Abstract
BACKGROUND
Vitamin D deficiency has been associated with cardiovascular risk factors, including type 2 diabetes mellitus (T2DM). Evidence shows that patients with low serum 25-hydroxyvitamin D (25OHD) concentrations have a higher risk of developing coronary artery disease.
OBJECTIVE
The objective of this study was to assess vitamin D as a predictor of the severity in diabetics with acute coronary syndrome (ACS).
METHODS
A total of 166 patients were diagnosed with ACS. Serum 25OHD concentrations were analyzed, and risk factors for ACS were evaluated.
RESULTS
Patients diagnosed as having acute myocardial infarction with elevation of the ST segment had a higher rate of 25OHD, <20 ng/mL compared to ≥30 ng/mL (47.8% × 13.4%, P = 0.03). Diabetics with vitamin D deficiency had more multivessel lesions in the coronary angiography than non-diabetics (69% × 31.8%, P = 0.007). After adjustments for confounders, serum 25OHD remained associated with more severe disease.
CONCLUSION
Vitamin D deficiency is associated with more severe ACS and is a predictor of more extensive coronary lesions in patients with T2DM.
Keywords: 25-hydroxyvitamin D, acute coronary syndrome, type 2 diabetes mellitus
Introduction
Vitamin D is involved in the functioning of pancreatic beta cells via the vitamin D receptor,1 and reduced serum 25-hydroxivitamin D (25OHD) concentrations are associated with a risk of developing type 2 diabetes mellitus (T2DM)2 and metabolic syndrome.3
Vitamin D acts on the renin–angiotensin–aldosterone system and effects changes in cardiomyocytes as a result of the presence of its receptor in this type of cell, which explains its direct effect on the cardiovascular system.4 Individuals with low serum 25OHD levels have a higher risk of developing an acute myocardial infarction.5 Patients with T2DM are more likely to develop ischemic cardiovascular disease, and vitamin D deficiency would appear to increase this risk by acting directly on the endothelial function, leading to more unstable plaques.6,7
Macrovascular disease is a cause of increased mortality in T2DM and likely to be further increased with vitamin D deficiency,8 although few studies have evaluated vitamin D status in individuals with T2DM and acute coronary artery disease (CAD). The aim of the present study was to measure vitamin D status (serum 25-OHD concentration) in diabetic patients with acute coronary syndrome (ACS) and its association with the severity of the disease.
Methods
Subjects
A total of 166 consecutive patients at the Coronary Care Unit of Agamenon Magalhães Hospital were studied. The inclusion criterion was ACS, and the diagnosis was made on the basis of a compatible clinical profile in combination with electrocardiographic criteria,9,10 while that of T2DM was made using the American Diabetes Association criteria.11 Patients were excluded if they had chronic kidney disease, heart failure, primary hyperparathyroidism, active malignant disease, and uncontrolled hyperthyroidism or hypothyroidism. No patients were on vitamin D supplements.
Physical examination and laboratory investigations
After providing the informed consent document, the patients had their histories taken and underwent a physical examination for measurement of their anthropometric features. The skin phototype was assessed using Fitzpatrick’s classification,12 and the sun index was calculated on the basis of exposed body surface, multiplied by hours weekly spent in the sun.13
Blood samples were collected in the fasting state within the first 24 hours after admission. Serum parathormone was measured using Immulite 200 chemiluminescence equipment, with intra- and interassay variation coefficients of 2–5.7% and 6.3–6.8%, respectively. Serum total 25OHD concentrations were measured by chemiluminometric assay (LIAISON; DiaSorin). Assay sensitivity, defined as the lowest value different from zero, was 1.8 ng/mL, and the interassay coefficient of variation was 5%. Serum glucose, triglycerides, creatinine, glycated hemoglobin, total cholesterol and fractions, and calcium were measured using an autoanalyzer (Johnson & Johnson).
