Abstract
Background
Advanced heart failure (HF) is commonly accompanied by central sleep apnea (CSA) with Cheyne-Stokes respiration (CSR). The aim of this study was to evaluate the relationship between CSA/CSR and other clinical features of HF, with particular emphasis on cardiovascular hemodynamics.
Material/Methods
In 161 stable HF patients with left ventricular ejection fraction (LVEF) ≤45% (NYHA class I–III; mean LVEF 32.8%) the clinical evaluation included: LVEF; left and right ventricular end-diastolic diameter (LVDd, RVDd); ratio of early transmitral flow velocity to early diastolic septal mitral annulus velocity (E/e’) assessed by echocardiography; stroke index (SI); heart rate (HR); cardiac index (CI); and systemic vascular resistance index (SVRI) assessed by impedance cardiography (ICG). The comparison was performed between 2 subgroups: one with moderate/severe CSA/CSR - CSR_ [+] (n=51), and one with mild or no CSA/CSR – CSR_ [−] (n=110).
Results
CSR_ [+] patients presented more advanced NYHA class (p<0.001) and more frequently had permanent atrial fibrillation (p=0.018). Moreover, they had: lower LVEF (p<0.0001); higher LVDd (p<0.0001), RVDd (p<0.001), and E/e’ (p<0.001); lower SI (p<0.001) and CI (p=0.009); and higher HR (p=0.044) and SVRI (p=0.016). The following predictors of CSR_ [+] were identified: NYHA class (OR=3.34 per class, p<0.001, which was the only independent predictor); atrial fibrillation (OR=2.29, p=0.019); RV enlargement (OR=2.75, p=0.005); LVEF<35% (OR=3.38, p=0.001); E/e’ (OR=3.15; p=0.003); and SI<35 ml/m2 (OR=2.96, p=0.003).
Conclusions
Presence of CSA/CSR in HF is associated with NYHA class, atrial fibrillation and more advanced impairment of cardiovascular structure and hemodynamics. Patient functional state remains the main determinant of CSR.
MeSH Keywords: Cardiography, Impedance; Echocardiography; Heart Failure; Prognosis; Sleep Apnea, Central
Background
Heart failure (HF) is often accompanied by disturbances of breathing during sleep (SDB, sleep-disordered breathing). The frequency of obstructive sleep apnea (OSA) in a population of patients with HF is estimated as 11–43% and central sleep apnea (CSA) as 28–40% [1–4]. Obstructive sleep apnea, mainly associated with hypertension and cardiac arrhythmias, dominates in milder forms of HF, whereas CSA of the Cheyne-Stokes type of respiration (CSR) is typical for advanced HF [5].
The main pathophysiological phenomena related to CSR are: chronic hyperventilation, hypocapnia, and hypersensitivity to carbon dioxide partial pressure [6–9]. Cheyne-Stokes respiration is usually associated with significant myocardial impairment and constitutes an independent negative prognostic factor [10]. There are also opinions that it is the compensation mechanism [11,12]. However, there is no doubt that the recognition of CSR in patients with HF has important therapeutic implications. In recent years, many studies confirmed the beneficial effect of SDB-specific treatment on cardiac function and quality of life [13–17]. Nevertheless, recent reports have called into question the benefits from adaptive servo-ventilation, which is a new method of non-invasive ventilation, on the cardiovascular system of patients with CSR and advanced HF [18]. Despite these controversies, it is still reasonable to look for indicators useful in identifying patients with SDB.
Identification of patients at high risk of CSR is not easy. The symptoms of CSR are usually masked by complaints typical of HF. Moreover, the recommendations on how to select patients to screen for CSR are still not clearly established. At the same time, the availability of polysomnography is limited. Thus, there is still a need for identification of non-invasive predictors of the occurrence of CSR, which will help to optimize the process of diagnosis.
Therefore, the aim of this study was to evaluate the relationship between CSA/CSR and other clinical features of HF, with particular emphasis on cardiovascular hemodynamics, and to identify those that may be useful in predicting the occurrence of CSR in this population.
Material and Methods
Study group
The study was designed and implemented as a prospective clinical trial. Consecutive patients with systolic HF (ischemic and non-ischemic) were recruited. The inclusion criteria were: 1) HF diagnosed at least 3 months before the study, with a left ventricular ejection fraction (LVEF) assessed by echocardiography at ≤45%; 2) stable condition, with the severity of clinical symptoms according to NYHA class I–III; and 3) optimal medical therapy for at least 1 month. The exclusion criteria were: 1) HF deterioration; 2) HF as a result of primary valvular defect; 3) acute coronary syndrome in the last 6 months; 4) revascularization treatment; 5) myocarditis; 6) pulmonary embolism; 7) cerebrovascular event or transient ischemic attack (TIA); 8) implanted cardiac resynchronization device in the last 6 months; 9) chronic obstructive pulmonary disease (COPD) with FEV1 <50%; and 10) respiratory failure (PaO2 <60 mmHg and/or PaCO2 >45 mmHg).
