versus 25.9) compared to the overall population. Fewer Japanese patients were current smokers (18% versus 37% of the overall population) and duration from diagnosis was 5 years compared to 7 years for the overall population. However, there was a slightly higher smoking history compared to the overall population (64 versus 46 pack-years). In total, 50% of the overall study population were GOLD 2 compared to 60% of the Japanese population. Furthermore, the proportion of patients using ICS and short-acting muscarinic antagonists, short-acting β-agonists, and LABAs was lower in the Japanese population compared to the overall population, while the proportion of patients using xanthines and LAMAs was higher (Table 1). Common baseline SGRQ scores were 33.7 for the Japanese population and 43.5 for the overall population. Generally, demographics were similar for Japanese patients across the treatment groups (Table S1).
Figure 2.
Patient disposition of the Japanese sub-population in Tonado®.
Abbreviation: AE, adverse event.
Table 1.
Demographic and baseline patient characteristics (treated population, combined data set)
| Characteristics | Japanese population (combined studies) (n=413) |
Overall population (combined studies) (n=5,162) |
|---|---|---|
| Male, n (%) | 386 (93.5) | 3,762 (72.9) |
| Mean (SD) age, years | 69.1 (7.0) | 64.0 (8.3) |
| Mean (SD) body mass index, kg/m2 | 22.4 (3.6) | 25.9 (5.5) |
| Smoking status, n (%) | ||
| Ex-smoker | 339 (82.1) | 3,254 (63.0) |
| Current smoker | 74 (17.9) | 1,908 (37.0) |
| Mean (SD) smoking history, pack-years | 63.8 (33.3) | 46.2 (25.5) |
| Mean (SD) duration of diagnosis, years | 4.7 (4.2) | 6.5 (5.9) |
| Comorbidities, n (%) | 381 (92.3) | 4,462 (86.4) |
| Cardiac | 65 (15.7) | 1,107 (21.4) |
| Vascular | 192 (46.5) | 2,481 (48.1) |
| Mean (SD) pre-bronchodilator screening | ||
| FEV1, L | 1.170 (0.454) | 1.203 (0.493) |
| Mean (SD) post-bronchodilator screening | ||
| FEV1, L | 1.334 (0.452) | 1.374 (0.511) |
| Change from pre- to post-bronchodilator | 164 (126) | 171 (145) |
| FEV1, mL | ||
| FEV1/FVC, % | 43.0 (11.3) | 45.0 (11.7) |
| % of predicted normal FEV1 | 53.2 (14.9) | 50.0 (15.3) |
| GOLD, n (%)a | ||
| 2 (50–<80) | 247 (59.8) | 2,588 (50.1) |
| 3 (30–<50) | 139 (33.7) | 1,989 (38.5) |
| 4 (<30) | 27 (6.5) | 581 (11.3) |
| Baseline pulmonary medication, n (%) | 342 (82.8) | 4,107 (79.6) |
| SAMAb | 8 (1.9) | 665 (12.9) |
| LAMAc | 267 (64.6) | 1,840 (35.6) |
| SABAd | 32 (7.7) | 2,079 (40.3) |
| LABAe | 112 (27.1) | 2,393 (46.4) |
| ICSf | 110 (26.6) | 2,446 (47.4) |
| Xanthineg | 57 (13.8) | 516 (10.0) |
| Mucolytics | 91 (22.0) | 752 (14.6) |
Notes:
Based on post-bronchodilator FEV1 % predicted;
ipratropium, ipratropium/fenoterol, ipratropium/salbutamol, oxitropium;
tiotropium;
including fenoterol, salbutamol, ipratropium/fenoterol, ipratropium/salbutamol, terbutaline;
including formoterol, salmeterol, indacaterol, formoterol/beclomethasone, formoterol/budesonide, salmeterol/fluticasone;
luticasone, mometasone, formoterol/beclomethasone, formoterol/budesonide, salmeterol/fluticasone;
including theophylline, aminophylline.
Abbreviations: SD, standard deviation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; SAMA, short-acting muscarinic antagonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β-agonist; LABA, long-acting β2-agonist; ICS, inhaled corticosteroid.
