Abstract
AIM: To quantify Toxoplasma gondii DNA using a specially constructed artificial template as competitor in a nested polymerase chain reaction (PCR). METHODS: The diagnostic assay was a nested PCR employing four primers that amplify part of the single copy gene for the P30 major surface antigen in T gondii. An artificial competitor containing the four primer binding sites was made first by creating a 216 bp deletion in the native 914 bp full length PCR product using restriction enzyme digestion, ligation of selected digestion fragments, and cloning the ligation product into an E coli plasmid vector for production. Competitive nested PCR using three different quantities of T gondii genomic DNA with four corresponding 10-fold dilutions of the artificial competitor was then performed, and the results visualised with agarose gel electrophoresis. A standard curve was drawn by plotting the T gondii to competitor ratio readings against log10 of the competitor readings. RESULTS: The band intensities on agarose gel showed quantitative amplification in competitive nested PCR. The amount of competitor required to achieve equal molar amounts of PCR products is calculated by reading off the value of the competitor where the T gondii to competitor ratio equals 1 on the standard curves. CONCLUSIONS: Competitive PCR is possible with a nested assay, and quantitative amplification is well preserved. The use of an artificial competitor containing the same primer binding sites as the target enables the absolute amount of T gondii DNA in unknown samples to be estimated. In addition, the competitor simultaneously serves as a control for detecting false negative results of failed reactions in individual assay runs.
Full text
PDF
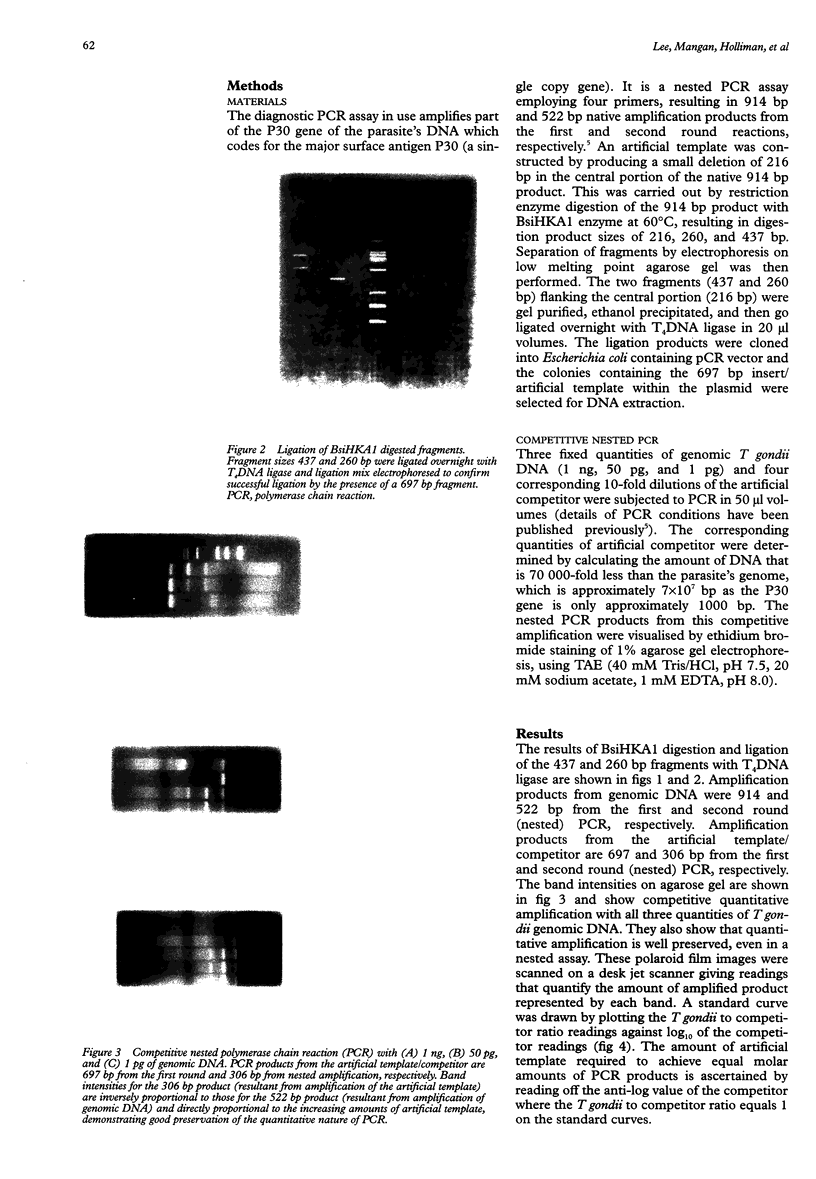
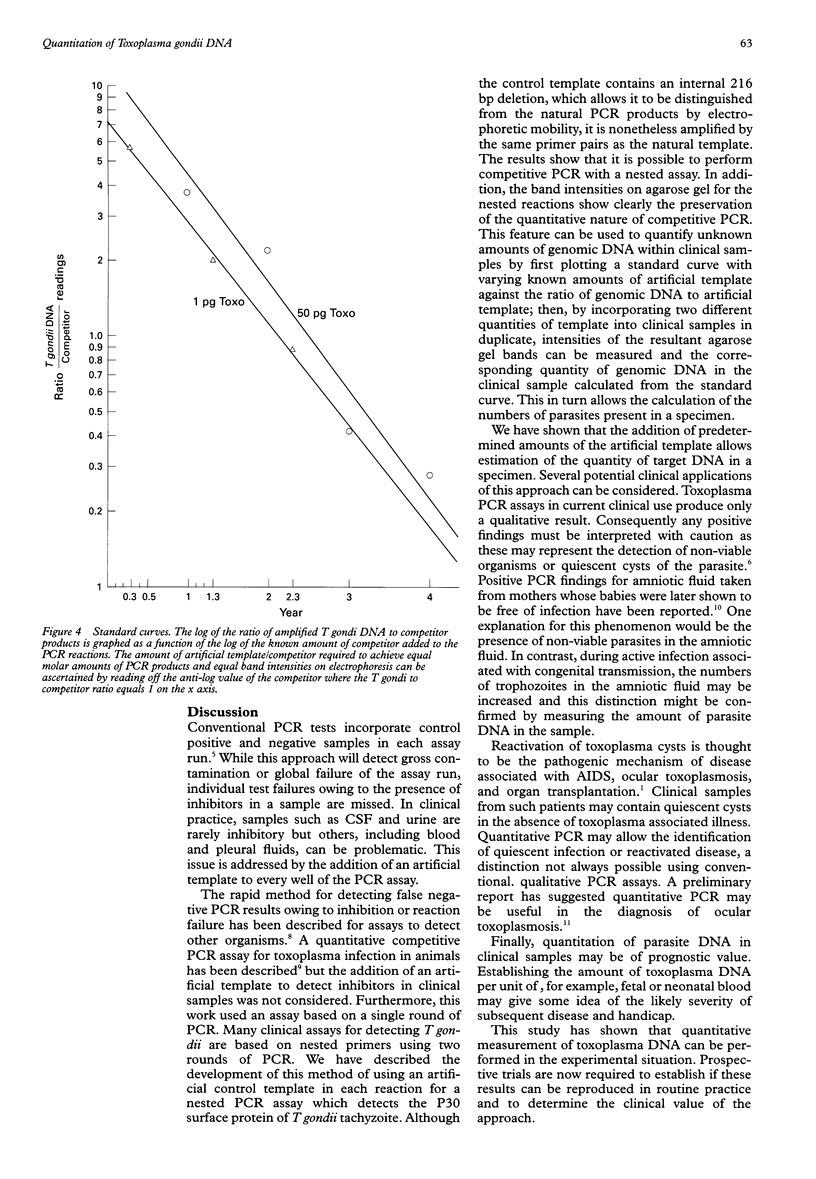