Creatine phosphokinase and creatine phosphokinase-MB were measured using the dry chemistry technique on the Johnson & Johnson® Chemistry System. Troponin was measured using an immunoassay on the Roche® Cardiac Reader. The scores for cardiovascular TIMI (Thrombolysis in Myocardial Infarction) risk,9,10 were obtained from the data in the medical records. Ventricular function was assessed by means of an echocardiogram on an Esaote® MyLab 30 Gold using the methods developed by Simpson and de Teicholtz to estimate the ejection fraction in the left ventricle. Coronary angiography was carried out using a Philips® Allura CV0246, and a lesion was considered significant when there was stenosis ≥70% in the main coronary artery or ≥50% in the left coronary artery trunk.14
Statistical analysis
The results were analyzed using the following descriptive statistical measurements: mean, standard deviation, median, and absolute and percentage distributions (percentages). The association between two category variables was assessed using the chi-square or Fisher’s exact test, and the odds ratio (OR) was calculated, along with the respective confidence interval. To confirm differences between categories in relation to numerical variables, Student’s t-test was used to compare two categories, and F-test (analysis of variance (ANOVA)) was used to compare three or more categories. In the case of a significant difference in the F-test (ANOVA), multiple Tukey comparisons were used for acceptance of the equality of variances hypothesis and the Tamhane comparisons were used for rejection of the equality of variances hypothesis. Verification of the equality of variances test was performed using the Levene’s F-test. The association between numerical variables was assessed using Pearson’s correlation coefficient. To assess the patients’ probability to present multivessel disease according to possible risk factors, it was adjusted to a multiple logistic regression analysis with backward selection. In the initial analysis, the 20% (P < 0.20) significant variables association was included. In the bivariate analysis and in the final analysis, the variables that showed P < 0.20 remained.
The statistical error used was 5%, and the confidence interval was 95.0%. The software used to input data and perform statistical calculations was SPSS Version 23.
The study was approved by the institution’s research ethics committee (Agamenon Magalhães Hospital, Recife/Brazil). The research was conducted in accordance with the principles of the Declaration of Helsinki.
Results
A total of 166 patients were included, of whom 66 were diabetics and 100 non-diabetics. The mean 25OHD was 22.33 ± 8.48 ng/mL. The prevalence of vitamin D deficiency (<20 ng/mL) was 41.6% and that of insufficiency (≥20 and <30 ng/mL) was 40.4%. Of those with T2DM, 54% had 25OHD <20 ng/mL compared with 33% of those with non-T2DM (P = 0.006). The overall mean for the sun index was 4.21 ± 5.04. The general characteristics of diabetic and non-diabetic patients are shown in Tables 1 and 2.
Table 1.
General characteristics of study patients.
| VARIABLE | DM | P VALUE | |
|---|---|---|---|
| T2DM | NON-T2DM | ||
| MEAN ± SD (MEDIAN) | MEAN ± SD (MEDIAN) | ||
| n = 66 | n = 100 | ||
| Age (years) | 64.64 ± 10.91 (65.50) | 57. 63 ± 11.20 (58.00) | P < 0.001* |
| Duration of hospitalization (days) | 14.00 ± 9.57 (12.00) | 9.08 ± 9.30 (6.00) | P = 0.032* |
| Sun index** | 4.18 ± 4.83 (2.91) | 4.22 ± 5.19 (2.66) | P = 0.963 |
| TIMI-risk | 4.27 ± 1.75 (4.00) | 3.03 ± 1.65 (3.00) | P < 0.001* |
| Echocardiogram-ejection fraction (%) | 57.91 ± 10.52 (61.50) | 58.24 ± 9.95 (61.00) | P = 0.838 |
| Creatine phosphokinase (U/L) | 614.27 ± 846.79 (293.00) | 797.22 ± 1440.30 (317.50) | P = 0.353 |
| Creatine phosphokinase-MB (U/L) | 75.99 ± 89.69 (43.00) | 87.91 ± 121.50 (43.00) | P = 0.495 |
| Troponin (ng/mL) | 27. 39 ± 35.41 (8.39) | 28.65 ± 37.67 (8.02) | P = 0.829 |
| Body mass index (kg/m2) | 27.17 ± 4.62 (27.00) | 27.99 ± 4.92 (27.30) | P = 0.284 |
| Waist circumference (cm) | 95.77 ± 13.41 (94.50) | 96.13 ± 12.85 (94.50) | P = 0.863 |
| Total cholesterol (mg/dL) | 180.20 ± 39.27 (177.50) | 201.58 ± 47.91 (198.00) | P = 0.003* |
| HDL (mg/dL) | 39.86 ± 11.03 (37.50) | 43.14 ± 12.28 (40.00) | P = 0.082 |
| LDL (mg/dL) | 102.59 ± 31.29 (101.50) | 125.51 ± 43.68 (124.00) | P < 0.001* |
| Triglycerides (mg/dL) | 189.27 ± 92.22 (171.50) | 177.05 ± 94.75 (150.00) | P = 0.412 |
| Parathormone (pg/mL) | 46.31 ± 34.11 (42.30) | 39.83 ± 27.89 (33.10) | P = 0.185 |
| Vitamin D (25OHD) (ng/mL) | 20.34 ± 8.17 (18.90) | 23.64 ± 8.63 (23.00) | P = 0.015* |
Notes:
P < 0.05 (significant), Student’s t-test.