Efficacy
Lung function
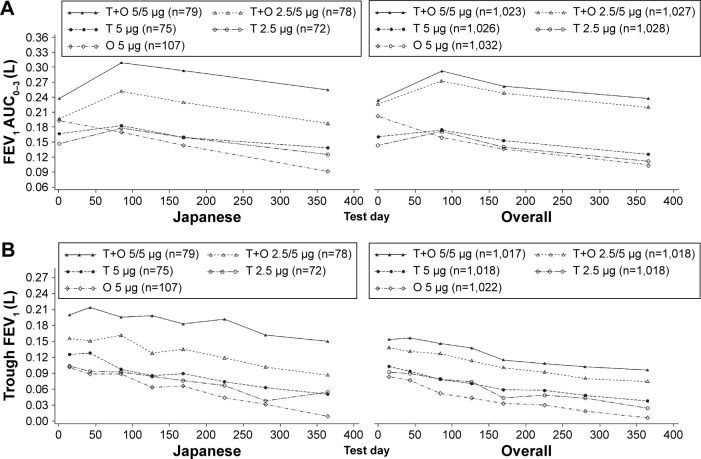
Improvements in lung function following treatment with tiotropium + olodaterol were slightly better in Japanese patients compared to the overall population. Analysis of the combined data from the two studies revealed improvements in the change from baseline in FEV1 AUC0–3 with both doses of tiotropium + olodaterol compared to monotherapies in Japanese patients. Improvements in mean change from baseline (95% confidence interval [CI]) in FEV1 AUC0–3 at Week 24 of treatment with tiotropium + olodaterol 5/5 μg were 151 mL (104, 198) greater than olodaterol 5 μg and 134 mL (83, 185) greater than tiotropium 5 μg (nominal P<0.0001 versus either monotherapy), while tiotropium + olodaterol 2.5/5 μg improved values by 87 mL (39, 134) more than olodaterol 5 μg and by 70 mL (18, 122) more than tiotropium 2.5 μg (nominal P<0.01) (Figure 3A and Tables S2, S3, and Figure S1A). These results were similar to those seen in the overall population; improvements in mean (95% CI) FEV1 AUC0–3 at Week 24 of treatment with tiotropium + olodaterol 5/5 μg were 128 mL (111, 144) greater than olodaterol 5 μg and 110 mL (93, 127) greater than tiotropium 5 μg. For tiotropium + olodaterol 2.5/5 μg improvements were 115 mL (98, 131) greater than olodaterol 5 μg and 111 mL (95, 128) greater than tiotropium 2.5 μg (P<0.0001 for all comparisons) (Figures 3A and S1A).
Figure 3.
Change from baseline in FEV1 AUC0–3 (A) and trough FEV1 (B) at Week 24 for the Japanese and overall populations.
Notes: Common baseline means (standard error) for the Japanese and overall populations, respectively, L: FEV1 AUC0–3, 1.120 (0.023) and 1.154 (0.007); trough FEV1, 1.120 (0.023) and 1.155 (0.007). Reproduced with permission of the European Respiratory Society ©: European Respiratory Journal. 2015;45(4):969–979; DOI: 10.1183/09031936.00136014.13
Abbreviations: FEV1, forced expiratory volume in 1 second; AUC0–3, area under the curve from 0–3 hours; T, tiotropium; O, olodaterol.
Change from baseline in mean (95% CI) trough FEV1 at Week 24 improved to a greater extent with tiotropium + olodaterol compared to the individual components in Japanese patients. Improvements with tiotropium + olodaterol 5/5 μg were 131 mL (85, 176) greater than olodaterol 5 μg and 108 mL (59, 156) greater than tiotropium 5 μg (nominal P<0.0001 versus either monotherapy). For tiotropium + olodaterol 2.5/5 μg increases were 60 mL (14, 105) greater than olodaterol 5 μg (nominal P<0.01) and 47 mL (−3, 97) greater than tiotropium 2.5 μg (nominal P-value not significant) (Figure 3B, Tables S2, S3, and Figure S1B). This is similar to the overall population: with tiotropium + olodaterol 5/5 μg improvements were 85 mL (67, 102) greater than olodaterol 5 μg and 60 mL (43, 77) greater than tiotropium 5 μg (P<0.0001 versus either monotherapy). For tiotropium + olodaterol 2.5/5 μg, improvements were 62 mL (45, 80) greater than olodaterol 5 μg and 45 mL (28, 62) greater than tiotropium 2.5 μg (P<0.0001 versus either monotherapy) (Figures 3B and S1B).
Further lung-function end points are shown up to Week 52 in Figure S2 and Tables S4 and S5.
Symptomatic benefit
SGRQ total scores improved in Japanese patients at Week 24 of treatment in all groups, with the largest reduction in symptom scores seen in the group receiving tiotropium + olodaterol 5/5 μg. Improvements in total SGRQ scores were greater in the Japanese population compared to the overall population, although Japanese patients had a much lower common baseline SGRQ score. The differences in total SGRQ scores between tiotropium + olodaterol 2.5/5 μg and each component were not statistically significant for the overall population or the Japanese sub-population (Table 2). The largest improvements for tiotropium + olodaterol compared to each component were in the symptom domain of the SGRQ (Figure S3).
