Abstract
Categorization of patients with COPD as reversible or nonreversible to a bronchodilator may change over time. This post hoc analysis aimed to determine if an individual’s reversibility, when treated as a continuous variable, could predict his/her future response to two short-acting bronchodilators: albuterol and ipratropium. The analysis was completed using data from a 4-week, randomized, open-label, two-period crossover study (NCT01691482; GSK study DB2114956). Patients received albuterol (doses: UK =4×100 μg/puff; US =4×90 μg/puff) followed 1 hour later by ipratropium (4×20 μg/puff) or vice versa during treatment Period 1. The order of treatments was reversed during Period 2. Predefined efficacy end points included pre- and post-bronchodilator forced expiratory volume in 1 second. The correlation coefficient between bronchodilator response on Days 1 and 10 was investigated, as well as the correlation between treatment response on Day 1 and the mean treatment response on Days 5–10, for each individual patient. Bronchodilator response to albuterol on Day 1 was strongly correlated with that on Day 10 (r=0.64; n=53). The correlation coefficient of bronchodilator treatment response on Day 1 and Days 5–10 was 0.78 (P<0.001; n=53) and 0.76 (P<0.001; n=54) for albuterol and ipratropium, respectively. A single measurement of the initial bronchodilator response to albuterol or ipratropium was, therefore, highly correlated with the subsequent mean bronchodilator response over 5–10 days, demonstrating its potential usefulness for future treatment decisions.
Keywords: bronchodilator responsiveness, FEV1, correlation, short-acting bronchodilators, spirometry
Introduction
COPD is characterized by persistent airflow limitation.1,2 Inhaled β2-agonists and antimuscarinics have become central to the management of patients with COPD, as they improve lung function.2 Improvements in exercise tolerance and quality of life, and reductions in COPD symptoms including exacerbations have also been demonstrated with these treatments.2 Long-acting bronchodilators are used as regular maintenance therapies in patients with moderate-to-severe COPD, while short-acting bronchodilators are generally used as required to provide symptomatic relief.2
Despite little evidence to suggest a bimodal distribution of bronchodilator response with two distinct groups of responders and nonresponders,3,4 the paradigm of the reversible and nonreversible COPD phenotype has been widely applied to classify patients in clinical trials.5–8 However, bronchodilator responsiveness may be considered a continuous variable,9 with the effect of bronchodilators on lung function varying substantially between patients.2,10 The 200 mL cut-off point used to establish reversibility6 is close to the mean forced expiratory volume in 1 second (FEV1) response of patients with COPD to albuterol (eg, Buhl et al reported mean FEV1 values of 164–177 mL in a population of 5,162 patients11), and therefore, the categorization of a patient as reversible or not reversible may not be consistent over time.9,12 Based on these findings, Calverley et al recommended that the dichotomized outcome of classifying patients as reversible or not reversible should be abandoned.9
As a result of the issues surrounding bronchodilator responsiveness, the relationship between baseline reversibility and clinical outcomes or diagnoses has been questioned.3,6,12 For example, in an analysis of the Understanding Potential Long-term Improvements in Function with Tiotropium (UPLIFT) study, the authors concluded that baseline bronchodilator responsiveness to ipratropium and albuterol, when assessed using three distinct categories, was not predictive of clinically important outcomes with tiotropium.6 In another study, when lack of reversibility to bronchodilators was used as a diagnostic marker for COPD, there was an underdiagnosis of COPD.3 It is now accepted that bronchodilator reversibility should not be included in diagnostic criteria for COPD.2 However, bronchodilator reversibility is still measured in many research studies and clinical trials to characterize patients, and is used routinely in clinical practice as the post-bronchodilator FEV1 is used for diagnosis of COPD.3,6–8,13
We recently reported that the free combination of the short-acting bronchodilators albuterol (a β2-agonist) and ipratropium (a muscarinic antagonist) led to lower day-to-day variability in FEV1 compared with either monotherapy.14 Here, we report a novel post hoc analysis of data from this clinical study, investigating the reproducibility of treatment response to a short-acting bronchodilator by considering reversibility as a continuous variable, rather than “reversible” or “nonreversible”.