Unit less.
Abbreviations: T2DM, type 2 diabetes mellitus; SD, standard deviation; DM, diabetes mellitus; HDL, High-density lipoprotein; LDL, low-density lipoprotein; 25OHD, 25-hydroxyvitamin D.
Table 2.
Gender, statin use, skin phototype, and risk factors for cardiovascular disease in the study population.
| VARIABLES | T2DM | P VALUE | OR (CI AT 95%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| YES | NO | GROUP TOTAL | |||||||
| N | % | N | % | N | % | ||||
| Total | 66 | 100.0 | 100 | 100.0 | 166 | 100.0 | |||
| Sex | |||||||||
| Male | 29 | 43.9 | 58 | 58.0 | 87 | 52.4 | P(1) = 0.076 | 1.00 | |
| Female | 37 | 56.1 | 42 | 42.0 | 79 | 47.6 | 1.76 (0.94–3.30) | ||
| Use of statin | |||||||||
| Yes | 28 | 42.4 | 13 | 13.0 | 41 | 24.7 | P(1) < 0.001* | 4.93 (2.31–10.55) | |
| No | 38 | 57. 6 | 87 | 87. 0 | 125 | 75.3 | 1.00 | ||
| History of CAD | |||||||||
| Yes | 22 | 33.3 | 15 | 15.0 | 37 | 22.3 | P(1) = 0.005* | 2.83 (1.34–6.00) | |
| No | 44 | 66.7 | 85 | 85.0 | 129 | 77.7 | 1.00 | ||
| Skin phototype | |||||||||
| I + II + III | 38 | 57.6 | 34 | 34.0 | 72 | 43.4 | P(1) = 0.003* | 2.63 (1.39–5.00) | |
| IV + V + VI | 28 | 42.4 | 66 | 66.0 | 94 | 56.6 | 1.00 | ||
| Smoking | |||||||||
| Yes | 10 | 15.2 | 34 | 34.0 | 44 | 26.5 | P(1) = 0.007* | 1.00 | |
| No | 56 | 84.8 | 66 | 66.0 | 122 | 73.5 | 2.88 (1.31–6.36) | ||
| Hypertension | |||||||||
| Yes | 53 | 80.3 | 65 | 65.0 | 11 8 | 71.1 | P(1) = 0.033* | 2.20 (1.06–4.57) | |
| No | 13 | 19.7 | 35 | 35.0 | 48 | 28.9 | 1.00 | ||
Note:
P < 0.05 (significant), Student’s t-test.
Abbreviations: T2DM, type 2 diabetes mellitus; OR, odd ratio; CI, confidence interval; CAD, coronary artery disease.
The diabetic patients had a lower mean 25OHD (20.34 ng/mL vs 23.64 ng/mL) when compared with non-diabetic patients, even with a lighter skin phototype, and there were no differences in the sun index between the two groups. The diabetic patients had a higher mean age (64.64 ± 10.91 years vs 57.53 ± 11.20 years), stayed longer in the hospital (14.0 ± 9.57 days vs 9.0 ± 9.3 days), had a higher mean TIMI risk (4.27 ± 1.75 vs 3.03 ± 1.65), and had a more frequent history of CAD (myocardial infarction and angina; P = 0.005) than non-diabetic patients. Despite this, when smoking was taken into account, patients with DM were less likely to smoke, and the mean total and low-density lipoprotein (LDL) cholesterol were also lower in these individuals (180.20 mg/dL vs 201.58 mg/dL; 102.59 mg/dL vs 125.51 mg/dL, respectively), probably because they used more statins (42% vs 13%), P < 0.001.