Table 2.
SGRQ total score and responders at Week 24 in the Japanese and overall populations (full analysis set, combined studies)
| Treatment | Japanese population (combined studies) (n=413) |
Overall population (combined studies) (n=4,837) |
||||
|---|---|---|---|---|---|---|
| Adjusted mean (SE) | Change from baseline | Responders, %a | Adjusted mean (SE) | Change from baseline | Responders, %a | |
| Common baseline | 33.71 (0.79) | 43.51 (0.26) | ||||
| Olodaterol 5 μg | 30.12 (0.99) | −3.59 | 36.9 | 38.37 (0.40) | −5.09 | 44.8 |
| Tiotropium 2.5 μg | 31.10 (1.18) | −2.62 | 35.3 | 37.79 (0.39) | −5.67 | 49.6 |
| Tiotropium 5 μg | 30.40 (1.08) | −3.32 | 40.5 | 37.91 (0.39) | −5.55 | 48.7 |
| Tiotropium + olodaterol 2.5/5 μg | 28.15 (1.11) | −5.56 | 55.3 | 37.34 (0.39) | −6.12 | 53.2 |
| Tiotropium + olodaterol 5/5 μg | 26.79 (1.16) | −6.92 | 57.9 | 36.67 (0.39) | −6.79 | 57.5 |
| Japanese population (combined studies)
|
Overall population (combined studies)
|
|||
|---|---|---|---|---|
| Adjusted mean (SE) treatment differenceb | 95% CI | Adjusted mean (SE) treatment difference | 95% CI | |
| Tiotropium + olodaterol 5/5 μg | ||||
| vs olodaterol 5 μg | −3.33 (1.53)* | −6.33, −0.33 | −1.69 (0.55)** | −2.78, −0.61 |
| vs tiotropium 5 μg | −3.60 (1.58)* | −6.71, −0.50 | −1.23 (0.55)* | −2.31, −0.15 |
| Tiotropium + olodaterol 2.5/5 μg | ||||
| vs olodaterol 5 μg | −1.97 (1.49) | −4.89, 0.95 | −1.03 (0.55) | −2.11, 0.05 |
| vs tiotropium 2.5 μg | −2.95 (1.62) | −6.13, 0.24 | −0.46 (0.55) | −1.53, 0.62 |
Notes: Adjusted mean (SE) obtained from fitting a mixed-effects model for repeated measurements including fixed effects of treatment, planned test day, treatment-by-test-day interaction, baseline, and baseline-by-test-day interaction; patient as a random effect; spatial power covariance structure for within-patient errors and Kenward–Roger approximation of denominator degrees of freedom.
Defined as having an SGRQ total score at Week 24 improved by ≥4.0 units over baseline SGRQ total score; responder analysis results are from fitting a logistic regression model with treatment as covariate and a logit link function. Number of patients contributing to SGRQ analysis in the Japanese population: olodaterol 5 μg n=103, tiotropium 2.5 μg n=68, tiotropium 5 μg n=74, tiotropium + olodaterol 2.5/5 μg n=76, tiotropium + olodaterol 5/5 μg n=76. Number of patients contributing to the SGRQ analysis in the overall population: olodaterol 5 μg n=954, tiotropium 2.5 μg n=960, tiotropium 5 μg n=954 (n=955 for the responder analysis), tiotropium + olodaterol 2.5/5 μg n=990, tiotropium + olodaterol 5/5 μg n=979;
nominal P-value.
P<0.05;
P<0.01.
Abbreviations: SGRQ, St George’s Respiratory Questionnaire; SE, standard error; CI, confidence interval.
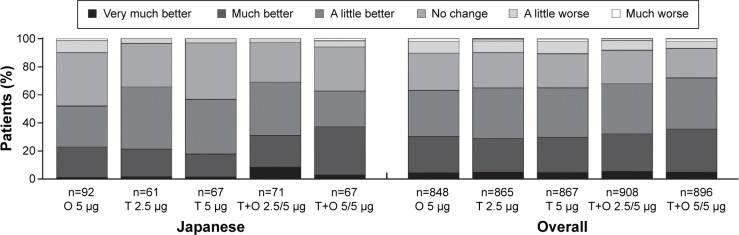
Baseline Dyspnea Index score was slightly higher (ie, lower symptoms) in Japanese patients compared to the overall population (7.75 versus 6.54, respectively). Mahler TDI focal score improved to >1 for both doses of tiotropium + olodaterol in Japanese patients compared to scores of 0.40–0.85 for the individual components. Tiotropium + olodaterol 5/5 μg improved Mahler TDI focal score by >0.7 units compared to the monotherapies (nominal P<0.05 for both comparisons) and tiotropium + olodaterol 2.5/5 μg by >0.8 units compared to olodaterol 5 μg and tiotropium 2.5 μg (nominal P<0.01 for both comparisons) at Week 24 (Table 3). Improvements with nominal P<0.05 were also seen in Patients’ Global Rating at Week 24 for both doses of tiotropium + olodaterol compared to individual components. The majority of patients rated their symptoms as improved up to Week 52 (Figure 4 and Tables S6 and S7).