Methods
Study design
This was a post hoc analysis of a 4-week, randomized, open-label, two-period crossover study performed at two study centers specializing in spirometry (one in the UK, one in the US), between July 2012 and February 2013 (NCT01691482; GSK study number DB2114956).14 Patients were ≥40 years of age, were current or former smokers with a smoking history of ≥10 pack-years, had a previous physician diagnosis of COPD,1 had a post-albuterol FEV1/forced vital capacity ratio <0.70, and a post-albuterol FEV1 of ≥30% and ≤70% of predicted normal. Concomitant use of inhaled corticosteroids at a stable dose was permitted. Patients with a current diagnosis of asthma or any clinical significant uncontrolled disease were excluded.14
Patients were randomized 1:1 to receive albuterol (GlaxoSmithKline, Middlesex, UK) via metered dose inhaler (doses: UK =4×100 μg/puff; US =4×90 μg/puff), followed by ipratropium 1 hour later (4×20 μg/puff; Boehringer Ingelheim, Ingelheim, Germany) or vice versa, during treatment Period 1 (10–14 days). The order of treatments was then reversed during treatment Period 2. Placebo was administered on Day 4 in both study periods. There was no washout stage between Periods 1 and 2.14 At each visit (except for Day 4 of each study period [Visits 5 and 15]), pre-dose spirometry was performed, followed by the administration of either albuterol or ipratropium. Spirometry was repeated 1 hour after administration (Figure S1). On Day 4 of each study period, spirometry readings were taken at approximately the same times (ie, at 0 and 1 hour). At each study visit, patients were asked to refrain from smoking for 1 hour prior to the first pulmonary function test and throughout the study visit, until after the last spirometry test had been performed.14 The analyses detailed in this study were completed for both albuterol and ipratropium. The study protocol and any amendments were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board (Manchester Evaluation Unit, Manchester, UK: D2012-2166-E02-UK; Chesapeake institutional review board, SC, USA: PRO0007141). Written informed consent was obtained from each patient prior to initiation of any study procedures.
Outcomes and assessments
Full details on outcomes and assessments for the DB2114956 study have been previously published.14 The post hoc analyses presented in this study focused on the bronchodilator treatment response in terms of FEV1, defined as the change in FEV1 at 1 hour (post-dose) from 0 hour (pre-dose) on each day. Initial exploratory post hoc analyses examined the correlation coefficient of FEV1 response between Day 1 and Day 10. The mean within-patient difference in FEV1 response (including the proportion of patients exhibiting differences of <100, ≥100 to <150, ≥150 to <200, and ≥200 mL) between these days was also calculated. The correlation coefficient of bronchodilator treatment response on Day 1 and the mean treatment response on Days 5–10, for each individual patient, were also investigated. Additional post hoc analyses included the following: 1) the correlation coefficient of the mean treatment response for Days 1–2 and Days 1–3 with the mean response during Days 5–10, for each individual patient; and 2) the stability of each individual patient’s status as reversible or nonreversible on Day 1 compared with Day 10. Reversibility was defined as an increase in FEV1 of ≥12% and ≥200 mL following administration of albuterol or ipratropium.
Statistical analyses
Time-dependent bronchodilator responses to albuterol or ipratropium were assessed by correlation coefficient analyses. The pairwise linear correlation coefficients of FEV1 at pre-dose (0 hour) and 1 hour post-dose were computed by treatment and by day to assess the individual effect of albuterol or ipratropium over time. These post hoc analyses did not take study period into consideration.
The overall analysis population was defined as all patients who were randomized and completed pre- and post-bronchodilator assessments for at least 17 visits, with no more than 3 consecutive missing days. All programming was performed using SAS version 9 (SAS Institute Inc., Cary, NC, USA) and S-Plus version 7. (TIBCO Software Inc, CA, USA).