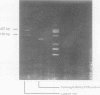
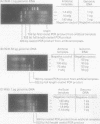
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg J. L., Grover C. M., Pouletty P., Boothroyd J. C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristina N., Liaud M. F., Santoro F., Oury B., Ambroise-Thomas P. A family of repeated DNA sequences in Toxoplasma gondii: cloning, sequence analysis, and use in strain characterization. Exp Parasitol. 1991 Jul;73(1):73–81. doi: 10.1016/0014-4894(91)90009-l. [DOI] [PubMed] [Google Scholar]
- Dupouy-Camet J., Bougnoux M. E., Lavareda de Souza S., Thulliez P., Dommergues M., Mandelbrot L., Ancelle T., Tourte-Schaefer C., Benarous R. Comparative value of polymerase chain reaction and conventional biological tests for the prenatal diagnosis of congenital toxoplasmosis. Ann Biol Clin (Paris) 1992;50(5):315–319. [PubMed] [Google Scholar]
- Johnson J. D., Butcher P. D., Savva D., Holliman R. E. Application of the polymerase chain reaction to the diagnosis of human toxoplasmosis. J Infect. 1993 Mar;26(2):147–158. doi: 10.1016/0163-4453(93)92788-x. [DOI] [PubMed] [Google Scholar]
- Luo W., Aosai F., Ueda M., Yamashita K., Shimizu K., Sekiya S., Yano A. Kinetics in parasite abundance in susceptible and resistant mice infected with an avirulent strain of Toxoplasma gondii by using quantitative competitive PCR. J Parasitol. 1997 Dec;83(6):1070–1074. [PubMed] [Google Scholar]
- Norose K., Tokushima T., Yano A. Quantitative polymerase chain reaction in diagnosing ocular toxoplasmosis. Am J Ophthalmol. 1996 Apr;121(4):441–442. doi: 10.1016/s0002-9394(14)70443-x. [DOI] [PubMed] [Google Scholar]
- Savva D., Morris J. C., Johnson J. D., Holliman R. E. Polymerase chain reaction for detection of Toxoplasma gondii. J Med Microbiol. 1990 May;32(1):25–31. doi: 10.1099/00222615-32-1-25. [DOI] [PubMed] [Google Scholar]