Table 3.
Mahler BDI/TDI focal scores at Week 24 for Japanese and overall populations (full analysis set, combined studies)
| Treatment | Japanese population
|
Overall population
|
|---|---|---|
| Adjusted mean Mahler (SE)a | Adjusted mean Mahler (SE)b | |
| Combined studies, BDI (common baseline) | 7.75 (0.10) | 6.54 (0.03) |
| TDI | ||
| Olodaterol 5 μg | 0.40 (0.20) | 1.56 (0.10) |
| Tiotropium 2.5 μg | 0.47 (0.24) | 1.69 (0.10) |
| Tiotropium 5 μg | 0.85 (0.23) | 1.63 (0.10) |
| Tiotropium + olodaterol 2.5/5 μg | 1.37 (0.23) | 1.98 (0.10) |
| Tiotropium + olodaterol 5/5 μg | 1.56 (0.23) | 1.98 (0.10) |
| Treatment differences Mahler TDI | Adjusted mean (SE) | 95% CI | Adjusted mean (SE) | 95% CI |
|---|---|---|---|---|
| Tiotropium + olodaterol 5/5 μg | ||||
| vs olodaterol 5 μg | 1.16 (0.31) | 0.56, 1.75 | 0.42 (0.14) | 0.16, 0.68 |
| vs tiotropium 5 μg | 0.71 (0.33) | 0.07, 1.35 | 0.36 (0.14) | 0.09, 0.62 |
| Tiotropium + olodaterol 2.5/5 μg | ||||
| vs olodaterol 5 μg | 0.97 (0.30) | 0.37, 1.56 | 0.42 (0.14) | 0.15, 0.68 |
| vs tiotropium 2.5 μg | 0.89 (0.33) | 0.24, 1.55 | 0.29 (0.13) | 0.03, 0.55 |
Notes: Adjusted mean (SE) obtained from fitting a mixed-effects model for repeated measurements including fixed effects of treatment, planned test day, treatment-by-test-day interaction, baseline, and baseline-by-test-day interaction; patient as a random effect; spatial power covariance structure for within-patient errors and Kenward–Roger approximation of denominator degrees of freedom.
Number of patients contributing to the mixed-effects model for repeated measurements for adjusted mean TDI across both studies in Japanese patients: tiotropium + olodaterol 5/5 μg n=77; tiotropium + olodaterol 2.5/5 μg n=76; tiotropium 5 μg n=74; tiotropium 2.5 μg n=70; olodaterol 5 μg n=104;
number of patients contributing to the mixed-effects model for repeated measurements for TDI score in the overall population: tiotropium + olodaterol 5/5 μg n=992; tiotropium + olodaterol 2.5/5 μg n=992; tiotropium 5 μg n=978; tiotropium 2.5 μg n=982; olodaterol 5 μg n=984.
Abbreviations: BDI, Baseline Dyspnea Index; TDI, Transition Dyspnea Index; SE, standard error; CI, confidence interval.
Figure 4.
Patients’ Global Rating after 52 weeks of treatment in the Japanese sub-population (full analysis set, combined data).
Abbreviations: T, tiotropium; O, olodaterol.
Adjusted weekly mean daily (24-hour) rescue medication use (puffs/day) was substantially reduced with both tiotropium + olodaterol 2.5/5 and 5/5 μg over the 52-week period of the study. Reductions in rescue medication use were observed for tiotropium + olodaterol 5/5 μg compared to both tiotropium 5 μg and olodaterol 5 μg, in particular in the second half of the study (Figure 5). A similar effect was observed in the use of rescue medication during the day and at night (Figures S4 and S5).
Figure 5.
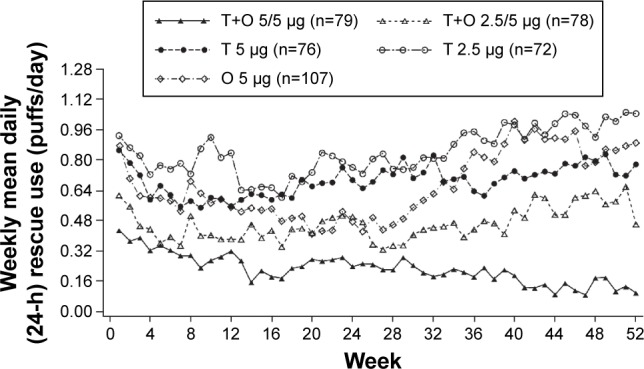
Twenty-four-hour rescue medication use for the Japanese sub-population of the combined Tonado® studies.