The study was conducted according to the Good Clinical Practice guidelines and the Declaration of Helsinki, with the approval of the local ethics committee. Each patient provided written informed consent to participate in the study. The recruited patients were examined with polygraphic test, and echocardiography and non-invasive assessment of hemodynamic parameters by means of ICG were performed.
Polygraphy test
Sleep-disordered breathing in all patients was diagnosed using the polygraphy system (Embletta, Embla, The Netherlands). During the examination, the following features were recorded: the flow of air through the upper airway, breathing movements of chest and abdomen, blood arterial oxygen saturation, pulse rate, snoring, and body position. The analysis was performed automatically using standard software (Somnologica for Embletta, Embla, The Netherlands), then verified manually.
Sleep-disordered breathing was defined according to the criteria of the American Academy of Sleep Medicine [19]. Apnea was diagnosed when the amplitude of the flow of air through airways dropped to ≤10% and an episode of respiratory arrest lasted at least 10 seconds. Hypopnea was diagnosed when there were at least 10 seconds of airflow reduction in the respiratory tracts to ≤70%, combined with decrease in saturation of at least 4%. Apneas were defined as obstructive or central, depending on the presence or absence of breathing movements of the chest and abdomen. Breathing disorders during sleep were ultimately classified as: central, when >50% of episodes of loss/reduction of air flow through the respiratory tracts were of central origin; or obstructive, when obstructive episodes dominated (they accounted for >50% of all episodes). The level of respiratory disorders was established by the number of indicators of apnea and hypopnea per hour of sleep (AHI, apnea/hypopnea index): mild form, AHI ≥5 and <15; moderate form, ≥15 and ≤30 episodes per hour; and severe form, >30 [20].
Cheyne-Stokes respiration was diagnosed when there were at least 3 consecutive cycles of respiration track with amplitude in the form of crescendo-decrescendo with central apnea or hypopnea, and at least 1 of the following conditions: 1) central AHI ≥5; or 2) cyclic change of crescendo-decrescendo lasting at least 10 minutes. Oxygen desaturation index (ODI) was defined as number of saturation drops by at least 4% per hour of sleep.
Echocardiographic studies
All patients underwent echocardiographic evaluation with the Vivid 7 Dimension system (GE Healthcare, USA) in a standard manner [21,22]. Left ventricle (LV) function was assessed by measuring left ventricular ejection fraction (LVEF) according to Simpson’s rule. The following parameters were obtained from the parasternal long axis view in the 2-dimensional presentation: left ventricular end-diastolic diameter (LVDd), left atrium dimension (LA), right ventricular end-diastolic diameter (RVDd) and intraventricular septum in diastole thickness (IVSd), and LV posterior wall thickness in diastole (PVd). Cardiac output (CO) was calculated by multiplying heart rate (HR) by LV stroke volume, which was determined from the product of velocity time integral through the outflow tract of the left ventricle and surface area of the LV outflow tract. The evaluation of E/e (defined as the ratio of the maximum velocity of early flow by mitral valve to early diastolic mitral annulus velocity in tissue Doppler) was also calculated.
Non-invasive monitoring of hemodynamic parameters by impedance cardiography
A non-invasive assessment of hemodynamic parameters by ICG (Niccomo, Medis, Germany) was performed during a 10-minute test in horizontal position, after not less than 10 minutes of rest. The measurements of HR, stroke index (SI), cardiac index (CI), systemic vascular resistance index (SVRI), and thoracic fluid content (TFC) were carried out with beat-to-beat method. Only arm systolic and diastolic blood pressure (SBP/DBP) measurement was performed (automatically) at 2-minute intervals. The parameters recorded in the 10th minute of recording were used for the analysis.
Statistical methods
The statistical analysis was performed using Statistica v.10 software (StatSoft, Inc., USA). The normality of data distribution was assessed using the Shapiro-Wilk test. Results are expressed as mean values and standard deviations (SD) for quantitative variables and frequencies and percentages for qualitative variables. We performed comparative analysis of 2 subgroups: patients with significant CSR (AHI ≥15/h) and patients with no significant CSR (AHI <15/h).
Comparisons for quantitative variables were performed using t tests for variables of normal distribution and non-parametric tests (Mann-Whitney U test) for variables of non-normal distribution. In case of ordinal variables, the Mann-Whitney U test was used, and for nominal variables we used the chi-square/V-square/chi-square test with a Yatesav amendment (depending on the numbers expected in subgroups). Then, the most representative nominal variables differentiating the 2 subgroups were tested as the predictors of significant CSR with use of logistic regression models (univariable and multivariable). Results with p<0.05 were considered statistically significant.
Results
The study included 161 patients with basic characteristics as presented in Table 1. Almost one-third of the subjects presented NYHA class III and permanent atrial fibrillation (AF) occurred in almost one-third of them. More than half of the patients (55.3%) presented LVEF<35%. Forty-one patients (25.5%) were free from SDB, 47 (29.2%) had OSA, and 73 (45.3%) had CSA of Cheyne-Stokes type (Figure 1). Moderate and severe forms of CSR were found in 51 patients (31.7%).