Results
Overall, 70 patients were screened, 56 were randomized to treatment and received at least one dose of bronchodilator in the treatment period (intent-to-treat population), and 53 patients completed the study. One patient in the intent-to-treat population was excluded from the efficacy population due to missing study visits for more than 3 consecutive days; therefore, data from 55 patients were analyzed. Baseline characteristics of the patients have been previously published.14 Briefly, patients had a mean age of 60.3 years (standard deviation [SD]: 7.42 years) and a mean predicted FEV1 post-albuterol at screening of 51.33% (SD: 9.87%). The mean FEV1 reversibility at the screening visit was 193 mL (SD: 188 mL), and the mean percentage reversibility was 19.54% (SD: 17.39%). Twenty-six (46%) patients were considered reversible (defined as an increase in FEV1 of ≥12% and ≥200 mL following administration of albuterol) at screening, and 30 (54%) were nonreversible.
The correlation coefficient of individual bronchodilator treatment response (change in FEV1 from 0 to 1 hour post-dose) to albuterol on Day 1 with that on Day 10 was 0.64 (Figure 1). The mean response to albuterol was 261 mL on Day 1 and 237 mL on Day 10, with a mean within-patient difference (Day 1 vs Day 10) of 47 mL (Table 1). The mean response to ipratropium was 253 mL on Day 1 and 241 mL on Day 10, with a mean within-patient difference (Day 1 vs Day 10) of 27 mL (Table 1). There was wide variability in the mean FEV1 responses to both treatments, as demonstrated by the associated SD values (Table 1). Out of 53 patients who received albuterol on Day 1 and Day 10, 31 (58%) had a response to albuterol on Day 10 that was <100 mL different to their response on Day 1; a similar proportion of patients (n=28; 52%) met this criterion for ipratropium at Day 10 (Table 2).
Figure 1.
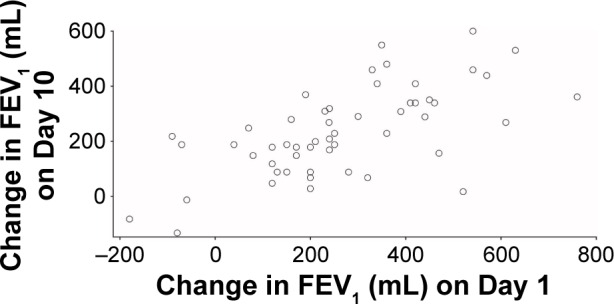
Response to albuterol on Day 1 compared with Day 10.
Abbreviation: FEV1, forced expiratory volume in 1 second.
Table 1.
Mean changes and mean difference between absolute change in FEV1 on Day 1 and Day 10
| Day 1 | Day 10 | |
|---|---|---|
| Albuterol, n | 55 | 53 |
| Mean (n) change in FEV1 (SD) (mL) | 261 (199) | 237 (158) |
| Mean (n) difference between Day 1 and Day 10 (SD) (mL)* | 47 (112) | |
| Ipratropium, n | 55 | 54 |
| Mean (n) change in FEV1 (SD) (mL) | 253 (180) | 241 (163) |
| Mean (n) difference between Day 1 and Day 10 (SD) (mL)* | 27 (170) |
Note:
The mean difference was calculated using the number of patients with data available on Day 10.
Abbreviations: FEV1, forced expiratory volume in 1 second; SD, standard deviation.
Table 2.
Difference in FEV1 response to albuterol and ipratropium between Day 1 and Day 10, stratified by specific thresholds
| Difference between Day 1 and Day 10 (mL) | Albuterol, n=53* | Ipratropium, n=54* |
|---|---|---|
| <100 | 31 | 28 |
| ≥100 to <150 | 12 | 8 |
| ≥150 to <200 | 4 | 9 |
| ≥200 | 6 | 9 |
Notes:
One patient completed the ipratropium study period but not the albuterol study period; therefore, n=53 for albuterol and n=54 for ipratropium.
Abbreviation: FEV1, forced expiratory volume in 1 second.
The correlation coefficient of bronchodilator treatment response on Day 1 and the mean of Days 5–10 was 0.78 (P<0.001) and 0.76 (P<0.001) for albuterol and ipratropium, respectively. The correlation coefficient of bronchodilator treatment response over the means of Days 1–2 (0.85 and 0.81 for albuterol and ipratropium, respectively) and Days 1–3 (0.88 and 0.84) with the means of Days 5–10 increased (compared with Day 1 and Days 5–10) for both bronchodilators.