Note: Common baseline mean (standard error), puffs/day: 1.08 (0.109).
Abbreviations: T, tiotropium; O, olodaterol; h, hour.
Safety
Overall, 82.1% of patients reported an AE. Of these, severe AEs (defined as incapacitating or causing inability to work or perform usual activities) were reported in 7.3% of Japanese patients and drug-related AEs (as assessed by the investigator) were reported in 8.5%. Incidences of AEs, serious AEs, and drug-related AEs were generally well-balanced across treatment groups and were mostly mild or moderate in intensity. Only two on-treatment deaths occurred.
Similarly to the overall population, the most frequently reported AEs in Japanese patients were from the MedDRA System Organ Classes of “respiratory, thoracic, and mediastinal disorders” and “infections and infestations”, with COPD (including COPD exacerbation/worsening) and nasopharyngitis being the most commonly reported. The incidence of these most common events was comparable across treatment groups (Table 4).
Table 4.
Summary of AEs: combined analysis of the Japanese population (treated set, combined studies)
| Olodaterol 5 μg, n (%) | Tiotropium 2.5 μg, n (%) | Tiotropium 5 μg, n (%) | Tiotropium + olodaterol 2.5/5 μg, n (%) | Tiotropium + olodaterol 5/5 μg, n (%) | Total, n (%) | |
|---|---|---|---|---|---|---|
| Patients | 108 (100.0) | 72 (100.0) | 76 (100.0) | 78 (100.0) | 79 (100.0) | 413 (100.0) |
| All AEs | 91 (84.3) | 55 (76.4) | 63 (82.9) | 66 (84.6) | 64 (81.0) | 339 (82.1) |
| Severe AEs | 8 (7.4) | 3 (4.2) | 4 (5.3) | 4 (5.1) | 11 (13.9) | 30 (7.3) |
| Treatment-related AEs | 10 (9.3) | 5 (6.9) | 4 (5.3) | 7 (9.0) | 9 (11.4) | 35 (8.5) |
| AEs leading to discontinuation | 9 (8.3) | 5 (6.9) | 6 (7.9) | 7 (9.0) | 10 (12.7) | 37 (9.0) |
| Serious AEs | 16 (14.8) | 7 (9.7) | 14 (18.4) | 18 (23.1) | 15 (19.0) | 70 (16.9) |
| Fatal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.5) | 2 (0.5) |
| Life-threatening | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Disabling/incapacitating | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Requiring hospitalization | 16 (14.8) | 7 (9.7) | 13 (17.1) | 15 (19.2) | 11 (13.9) | 62 (15.0) |
| Prolonging hospitalization | 1 (0.9) | 1 (1.4) | 0 (0.0) | 1 (1.3) | 1 (1.3) | 4 (1.0) |
| Other | 0 (0.0) | 1 (1.4) | 1 (1.3) | 6 (7.7) | 1 (1.3) | 9 (2.2) |
| Specific AEs with an incidence of SOC | ||||||
| PT >3% in any one group (SOC/PT) | ||||||
| Respiratory, thoracic, and mediastinal disorders | 38 (35.2) | 24 (33.3) | 25 (32.9) | 22 (28.2) | 31 (39.2) | 140 (33.9) |
| COPD (including COPD exacerbation/worsening) | 21 (19.4) | 14 (19.4) | 13 (17.1) | 9 (11.5) | 19 (24.1) | 76 (18.4) |
| Upper respiratory tract inflammation | 7 (6.5) | 3 (4.2) | 1 (1.3) | 4 (5.1) | 3 (3.8) | 18 (4.4) |
| Cough | 3 (2.8) | 1 (1.4) | 0 (0.0) | 3 (3.8) | 1 (1.3) | 8 (1.9) |
| Oropharyngeal pain | 1 (0.9) | 2 (2.8) | 3 (3.9) | 2 (2.6) | 1 (1.3) | 9 (2.2) |
| Infections and infestations | 61 (56.5) | 38 (52.8) | 36 (47.4) | 33 (42.3) | 42 (53.2) | 210 (50.8) |
| Nasopharyngitis | 28 (25.9) | 23 (31.9) | 15 (19.7) | 20 (25.6) | 24 (30.4) | 110 (26.6) |