Table 1.
Basic characteristics of the study group (n=161).
| Variable | Mean ±SD or n (%) |
|---|---|
| Age (years) | 64±11 |
|
| |
| Male gender | 150 (93.2) |
|
| |
| Height [cm] | 173±6.9 |
|
| |
| Weight [kg] | 88.6±19.8 |
|
| |
| BMI [kg/m2] | 29.4±5.9 |
|
| |
| SBP/DBP [mm Hg] | 124.2/74.6±17.4/12/0 |
|
| |
| Ischaemic etiology of HF | 106 (65.8) |
|
| |
| NYHA Class: | |
| NYHA I | 14 (8.7) |
| NYHA II | 94 (58.4) |
| NYHA III | 53 (32.9) |
|
| |
| Hypertension | 140 (87) |
|
| |
| Atrial fibrillation: | |
| Paroxysmal | 27 (16.8) |
| Permanent | 52 (32.3) |
|
| |
| Stimulation in ECG | 27 (16.8) |
|
| |
| ICD | 75 (46.6) |
|
| |
| CRT | 22 (13.7) |
|
| |
| Cardiac stimulator different from ICD/CRT | 16 (9.9) |
|
| |
| Medical history: | |
| Stroke/TIA | 10 (6.2) |
| Diabetes | 55 (34.2) |
| COPD | 23 (14.3) |
| eGFR <60 ml/min/1.73 m2 | 41 (25.9) |
|
| |
| Polygraphy | |
|
| |
| AHI [1/h] | 18.4±15.9 |
|
| |
| Mean SaO2 [%] | 92.8±1.9 |
|
| |
| ODI [1/h] | 18.6±15.4 |
|
| |
| No SDB or AHI <15/h | 89 (55.3) |
|
| |
| OSA with AHI ≥15/h | 21 (13) |
|
| |
| CSA/CSR with AHI ≥15/h | 51 (31.7) |
|
| |
| Echocardiographic parameters | |
| LVEF [%] | 32.8±7.0 |
| LVDd [cm] | 6.43±0.70 |
| LA [cm] | 4.78±0.57 |
| RVDd [cm] | 3.20±0.53 |
| E/e’ | 15.4±6.1 |
|
| |
| Haemodynamic parameters (ICG) | |
| SI [ml/m2] | 38.3±8.3 |
| CI [l/min/m2] | 2.54±0.52 |
| SVRI [dyn·s·cm−5·m2] | 2529.3±662.0 |
| TFC [1/kOhm] | 31.2±6.4 |
|
| |
| Pharmacotherapy | |
| B-blockers | 157 (97.5) |
| ACE-I | 148 (91.9) |
| ARB | 12 (7.5) |
| Aldosterone antagonists | 128 (79.5) |
| Diuretics | 144 (89.4) |
| Digoxin | 7 (4.3) |
| Amiodarone | 27 (16.8) |
Data presented as means ±SD or as a number (percentage). BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; HF – heart failure; NYHA – New York Heart Association; ICD – implantable cardioverter-defibrilator; CRT – cardiac resynchronization therapy; TIA – transient ischemic attack; COPD – Chronic Obstructive Pulmonary Disease; eGFR – estimated glomerular filtration rate; AHI – apnea/hypopnea index; ODI – oxygen desaturation index; SDB – sleep-disordered breathing; OSA – obstructive sleep apnea, CSA – central sleep apnea, CSR – Cheyne-Stokes respiration; LVEF – left ventricular ejection fraction; LVDd – left ventricular end diastolic diameter; LA – left atrium; RVDd – right ventricular end diastolic diameter; E/e’ – the ratio of maximum velocity of the early inflow through the mitral valve and protodiastolic mitral annulus velocity; SI – stroke index; CI – cardiac index; SVRI – systemic vascular resistance index; TFC – thoracic fluid content; ACE-I – angiotensin-converting enzyme inhibitor; ARB – angiotensin receptor blocker.
Figure 1.
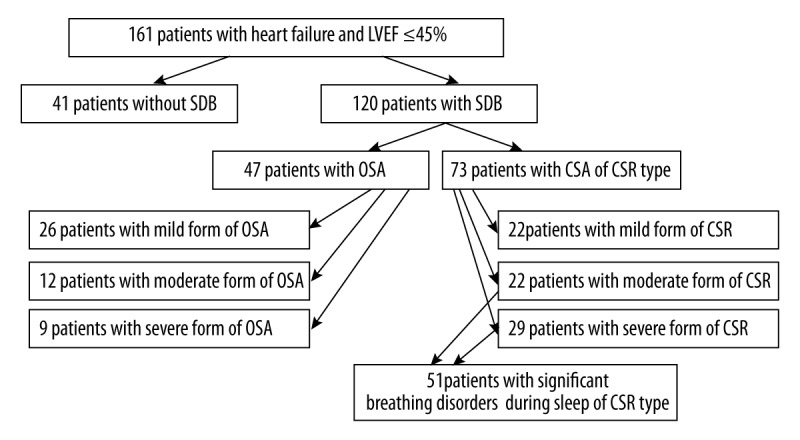
The prevalence of sleep breathing disorders in the study group. LVEF – left ventricular ejection fraction; SDB – sleep-disordered breathing; OSA – obstructive sleep apnea; CSA – central sleep apnea; CSR – Cheyne-Stokes respiration.