The prevalence of serum 25OHD levels <20 ng/dL was higher than the levels between 20 ng/dL and 30 ng/dL in diabetic patients. This higher frequency was also found when using the upper and lower 25OHD quartiles.
In the present study comparing patients with a deficiency with those with sufficiency and the upper and lower 25OHD quartiles, there was no evidence of differences between the groups in terms of age, the presence of metabolic syndrome, LDL, obesity, TIMI risk, ejection fraction on the echocardiogram, or cardiac enzymes, although there was a greater prevalence of lower serum 25OHD levels in individuals diagnosed with acute myocardial infarction with elevation of the ST segment (STEMI).
Patients diagnosed as having acute myocardial infarction with elevation of the ST segment had a higher rate of 25OHD, <20 ng/dL compared to ≥30 ng/dL (47.8% vs 13.4%, P = 0.03), regardless of cardiovascular risk factors.
The number of lesions in the coronary artery revealed by angiography varied from group to group: patients with T2DM and vitamin D deficiency had a higher frequency of multivessel lesions compared to non-T2DM patients (P = 0.007). This difference was not found between T2DM patients and non-T2DM patients with 25OHD >20 ng/mL and was independent of age (Fig. 1).
Figure 1.
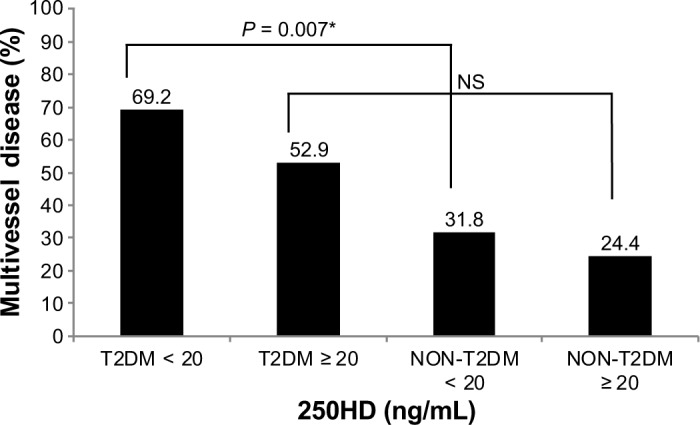
Prevalence of multivessel disease according to vitamin D status and the presence of diabetes.
Note: *P < 0.05 (significant).
Abbreviations: T2DM, Type 2 Diabetes mellitus; NS, non-significant; 25OHD, 25-hydroxyvitamin D.
Discussion
The high prevalence of low serum 25OHD levels is in agreement with previous findings for this type of population,2 although it is noteworthy that the present study comprised only individuals living in the tropics, where there is more sunlight. A study by our group,15 in the city of Recife, Brazil (latitude 8°S) with 284 elderly men with a mean age of 69.44 years found vitamin D deficiency in 31.5% with a mean 25OHD of 27.86 ± 13.52 ng/mL. The high prevalence of 25OHD <20 ng/mL in the present study, mainly among diabetic patients, corroborates the association between T2DM and vitamin D found in the literature.16 Another study17 conducted in Salvador, Brazil (latitude 12°S) in individuals with ACS found a mean 25OHD of 19.5 ng/mL, although, in this population, the mean age was greater (70 years). Most patients had a more pigmented skin phototype, and a different method was used for the 25OHD assay.
Another interesting finding is that, despite the mean sun index in our study being high (4.21 ± 5.04), with a mean daily exposure to sunlight of three hours, most patients did not have adequate serum 25OHD levels. In the abovementioned study conducted by Cabral et al,15 the mean sun index was 5.49 ± 5.05, with most of them being vitamin D deficient. These results draw attention to the need for vitamin D supplements in regions with abundant sunlight, since routine daily sunlight exposure is usually not sufficient to attain optimal serum 25OHD levels in most individuals.
One important finding was the higher prevalence of low serum 25OHD levels in patients with STEMI, regardless of the presence of traditional cardiovascular risk factors such as metabolic syndrome, dyslipidemia, obesity, hypertension, and smoking. These patients with low serum 25OHD levels are likely to be affected by T2DM, although the lower the level, the lower the proportion of T2DM, suggesting that vitamin D plays a more significant role in determining the severity of ACS the more extreme the deficiency. To our knowledge, this is the first study demonstrating that severity of ACS associated with vitamin D deficiency, making the latter a factor in predicting severity. Recently, Correia et al17 showed that patients with ACS and severe vitamin D deficiency (<10 ng/mL) exhibited higher hospital mortality, although STEMI, non-STEMI, and unstable angina remained unchanged. There were no deaths in this cohort during the period of evaluation.