| Bronchitis | 9 (8.3) | 1 (1.4) | 9 (11.8) | 5 (6.4) | 12 (15.2) | 36 (8.7) |
| Pneumonia | 9 (8.3) | 2 (2.8) | 2 (2.6) | 4 (5.1) | 4 (5.1) | 21 (5.1) |
| Influenza | 3 (2.8) | 5 (6.9) | 3 (3.9) | 2 (2.6) | 1 (1.3) | 14 (3.4) |
| Upper respiratory tract infection | 6 (5.6) | 2 (2.8) | 3 (3.9) | 0 (0.0) | 3 (3.8) | 14 (3.4) |
| Pharyngitis | 1 (0.9) | 1 (1.4) | 4 (5.3) | 1 (1.3) | 2 (2.5) | 9 (2.2) |
| Gastroenteritis | 2 (1.9) | 3 (4.2) | 2 (2.6) | 3 (3.8) | 1 (1.3) | 11 (2.7) |
| Herpes zoster | 1 (0.9) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 3 (3.8) | 5 (1.2) |
| Gastrointestinal disorders | 23 (21.3) | 14 (19.4) | 20 (26.3) | 20 (25.6) | 13 (16.5) | 90 (21.8) |
| Constipation | 7 (6.5) | 2 (2.8) | 4 (5.3) | 7 (9.0) | 1 (1.3) | 21 (5.1) |
| Diarrhea | 6 (5.6) | 2 (2.8) | 3 (3.9) | 1 (1.3) | 3 (3.8) | 15 (3.6) |
| Gastro-esophageal reflux | 1 (0.9) | 3 (4.2) | 3 (3.9) | 1 (1.3) | 1 (1.3) | 9 (2.2) |
| Abdominal discomfort | 0 (0.0) | 0 (0.0) | 3 (3.9) | 1 (1.3) | 1 (1.3) | 5 (1.2) |
| Vomiting | 0 (0.0) | 0 (0.0) | 3 (3.9) | 1 (1.3) | 1 (1.3) | 5 (1.2) |
| Dry mouth | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (3.8) | 4 (1.0) |
| Musculoskeletal and connective tissue disorders | 15 (13.9) | 7 (9.7) | 8 (10.5) | 13 (16.7) | 8 (10.1) | 51 (12.3) |
| Back pain | 5 (4.6) | 1 (1.4) | 1 (1.3) | 3 (3.8) | 1 (1.3) | 11 (2.7) |
| Muscle spasms | 2 (1.9) | 0 (0.0) | 1 (1.3) | 3 (3.8) | 2 (2.5) | 8 (1.9) |
| Metabolism and nutrition disorders | 8 (7.4) | 8 (11.1) | 6 (7.9) | 4 (5.1) | 6 (7.6) | 32 (7.7) |
| Diabetes mellitus | 1 (0.9) | 3 (4.2) | 1 (1.3) | 2 (2.6) | 1 (1.3) | 8 (1.9) |
| Hyperuricemia | 2 (1.9) | 1 (1.4) | 1 (1.3) | 1 (1.3) | 3 (3.8) | 8 (1.9) |
| Vascular disorders | 7 (6.5) | 4 (5.6) | 3 (3.9) | 4 (5.1) | 5 (6.3) | 23 (5.6) |
| Hypertension | 7 (6.5) | 2 (2.8) | 3 (3.9) | 3 (3.8) | 1 (1.3) | 16 (3.9) |
| Skin and subcutaneous tissue disorders | 12 (11.1) | 8 (11.1) | 10 (13.2) | 12 (15.4) | 10 (12.7) | 52 (12.6) |
| Dermatitis (contact) | 2 (1.9) | 1 (1.4) | 4 (5.3) | 0 (0.0) | 1 (1.3) | 8 (1.9) |
| Eczema | 3 (2.8) | 1 (1.4) | 1 (1.3) | 4 (5.1) | 1 (1.3) | 10 (2.4) |
| Eczema (asteatotic) | 0 (0.0) | 1 (1.4) | 1 (1.3) | 0 (0.0) | 3 (3.8) | 5 (1.2) |
| Pruritus | 2 (1.9) | 0 (0.0) | 2 (2.6) | 3 (3.8) | 0 (0.0) | 7 (1.7) |
| Reproductive system and breast disorders | 4 (3.7) | 3 (4.2) | 1 (1.3) | 3 (3.8) | 3 (3.8) | 14 (3.4) |
| Benign prostatic hyperplasia | 1 (0.9) | 3 (4.2) | 1 (1.3) | 3 (3.8) | 2 (2.5) | 10 (2.4) |
| Psychiatric disorders | 7 (6.5) | 1 (1.4) | 4 (5.3) | 3 (3.8) | 3 (3.8) | 18 (4.4) |
| Insomnia | 6 (5.6) | 1 (1.4) | 3 (3.9) | 2 (2.6) | 3 (3.8) | 15 (3.6) |
| Investigations | 11 (10.2) | 6 (8.3) | 5 (6.6) | 9 (11.5) | 2 (2.5) | 33 (8.0) |
| Blood urine present | 2 (1.9) | 0 (0.0) | 3 (3.9) | 2 (2.6) | 0 (0.0) | 7 (1.7) |
| Injury, poisoning, and procedural complications | 10 (9.3) | 7 (9.7) | 8 (10.5) | 4 (5.1) | 7 (8.9) | 36 (8.7) |
| Ligament sprain | 0 (0.0) | 3 (4.2) | 2 (2.6) | 0 (0.0) | 0 (0.0) | 5 (1.2) |
Abbreviations: AE, adverse event; SOC, System Organ Class; PT, preferred term.