Comparison of groups in terms of basic clinical parameters
Comparative analysis revealed significantly greater severity of clinical symptoms (NYHA classification), higher prevalence of AF and implanted ICDs, lower SBP, and more frequent use of diuretics in patients with significant CSR (Table 2, Figure 2).
Table 2.
Basic comparative characteristics depending on the presence of significant Cheyne-Stokes respiration (CSR).
| Variable | Mean ±SD or n (%) | p | |
|---|---|---|---|
| Significant CSR (n=51) | Without significant CSR (n=110) | ||
| Age (years) | 65±10 | 63±11 | 0.251 |
|
| |||
| Male gender | 50 (98) | 100 (90.9) | 0.183 |
|
| |||
| Height [cm] | 174.8±6.7 | 172.7±7.0 | 0.073 |
|
| |||
| Weight [kg] | 88.4±20.2 | 88.7±19.7 | 0.450 |
|
| |||
| BMI [kg/m2] | 29.0±6.5 | 29.6±5.6 | 0.962 |
|
| |||
| HF ischemic etiology | 33 (64.7) | 73 (66.4) | 0.837 |
|
| |||
| Hypertension | 43 (84.3) | 97 (88.2) | 0.497 |
|
| |||
| Permanent atrial fibrillation | 23 (45.1) | 29 (26.4) | 0.018 |
|
| |||
| Stimulation in ECG | 9 (17.6) | 18 (16.4) | 0.839 |
|
| |||
| ICD | 30 (58.8) | 45 (40.9) | 0.034 |
|
| |||
| CRT | 7 (13.7) | 15 (13.6) | 0.988 |
|
| |||
| Cardiac stimulator different from ICD/CRT | 6 (11.8) | 10 (9.1) | 0.597 |
|
| |||
| Blood pressure: | |||
| SBP [mm Hg] | 119.5±15.5 | 126.5±17.9 | 0.031 |
| DBP [mm Hg] | 75.0±11.7 | 74.4±12.2 | 0.674 |
|
| |||
| Medical history (outside cardiology): | |||
| Stroke/TIA | 4 (7.8) | 6 (5.5) | 0.816 |
| Diabetes | 17 (33.3) | 38 (34.6) | 0.880 |
| CODP | 3 (5.9) | 20 (18.2) | 0.067 |
| GFR <60 ml/min/1.73 m2 | 17 (34.7) | 24 (22) | 0.093 |
|
| |||
| Pharmacotheraphy: | |||
| B-blockers | 50 (98) | 107 (97.3) | 0.780 |
| ACE-I | 46 (90.2) | 102 (92.7) | 0.812 |
| ARB | 5 (9.8) | 7 (6.4) | 0.652 |
| Aldosterone antagonists | 41 (80.4) | 87 (79.1) | 0.849 |
| Duretics | 50 (98) | 94 (85.5) | 0.032 |
| Digoxin | 3 (5.9) | 4 (3.6) | 0.815 |
| Amiodarone | 9 (17.7) | 18 (16.4) | 0.839 |
Data presented as means ±SD or as a number (percentage). BMI – body mass index; HF – heart failure; ICD – implantable cardioverter-defibrilator; CRT – cardiac resynchronization therapy; SBP – systolic blood pressure; DBP – diastolic blood pressure; TIA – transient ischemic attack; CODP – chronic obstructive pulmonary disease; eGFR – estimated glomerular filtration rate; ACE-I – angiotensin-converting enzyme inhibitor; ARB – angiotensin receptor blocker.
Figure 2.
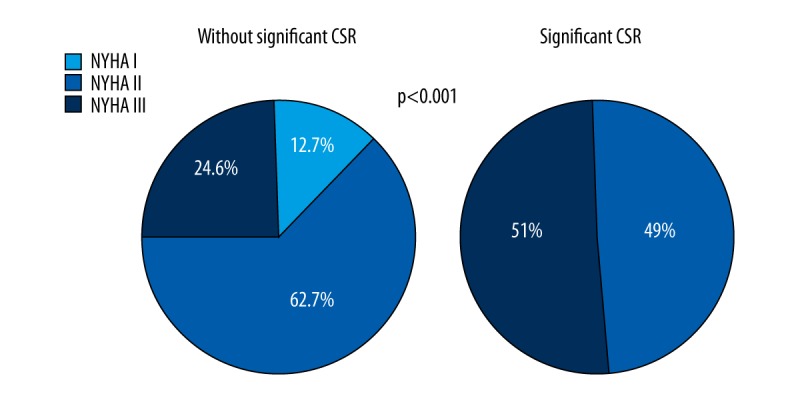
The severity of heart failure symptoms according to NYHA classification depending on the presence of significant Cheyne-Stokes respiration (CSR).