A study with asymptomatic individuals has shown that patients with T2DM and a high risk of CAD tend to have higher coronary calcium scores when they have severe vitamin D deficiency (<5 ng/mL),18 while Hartaigh et al19 assessed individuals with T2DM and a high risk of cardiovascular events undergoing coronary angiography and found that patients with lower serum 25OHD levels had a poorer glycemic control and higher serum inflammatory markers. These data suggest a role of vitamin D in the development of CAD, especially in patients with T2DM.
Few studies have reported coronary angiography findings and their association with serum 25OHD levels, and these were conducted mainly in non-diabetic patients. Pilz et al20 examined patients undergoing angiography, but did not associate coronary lesions with vitamin D deficiency. Recently, Lim et al21 have shown an association between less good vitamin D status and higher risk of coronary stenosis on angiotomography, although the mean age of patients was higher than in the present study and they had not undergone an acute coronary event. Shor et al22 have shown that serum 25OHD levels <20 ng/mL are predictors of significant coronary lesions, although they did not find any association with the number of vessels affected and did not differentiate between T2DM and non-T2DM patients. Syal et al,23 in a 100-patient study, identified an association of vitamin D with a higher number of lesions and degree of stenosis in patients undergoing a cardiac catheterization, although their study involved a population with low mean serum 25OHD compared to ours (14.8 ng/mL vs 20.34 ng/mL) and in their group of patients, 44% had stable arterial disease. We did not find any studies in the literature evaluating the number of coronary lesions and their association with vitamin D status in diabetic patients who had experienced an acute coronary event.
Patients with T2DM have been reported to have more coronary lesions than non-T2DM ones,24 although the present study showed that this difference was significant only in patients with vitamin D deficiency. In patients with 25OHD >20 ng/mL, there were no significant differences between T2DM and non-T2DM regarding the number of lesions. These data reinforce the potential role of vitamin D in exacerbating coronary disease, probably through a direct effect in inflammation and consequently plaque rupture.7,25
However, some other data in the ACS patients without stratification for T2DM have not shown an association between vitamin D deficiency with markers of inflammation.26 Likewise, Goleniewska et al27 evaluating ACS patients with very low serum 25OHD concentrations, from 6 ng/mL to 17 ng/mL (lower to upper quartile), showed no association with the extent of coronary lesions.
Finally, data from Norway in the ACS patients with serum 25OHD from 12 ng/mL to 31 ng/mL (lower to upper quartile), who were followed up to seven years, showed improved survival in those in the highest quartile.28
As limitation of the present study, we emphasize its cross-sectional design not allowing the establishment of general causality. There is a need for prospective or controlled studies with larger sample sizes where vitamin D replacement and the impact of the 25OHD levels on severity of CAD are observed.
In conclusion, vitamin D deficiency was independently associated with more severe ACS and was predictive of more extensive coronary lesions of patients with T2DM.