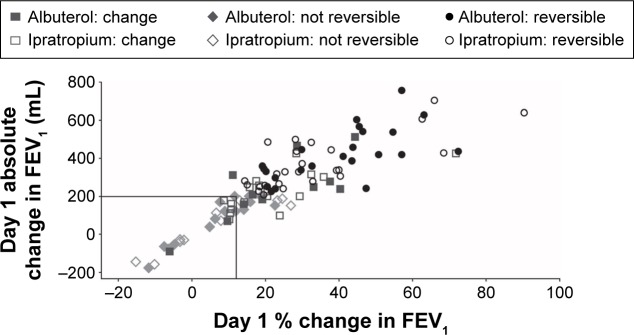
At Day 10, 42 out of 53 (79%) patients receiving albuterol had not changed their reversibility status (defined as an increase in FEV1 of ≥12% and ≥200 mL following administration of albuterol or ipratropium) from Day 1 (reversible at both days: n=24 [45%]; nonreversible at both days: n=18 [34%]; Figure 2). For ipratropium, at Day 10, 37 out of 54 (69%) patients had not changed their status from Day 1 (reversible at both days: n=25 [46%]; nonreversible at both days: n=12 [22%]). Patients who had a change in reversibility status tended to have FEV1 values clustered around the cut-off point used to define reversibility in this study (Figure 2).
Figure 2.
Change in FEV1 on Day 1 for albuterol and ipratropium.
Notes: Symbols demonstrate “reversibility” status on Day 10. Reversibility was defined as an increase in FEV1 of ≥12% and ≥200 mL following administration of albuterol or ipratropium. The square symbols represent patients whose reversibility status changed between Day 1 and Day 10.
Abbreviation: FEV1, forced expiratory volume in 1 second.
Discussion
There was substantial variability of the FEV1 response to albuterol and ipratropium between patients. However, the mean within-patient difference in response to albuterol or ipratropium between Days 1 and 10 was relatively small: 47 mL and 27 mL, respectively. The bronchodilator response on Day 1 showed a good correlation with the response on Day 10 (r=0.64) which improved when the average response on subsequent occasions (Days 5–10) was used. Overall, these results suggest that the short-term repeatability of bronchodilator reversibility tests is generally good with stable responses being observed for most measurements. This was supported by the observation that the majority of patients did not change reversibility status between Day 1 and Day 10.
As might be expected, those patients who changed reversibility status in this study had values clustered around the binary cut-off point for reversibility. Previous studies have reported varying proportions of patients changing reversibility status, dependent on the criteria employed: 75%–79% by Hanania et al,6 38% and 52% by Calverley et al,9 and 11% and 18% by Albert et al.12 The different proportions reported across these studies could be due to a variety of factors, including the exclusion of patients with a higher magnitude of response,9 the use of different cut-off points,6,9 the use of long-acting bronchodilators within the trials, and potential variability due to spirometric procedures between centers. In order to limit any spirometric measurement variations, this study was conducted in two centers specializing in spirometric measurements.
The issues with selecting a cut-off point can be illustrated with the data from a large study.11 In this study, the mean response in FEV1 to albuterol was 164–177 mL but with SD values of 138–150 mL. This implied that 68% of patients (the percent of patients in any normal population who are within one SD of the mean15) were <138–150 mL from the mean response.11 It should therefore be no surprise that a large number of patients when retested would cross any arbitrary boundary (eg, 200 mL) near the mean value of this distribution due solely to the error inherent in the measurement of FEV1. Three previous studies that tested different cut-off points for bronchodilator response found that all criteria produced inconsistent results.6,9,12 Consequently, the Global initiative of chronic Obstructive Lung Disease guidelines no longer recommend the use of reversibility for diagnosis of COPD or for prediction of future clinical outcomes.2 As our study results support the findings of Calverley et al,9 we suggest that future studies investigating the association of bronchodilator response with clinical outcomes should include bronchodilator response as a continuous, not dichotomized, variable.