Comparison of groups in polygraphy tests
In patients with significant CSR, as compared to the rest, the desaturation index (ODI) was significantly higher, and the average desaturation and the minimum saturation were lower (Table 3).
Table 3.
The comparison of polygraphy parameters depending on the presence of significant Cheyne-Stokes respiration (CSR).
| Variable | Mean ±SD | p | |
|---|---|---|---|
| Significant CSR (n=51) | Without significant CSR (n=110) | ||
| AHI [1/h] | 33.4±11.7 | 11.4±12.4 | <0.0001 |
| AHI obstructive [1/h] | 4.8±4.4 | 7.7±10.8 | 0.480 |
| AHI central [1/h] | 28.6±11.0 | 3.7±4.2 | <0.0001 |
| ODI [1/h] | 32.0±13.0 | 12.7±12.4 | <0.0001 |
| Minimal SaO2 [%] | 78.7±6.8 | 82.6±5.7 | <0.001 |
| Mean SaO2 [%] | 92.5±2.0 | 92.9±1.9 | 0.274 |
| Mean desaturation [%] | 7.4±2.4 | 5.8±3.6 | <0.0001 |
Data presented as means ±SD or as a number (percentage). AHI – apnea/hypopnea index; ODI – oxygen desaturation index; SaO2 – arterial oxygen saturation.
Comparison of groups within echocardiographic studies
Patients with important CSR in comparison to other patients had statistically significantly lower LVEF, larger dimensions of left and right ventricle and left atrium, and higher E/e’ index (Table 4).
Table 4.
The comparison of echocardiographic parameters depending on the presence of significant Cheyne-Stokes respiration (CSR).
| Variable | Significant CSR | Without significant CSR | p | |||
|---|---|---|---|---|---|---|
| N | Mean ±SD or n (%) | N | Mean ±SD or n (%) | |||
| LV systolic function | LVEF [%] | 51 | 29.2±7.2 | 110 | 34.5±6.4 | <0.0001 |
| LVEF <35% | 51 | 38 (74.5) | 110 | 51 (46.4) | <0.001 | |
| CO [ml/min] | 50 | 5.01±1.76 | 110 | 4.85±0.85 | 0.917 | |
| heart chambers (dimensions) | LVDd [cm] | 51 | 6.77±0.64 | 110 | 6.27±0.67 | <0.0001 |
| LVDd ≥6 cm (PLAX) | 51 | 45 (88.2) | 110 | 82 (74.5) | 0.048 | |
| LA [cm] | 51 | 5.14±0.58 | 110 | 4.61±0.48 | <0.0001 | |
| LA >4 cm (PLAX) | 51 | 51 (100) | 110 | 99 (90) | 0.045 | |
| RVDd [cm] | 50 | 3.40±0.55 | 110 | 3.10±0.50 | <0.001 | |
| RVDd >3.3 cm (PLAX) | 50 | 23 (46) | 110 | 26 (23.6) | 0.004 | |
| LV thickness | IVSd [cm] | 50 | 1.09±0.16 | 110 | 1.06±0.19 | 0.368 |
| IVSd >1.1 cm (PLAX) | 50 | 17 (34) | 110 | 36 (32.7) | 0.874 | |
| PWd [cm] | 50 | 1.07±0.15 | 110 | 1.05±0.17 | 0.339 | |
| LV filling pressure | E/e’ | 43 | 18.2±6.4 | 94 | 14.14±5.56 | <0.001 |
| E/e’ >15 | 43 | 28 (65.1) | 94 | 35 (37.2) | 0.002 | |
Data presented as means ±SD or as a number (percentage). N-number of patients in whom a given parameter was assessed. CO – cardiac output; LV – left ventricle; LVEF – left ventricular ejection fraction; LVDd – left ventricular end diastolic diameter; PLAX – parasternal long axis; LA – left atrium; RVDd – right ventricular end diastolic diameter; IVSd – intraventricular septum in diastole; PWd – posterior wall in diastole; E/e’ – the ratio of maximum velocity of the early inflow through the mitral valve and protodiastolic mitral annulus velocity.
Comparison of groups within hemodynamic assessment using impedance cardiography
Patients with moderate and severe respiratory disorder of CSR type presented significantly lower SI and CI, higher SVRI, and higher HR (Table 5).
Table 5.