Although AEs occurred at a slightly higher rate in the Japanese population compared to the overall population (82.1% versus 74.4%), there was generally no increase in the incidence of AEs with tiotropium + olodaterol compared to the mono-components in both populations.
Discussion
Although the burden of disease for COPD is worldwide, the efficacy and safety of therapeutic agents may differ between countries due to variable patient characteristics, such as body mass index, as well as differing baseline treatments. Japanese patients with COPD tended to be older with a lower body mass index. In addition, fewer Japanese patients were current smokers, yet with higher pack-year smoking history, and fewer used ICS, short-acting β-agonists, LABAs, and short-acting muscarinic antagonists at baseline, although a higher proportion of Japanese patients were using LAMAs or mucolytics at baseline. Therefore, we assessed the efficacy and safety of tiotropium + olodaterol in a Japanese sub-population.
There were nominally statistically significant and clinically relevant improvements in lung function as measured by the change from baseline in trough FEV1 and FEV1 AUC0–3 with tiotropium + olodaterol compared to either monotherapy, with slightly greater improvements in pulmonary function in Japanese patients following treatment with tiotropium + olodaterol compared to the overall population. One explanation for this could be the greater proportion of patients with slightly milder disease in the Japanese sub-population. Furthermore, improvements were seen in all treatment groups for SGRQ total scores (change from baseline), above the minimum clinically important difference of four units.14 These improvements were nominally statistically significant for tiotropium + olodaterol 5/5 μg compared to individual components, with Japanese patients reporting much less severe SGRQ scores in their stable periods and superior improvements in SGRQ scores following treatment. Similarly, TDI focal score improved with tiotropium + olodaterol to a greater extent than with the individual components.
Although these studies were not designed to assess the impact of tiotropium + olodaterol on COPD exacerbations (data not shown), it was interesting to note that fewer acute exacerbations were reported for Japanese patients compared to the overall population. This may have been due to the inclusion of fewer patients with severe COPD in the Japanese arm of the study and earlier treatment of respiratory symptoms, as well as a lower proportion of current smokers.
There was a slightly higher incidence of AEs including infection in the Japanese sub-population compared to the overall population but a lower incidence of COPD (including COPD exacerbation/worsening). Similar to the overall population and as expected in patients with COPD, the most frequent events occurred in the System Organ Classes of “respiratory, thoracic, and mediastinal disorders” and “infections and infestations”. Overall, incidence of AEs was balanced across treatment groups in Japanese patients and no major safety signals were identified with tiotropium + olodaterol compared to the mono-components. There was no additive adverse effect by combining the two different mechanisms of action. The safety profile of tiotropium + olodaterol in the Japanese population was comparable to that of the overall population, with no additional safety risks.
This sub-analysis broadly mirrored the results observed in the previously reported overall population13 and reflects those of other studies. To date, no studies have demonstrated any differences between ethnic groups in systemic exposure to inhaled medications such as β-agonists.12,15 While there were key differences between Japanese patients and the overall population in this study, such as lower body mass index scores, systemic exposure to olodaterol has been shown to be unaffected by age, sex, weight, or renal, liver, or lung function.12
There were several limitations to these studies. Due to the long duration of 52 weeks, we decided not to include a placebo arm because it was considered unethical to deny treatment to symptomatic patients, as LAMAs are standard maintenance therapy for COPD. Although the number of patients included in the Japanese arm was low (~8% of the overall population), the data generally mirror the results seen in the overall population, indicating consistent efficacy and safety for this population.
Once-daily dosing is more convenient for patients and could increase medication compliance,16 the monitoring of which is considered one of the most important factors by Japanese health care providers when selecting an inhaler device.17 In this study, tiotropium + olodaterol was administered via one device – the Respimat® inhaler – which contains a liquid suspension and could provide physicians, as well as patients, with greater choice in their maintenance treatment options.