This study had limitations. It was a post hoc analysis of data from an exploratory study, and as such should be treated with a degree of caution, but given that the correlations seen were statistically significant to a very high degree and other components of the analysis were purely descriptive, the findings warrant consideration and further investigation. A drawback of the study was its short duration. Long-term studies are required to determine if these findings are transferable over longer time frames.
Overall, we believe our study shows that bronchodilator responsiveness is a consistent variable within an individual patient over the short term, but highly variable between patients. With the measurement error inherent in spirometry in routine clinical practice, or even across multiple centers in large studies, we are cautious of suggesting that measuring a spirometric response may predict an individual’s likelihood of achieving sustained benefit from bronchodilators. However, the results presented in this study do suggest that accurate measures of bronchodilation may be able to predict future response at the individual level over the short term.
Supplementary material
Schematic diagram of the study design.
Notes: *Drugs administered immediately after spirometry. S1: pre-dose spirometry; S2: spirometry 1 hour after drug 1; S3: spirometry 1 hour after drug 2.
Footnotes
Disclosure
This study was funded by GSK (DB2114956). Editorial support in the form of developing the first draft based on author input, referencing, editing, and incorporation of author comments was provided by Gillian Groeger, PhD, and Natasha Thomas, PhD, Fishawack Indicia Ltd, UK, and funded by GSK. S Pascoe and C-Q Zhu are employees of GSK and own GSK stock. W Wu was an employee of GSK at the time this study was completed but is currently employed by PAREXEL International. D Singh declares he has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards, and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, Glenmark, GSK, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Skypharma, Takeda, Teva, Theravance, and Verona. The authors report no other conflicts of interest in this work.
References
- 1.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease. 2016. [Accessed March 18, 2016]. Available from: http://www.goldcopd.org/
- 3.Kjeldgaard P, Dahl R, Lokke A, Ulrik CS. Detection of COPD in a high-risk population: should the diagnostic work-up include bronchodilator reversibility testing? Int J Chron Obstruct Pulmon Dis. 2015;10:407–414. doi: 10.2147/COPD.S76047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest. 2003;123(5):1441–1449. doi: 10.1378/chest.123.5.1441. [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, Tashkin DP, Rennard SI, McElhattan J, Martin UJ. Bronchodilator responsiveness and onset of effect with budesonide/formoterol pMDI in COPD. Respir Med. 2011;105(8):1176–1188. doi: 10.1016/j.rmed.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Hanania NA, Sharafkhaneh A, Celli B, et al. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12(1):6. doi: 10.1186/1465-9921-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh D, Pujol H, Ribera A, et al. A dose-ranging study of the bronchodilator effects of abediterol (LAS100977), a long-acting beta2-adrenergic agonist, in asthma; a Phase II, randomized study. BMC Pulm Med. 2014;14:176. doi: 10.1186/1471-2466-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–750. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 9.Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58(8):659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthonisen NR, Lindgren PG, Tashkin DP, Kanner RE, Scanlon PD, Connett JE. Bronchodilator response in the lung health study over 11 yrs. Eur Respir J. 2005;26(1):45–51. doi: 10.1183/09031936.05.00102604. [DOI] [PubMed] [Google Scholar]
- 11.Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4) Eur Respir J. 2015;45(4):969–979. doi: 10.1183/09031936.00136014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 13.Montes de Oca M, Perez-Padilla R, Talamo C, et al. Acute bronchodilator responsiveness in subjects with and without airflow obstruction in five Latin American cities: the PLATINO study. Pulm Pharmacol Ther. 2010;23(1):29–35. doi: 10.1016/j.pupt.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Singh D, Zhu CQ, Sharma S, Church A, Kalberg CJ. Daily variation in lung function in COPD patients with combined albuterol and ipratropium: results from a 4-week, randomized, crossover study. Pulm Pharmacol Ther. 2014;31:85–91. doi: 10.1016/j.pupt.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 15.StatSoft Inc . Electronic Statistics Textbook. Tulsa, OK: StatSoft; 2013. [Accessed March 18, 2016]. Available from: http://www.statsoft.com/textbook/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic diagram of the study design.
Notes: *Drugs administered immediately after spirometry. S1: pre-dose spirometry; S2: spirometry 1 hour after drug 1; S3: spirometry 1 hour after drug 2.