The comparison of haemodynamic parameters assessed by impedance cardiography (ICG) depending on the presence of significant Cheyne-Stokes respiration (CSR).
| Mean ±SD | Statistical significance (p) | ||
|---|---|---|---|
| Significant CSR (n=46*) | Without significant CSR (n=104*) | ||
| HR [1/min] | 67.7±11.5 | 66.0±9.6 | 0.044 |
| SBP [mm Hg] | 112.0±13.4 | 113.9±17.9 | 0.545 |
| DBP [mm Hg] | 71.6±15.0 | 70.3±10.8 | 0.513 |
| SI [ml/m2] | 34.8±7.6 | 39.8±8.1 | <0.001 |
| CI [l/min/m2] | 2.37±0.51 | 2.61±0.52 | 0.009 |
| SVRI [dyn·s·cm−5·m2] | 2768±778 | 2423±576 | 0.016 |
| TFC [1/kOhm] | 30.6±6.5 | 31.5±6.4 | 0.255 |
| TFC >35 1/kOhm | 8 (18.2) | 20 (19.2) | 0.861 |
| SI <35 ml/m2 | 24 (52.2) | 28 (26.9) | 0.003 |
| HR >70/min | 21 (45.7) | 31 (29.8) | 0.066 |
Reliable measurement of ICG was recorded in 150 patients. Data presented as means ±SD or as a number (percentage).
HR – heart rate; SBP – systolic blood pressure; DBP – diastolic blood pressure; SI – stroke index; CI – cardiac index; SVRI – systemic vascular resistance index; TFC – thoracic fluid content.
Predictors of the presence of significant CSR
In the univariate regression analysis, advanced NYHA class, permanent AF, right ventricular enlargement, LVEF <35%, E/e’ >15, and SI <35 ml/m2 proved to be predictors of presence of significant CSR (Table 6). However, only NYHA class turned out to be an independent predictor.
Table 6.
The results of uni- and multivariate logistic regression (predictors of significant CSR).
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| NYHA class (per one class) | 3.34 | 1.77–6.30 | <0.001 | 2.33 | 1.05–5.19 | 0.036 |
| Permanent AF | 2.29 | 1.14–4.63 | 0.019 | – | – | |
| LVEF < 35% | 3.38 | 1.61–7.08 | 0.001 | – | – | |
| LV enlargement (LVDd ≥6 cm; PLAX) | 2.56 | 0.98–6.70 | 0.053 | – | – | |
| RV enlargement (RVDd >3.3cm; PLAX) | 2.75 | 1.35–5.62 | 0.005 | – | – | |
| E/e’ >15 | 3.15 | 1.47–6.73 | 0.003 | – | – | |
| SI <35 ml/m2 | 2.96 | 1.43–6.14 | 0.003 | – | – | |
NYHA – New York Heart Association; AF – atrial fibrillation; LVEF – left ventricular ejection fraction; LV – left ventricle; LVDd – left ventricular end diastolic diameter; PLAX – parasternal long axis; RV – right ventricle; RVDd – right ventricular end diastolic diameter; E/e’ – the ratio of maximum velocity of the early inflow through the mitral valve and protodiastolic mitral annulus velocity; SI – stroke index.
Discussion
Our results show wide prevalence of SDB in patients with systolic HF, despite optimal drug therapy. It appears that this problem is probably not adequately appreciated in this population. Sleep apnea is rarely recognized and frequently remains untreated. The insufficient awareness of the role of SDB in the pathogenesis of HF still plays an important role. However, the limited availability of diagnostic tools also is important. Therefore, a search for simple diagnostic parameters that increase the ability to identify patients with severe CSR can provide clinical benefits. We identified some prognostic variables, including those related with anamnesis (e.g., NYHA class and atrial fibrillation), heart morphology, and hemodynamics. Our results confirm the correlation between CSA and more advanced cardiovascular dysfunction.
Clinical characteristics of the population
Demographic profile and medical history of the recruited patients were similar to those of participants of other studies, concerning SDB and HF [1–3,23]. We confirmed wide dissemination of unrecognized SDB in patients with systolic HF, despite optimal drug therapy. Significant SDB (AHI ≥15/h) occurred in 44.7% of patients and more than twice as many patients had significant CSA than had OSA. Our data are similar to those presented by Oldenburg et al. [2], who diagnosed SDB in 76% of 700 patients with HF (LVEF ≤40%), also with a higher proportion of CSA. Likewise, other authors reported similar frequencies of CSA [1,3,23].
Central sleep apnea, clinical state, and co-existing diseases
Our results confirm that CSR is strongly associated with advanced HF. Features indirectly characterizing the severity of HF (advanced NYHA class, lower SBP, a greater need for diuretics, and a proportion of ICD implantations) corresponded with the occurrence of significant CSA/CSR. The obtained observations are compatible with the results of Oldenburg et al. [2], who compared patients with CSA to those with OSA. Patients with CSA had significantly lower SBP and DBP, as well as a greater severity of symptoms according to NYHA. Javaheri et al. [1] also observed lower values of DBP in patients with CSA. However, some studies did not confirm the correlation of CSR with NYHA class [3,24].