Conclusion
The combination of tiotropium + olodaterol 5/5 μg significantly improved lung function and quality of life and provided symptomatic benefit over tiotropium or olodaterol monotherapies in Japanese patients with COPD. This was generally consistent with the overall population, although a slightly improved benefit was observed for Japanese patients. The frequency of AEs was balanced across treatment groups, with no increased incidence with tiotropium + olodaterol compared to monotherapies. The combination of tiotropium + olodaterol was well tolerated in Japanese patients with COPD with a similar safety profile to the overall population.
Acknowledgments
This work was supported by Nippon Boehringer Ingelheim Co. Ltd. We would like to thank Shuhei Nakamura of Nippon Boehringer Ingelheim Co. Ltd for assistance with writing, and also Daisuke Kuroki and Mami Mori of Nippon Boehringer Ingelheim Co. Ltd for publication management and editorial support. Medical writing assistance was provided by Laura George, PhD, on behalf of Complete HealthVizion, and was contracted and compensated by Nippon Boehringer Ingelheim Co. Ltd.
Footnotes
Author contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of, and editorial decisions relating to the manuscript, were involved at all stages of development, and have approved the submitted manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors received no compensation related to the development of the manuscript. MI reports lecture fees from AstraZeneca, GlaxoSmithKline, Nippon Boehringer Ingelheim, and Novartis Pharma. HT reports lecture fees from AstraZeneca, GlaxoSmithKline, KYORIN Pharmaceutical, Meiji Seika Pharma, Nippon Boehringer Ingelheim, and Novartis Pharma. YF reports honoraria for consultancy from Eisai, Otsuka Pharmaceuticals, and Nippon Boehringer Ingelheim. AT, LG, LL, FV, and YZ are all employees of Boehringer Ingelheim. Interim findings from this analysis were presented at the Asian Pacific Society of Respirology Congress in Kuala Lumpur, Malaysia, December 3–6, 2015 as a poster presentation. The authors report no other conflicts of interest in this work.
References
- 1.Teramoto S, Yamamoto H, Yamaguchi Y, Matsuse T, Ouchi Y. Global burden of COPD in Japan and Asia. Lancet. 2003;362(9397):1764–1765. doi: 10.1016/S0140-6736(03)14865-9. [DOI] [PubMed] [Google Scholar]
- 2.Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458–465. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [Accessed January 29, 2016]. Updated 2016. Available from: http://goldcopd.org/
- 4.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 5.Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta-analysis of clinically relevant outcomes. Respir Care. 2011;56(4):477–487. doi: 10.4187/respcare.00852. [DOI] [PubMed] [Google Scholar]
- 6.Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigo GJ, Nannini LJ. Tiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysis. Pulm Pharmacol Ther. 2007;20(5):495–502. doi: 10.1016/j.pupt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bouyssou T, Casarosa P, Naline E, et al. Pharmacological characterization of olodaterol, a novel inhaled β2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models. J Pharmacol Exp Ther. 2010;334(1):53–62. doi: 10.1124/jpet.110.167007. [DOI] [PubMed] [Google Scholar]
- 9.Beeh KM, Westerman J, Kirsten AM, et al. The 24-h lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;32:53–59. doi: 10.1016/j.pupt.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. doi: 10.1016/j.rmed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO® study) Int J Chron Obstruct Pulmon Dis. 2016;11:193–205. doi: 10.2147/COPD.S95055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose M, Takizawa A, Izumoto T, et al. Efficacy and safety of the long-acting β2-agonist olodaterol over 4 weeks in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1673–1683. doi: 10.2147/COPD.S86002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4) Eur Respir J. 2015;45(4):969–979. doi: 10.1183/09031936.00136014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones PW. Estimation and application of the minimum clinically important difference in COPD. Lancet Respir Med. 2014;2(3):167–169. doi: 10.1016/S2213-2600(14)70038-4. [DOI] [PubMed] [Google Scholar]
- 15.Hosoe M, Woessner R, Matsushima S, Lawrence D, Kramer B. Efficacy, safety and pharmacokinetics of indacaterol in Caucasian and Japanese patients with chronic obstructive pulmonary disease: a comparison of data from two randomized, placebo-controlled studies. Clin Drug Investig. 2011;31(4):247–255. doi: 10.2165/11586520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Muruganandan S, Jayaram L. Profile of a fixed-dose combination of tiotropium/olodaterol and its potential in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1179–1189. doi: 10.2147/COPD.S54154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28(3):219–228. doi: 10.1089/jamp.2014.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]