Footnotes
ACADEMIC EDITOR: Nigel Irwin, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2456 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: FG, ADS and KFV. Analyzed the data: FG, AC, ADS and KFV. Wrote the first draft of the manuscript: FG and AC. Contributed to the writing of the manuscript: FG. Agreed with manuscript results and conclusions: FG. Jointly developed the structure and arguments for the paper: FG and FB. Made critical revisions and approved the final version: FG and FB. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Kayaniyil S, Retnakaran R, Harris SB, et al. Prospective associations of vitamin D with β-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes. 2011;60(11):2947–2953. doi: 10.2337/db11-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–232. doi: 10.1016/j.ecl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayaniyil S, Vieth R, Harris SB, et al. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab. 2011;96(1):168–175. doi: 10.1210/jc.2010-1439. [DOI] [PubMed] [Google Scholar]
- 4.Pilz S, Tomaschitz A, Drechsler C, Dekker JM, Marz W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010;54:1103–1113. doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strawbridge RJ, Deleskog A, McLeod O, et al. A serum 25-hydroxyvitamin D concentration-associated genetic variant in DHCR7 interacts with type 2 diabetes status to influence subclinical atherosclerosis (measured by carotid intimamedia thickness) Diabetologia. 2014;57(6):1159–1172. doi: 10.1007/s00125-014-3215-y. [DOI] [PubMed] [Google Scholar]
- 7.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95(12):787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 8.Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33(10):2238–2243. doi: 10.2337/dc10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 13.Barger-Lux MJ, Reaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87:4952–4956. doi: 10.1210/jc.2002-020636. [DOI] [PubMed] [Google Scholar]
- 14.Smith SC, Jr, Dove JT, Jacobs AK, et al. American College of Cardiology. American Heart Association Task Force on Practice Guidelines Committee to Revise the 1993 Guidelines for Percutaneous Transluminal Coronary Angioplasty. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines)—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) J Am Coll Cardiol. 2001;37(8):2215–2239. doi: 10.1016/s0735-1097(01)01344-4. [DOI] [PubMed] [Google Scholar]
- 15.Cabral MA, Borges CN, Maia JN, Aires CA, Bandeira F. Prevalence of vitamin D deficiency during the summer and its relationship with sun exposure and skin phototype in elderly men living in the tropics. Clin Interv Aging. 2013;8:1347–1351. doi: 10.2147/CIA.S47058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29(3):722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 17.Correia LC, Sodré F, Garcia G, et al. Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. Am J Cardiol. 2013;111(3):324–327. doi: 10.1016/j.amjcard.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Joergensen C, Reinhard H, Schmedes A, et al. Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care. 2012;35:168–172. doi: 10.2337/dc11-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartaigh B, Thomas GN, Silbernagel G, et al. Association of 25-hydroxyvitamin D with type 2 diabetes among patients undergoing coronary angiography: cross-sectional findings from the Ludwigshafen RIsk and Cardiovascular Health (LURIC) Study. Clin Endocrinol (Oxf) 2013;79(2):192–198. doi: 10.1111/cen.12024. [DOI] [PubMed] [Google Scholar]
- 20.Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 21.Lim S, Shin H, Kim MJ, et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: the Korean Longitudinal Study on Health and Aging. J Clin Endocrinol Metab. 2012;97(1):169–178. doi: 10.1210/jc.2011-1580. [DOI] [PubMed] [Google Scholar]
- 22.Shor R, Tirosh A, Shemesh L, et al. 25 hydroxyvitamin D levels in patients undergoing coronary artery catheterization. Eur J Intern Med. 2012;23(5):470–473. doi: 10.1016/j.ejim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Syal SK, Kapoor A, Bhatia E, et al. Vitamin D deficiency, coronary artery disease, and endothelial dysfunction: observations from a coronary angiographic study in Indian patients. J Invasive Cardiol. 2012;24(8):385–389. [PubMed] [Google Scholar]
- 24.Pajunen P, Nieminen MS, Taskinen MR, Syvanne M. Quantitative comparison of angiographic characteristics of coronary artery disease in patients with non insulin-dependent diabetes mellitus compared with matched non diabetic control subjects. Am J Cardiol. 1997;80:550–556. doi: 10.1016/s0002-9149(97)00420-7. [DOI] [PubMed] [Google Scholar]
- 25.Thorand B, Zierer A, Huth C, et al. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care. 2011;34(10):2320–2322. doi: 10.2337/dc11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eren E, Ellidag HY, Yılmaz A, Aydın Ö, Yılmaz N. No association between vitamin D levels and inflammation markers in patients with acute coronary syndrome. Adv Med Sci. 2015;60(1):89–93. doi: 10.1016/j.advms.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Goleniewska B, Kacprzak M, Zielińska M. Vitamin D level and extent of coronary stenotic lesions in patients with first acute myocardial infarction. Cardiol J. 2014;21(1):18–23. doi: 10.5603/CJ.a2013.0048. [DOI] [PubMed] [Google Scholar]
- 28.Naesgaard PA, Pönitz V, Aarsetoey H, et al. Prognostic utility of vitamin D in acute coronary syndrome patients in coastal Norway. Dis Markers. 2015;2015:283178. doi: 10.1155/2015/283178. [DOI] [PMC free article] [PubMed] [Google Scholar]