The present study showed a strong relationship between CSR and AF. It confirms that CSA/CSR is etiologically associated with this form of arrhythmia. In a study of more than 2900 older men, a strong relationship of CSA with AF was demonstrated, and OSA was more associated with ventricular arrhythmia (bigeminy, trigeminy, and non-sustained ventricular tachycardia) [25]. Another study revealed that, even in the absence of HF, AF is very strongly associated with CSA [26]. Sin et al. [23] and Schulz et al. [3] also showed a higher incidence of AF in patients with CSA. It seems that the pathophysiological backgrounds of arrhythmia in OSA and CSA are different. OSA can lead to the occurrence of AF as a result of the dysfunction of autonomic system and increased atrial dimensions and pressure. The risk of ventricular arrhythmias related to OSA appears to result primarily from hypoxia [1,27,28]. In contrast, CSR supraventricular and ventricular arrhythmias are a priori associated with a significant impairment of myocardial function, and to a lesser extent result from the disordered breathing.
Central sleep apnea and the function of cardiovascular system
The results of our study show that the presence of CSR is associated with worse hemodynamic and morphologic parameters assessed by echocardiography and ICG. Patients with CSA had more advanced left ventricular systolic (lower LVEF) and diastolic dysfunction (higher E/e’) and larger heart chambers. Likewise, Sin et al. [23] found lower LVEF in patients with CSA than with OSA. Javaheri et al. [1] found no differences within LVEF between subjects with CSA and OSA, but in relation to those without SDB, they presented lower LVEF [1]. Those observations are also convergent with the findings of Oldenburg et al. [2].
However, it should be noted that not all researchers observed the relation of CSR with the essential LV dysfunction [3,24,29]. Schulz et al. [3] did not show any differences within LVEF among subjects with CSR in comparison to patients with OSA or no SDB. Carmona-Bernali et al. [24] did not confirm vital differences in echocardiographic parameters (LVEF, LVDd, LV end-diastolic volume, and end-systolic volume) with respect to CSR.
Impedance cardiography provided more detailed hemodynamic assessment of our patients. The presence of significant CSR was associated with worse hemodynamic parameters: lower SI and CI, and higher SVRI. The obtained results confirmed that CSA/CSR is related with impaired LV function as a blood pump, which partly explains the worse prognosis of such patients [30]. Oldenburg et al. [31] observed a correlation of AHI with pulmonary capillary wedge pressure (positive), pulmonary artery pressure (positive), and CI (negative) in patients with HF and CSA. Such correlations were not noticed in patients with OSA [31]. Giannoni et al. [32] reported that CSR may also contribute to increased pulmonary artery pressure and right chamber remodeling.
A significant cardiovascular dysfunction supports the idea of a strong relationship between HF and pathogenesis of CSA. Hyperventilation, hypocapnia, and impaired central regulation of breathing rhythm are strictly related to pulmonary circulatory overload. On the other hand, the studies of Oldenburg et al. [11] call attention to another hypothesis, that breathing disorders may be a compensation mechanism. CSR may result in a temporary increase in CI, mainly due to increasing the frequency of HR. Unfortunately, it is detrimental for a damaged heart in long-term prognosis [11]. This observation agrees with our observation of higher resting HR in the group of patients with significant CSR.
Predictors of central sleep apnea
The aggravation of HF symptoms (NYHA class) was revealed to be the only predictor of the occurrence of significant CSR independent of other variables such as permanent AF, the enlargement of LV and RV, LVEF <35%, E/e’ >15, and SI <35 ml/m2. Other researchers [23,33] also reported advanced HF, low LVEF, and AF as the predictors of the CSR. They also add hypocapnia (OR 4.33), male sex (OR 3.5), and advanced age >60 years (OR 2.37) [23]. We conclude that the core determinant of CSR is a severe myocardial dysfunction reflecting patient clinical state. However, the assessment of other cardiovascular parameters helps to objectify the reported symptoms and increases the chances to identify patients with CSR, especially in view of the fact that NYHA classification is subjective and can change over time.
Clinical implications and perspectives
The clinical importance of CSR in HF patients requires deeper knowledge and further research. As an expression of breathing control system instability, CSR seems to be a reflection of cardiac function, which was confirmed by our results. However, one cannot exclude that CSR is an accompanying disease worsening prognosis, even if activated as a mechanism compensating for co-existing hemodynamic disorders. These doubts indicate the need to consolidate principles of SDB diagnostics and therapy in HF patients. It might be expected that more detailed assessment of the influence of non-invasive ventilation methods (including adaptive servo-ventilation) on hemodynamic disorders related to CSR can contribute to the explanation of discrepancies concerning the prognosis for patients undergoing such therapy [14,17,18,34–36].
Limitations
The basic limitation of our work is the small number of subjects included, which could result in underestimation of differences within studied variables. Moreover, our group consisted mostly of men. Thus, the obtained results should not be extrapolated to women directly. Moreover, we used polygraphy to assess SDB instead of a more detailed method, polysomnography. However, polygraphy meets diagnostic requirements (high sensitivity and specificity) and was also used in many of the cited studies [2,3,37]. The more frequent use of diuretics in patients with significant CSR should be interpreted carefully in view of the fact that we did not provide detailed data regarding classes of diuretics and average doses. We are also aware that more detailed assessment, including of some laboratory tests (e.g., natriuretic peptides) and cardiopulmonary exercise test would complement our methodology.
Conclusions
The extended non-invasive diagnostics confirmed the relationship of CSR with severe cardiovascular alterations and functional state. A detailed hemodynamic assessment may be useful in identification of CSR, but the aggravation of HF symptoms (NYHA class) seems to be the strongest predictor. Changes in hemodynamic profile can be tested as surrogate markers of effectiveness of SDB-specific treatment in future studies.
Footnotes
Source of support: This publication was partly supported by the National Centre of Science (grant no. NN 402489840) and the Ministry of Science and Higher Education/Military Institute of Medicine (grant no. 213/WIM)
Conflict of interest
The authors report no conflicts of interest to disclose.
References
- 1.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–59. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–57. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Blau A, Börgel J, et al. Sleep apnoea in heart failure. Eur Respir J. 2007;29:1201–5. doi: 10.1183/09031936.00037106. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, et al. Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016 doi: 10.1002/ejhf.592. pii: ehw128. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Naughton M, Benard D, Tam A, et al. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–38. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19:37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- 8.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–54. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 9.Francis DP, Willson K, Davies LC, et al. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–21. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 10.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–40. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg O, Spießhöfer J, Fox H, et al. Cheyne-Stokes respiration in heart failure: friend or foe? Hemodynamic effects of hyperventilation in heart failure patients and healthy volunteers. Clin Res Cardiol. 2015;104:328–33. doi: 10.1007/s00392-014-0784-1. [DOI] [PubMed] [Google Scholar]
- 12.Naughton MT. Cheyne-Stokes respiration: friend or foe? Thorax. 2012;67:357–60. doi: 10.1136/thoraxjnl-2011-200927. [DOI] [PubMed] [Google Scholar]
- 13.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10:581–86. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Oldenburg O, Bitter T, Lehmann R, et al. Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol. 2011;100:107–15. doi: 10.1007/s00392-010-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasai T, Usui Y, Yoshioka T, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3:140–48. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 16.Koyama T, Watanabe H, Kobukai Y, et al. Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ J. 2010;74:2118–24. doi: 10.1253/circj.cj-10-0082. [DOI] [PubMed] [Google Scholar]
- 17.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: Practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson AL. The AASM manual for scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. pp. 45–47. [Google Scholar]
- 20.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 21.Echocardiography in clinical practice – Echocardiography Standards Section Polish Cardiology Society 2007. Kardiol Pol. 2007;65:1142–62. [PubMed] [Google Scholar]
- 22.Płońska-Gościniak E. Kompendium echo. Warsaw: Medical Tribune Poland; 2011. [in Polish] [Google Scholar]
- 23.Sin DD, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 24.Carmona-Bernal C, Ruiz-García A, Villa-Gil M, et al. Quality of life in patients with congestive heart failure and central sleep apnea. Sleep Med. 2008;9:646–51. doi: 10.1016/j.sleep.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–55. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung RS, Huber MA, Rogge T, et al. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28:1543–46. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 27.Fichter J, Bauer D, Arampatzis S, et al. Sleep-related breathing disorders are associated with ventricular arrhythmias in patients with an implantable cardioverter-defibrillator. Chest. 2002;122:558–61. doi: 10.1378/chest.122.2.558. [DOI] [PubMed] [Google Scholar]
- 28.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 29.Skobel E, Norra C, Sinha A, et al. Impact of sleep-related breathing disorders on health-related quality of life in patients with chronic heart failure. Eur J Heart Fail. 2005;7:505–11. doi: 10.1016/j.ejheart.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Ng ACC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev. 2009;14:89–99. doi: 10.1007/s10741-008-9096-8. [DOI] [PubMed] [Google Scholar]
- 31.Oldenburg O, Bitter T, Wiemer M, et al. Pulmonary capillary wedge pressure and pulmonary arterial pressure in heart failure patients with sleep-disordered breathing. Sleep Med. 2009;10:726–30. doi: 10.1016/j.sleep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Giannoni A, Raglianti V, Mirizzi G, et al. Influence of central apneas and chemoreflex activation on pulmonary artery pressure in chronic heart failure. Int J Cardiol. 2016;202:200–6. doi: 10.1016/j.ijcard.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Sharma B, Owens R, Malhotra A. Sleep in congestive heart failure. Med Clin North Am. 2010;94:447–64. doi: 10.1016/j.mcna.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 35.Randerath WJ, Nothofer G, Priegnitz C, et al. Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest. 2012;142(2):440–47. doi: 10.1378/chest.11-2089. [DOI] [PubMed] [Google Scholar]
- 36.Sharma BK, Bakker JP, McSharry DG, et al. Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: A systematic review and meta-analysis. Chest. 2012;142(5):1211–21. doi: 10.1378/chest.12-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintana-Gallego E, Villa-Gil M, Carmona-Bernal C, et al. Home respiratory polygraphy for diagnosis of sleep-disordered breathing in heart failure. Eur Respir J. 2004;24:443–48. doi: 10.1183/09031936.04.00140603. [DOI] [PubMed] [Google Scholar]


