Abstract
Rhodacyclopentanones, an “sp3-rich” class of metallacycle, underpin an emerging range of catalytic methodologies for the direct generation of complex scaffolds. This review highlights strategies for accessing rhodacyclopentanones (and related species) by C-C bond activation of cyclobutanones or cyclopropanes. The scope and mechanism of methodologies that exploit these activation modes is outlined.
Introduction
Synthetic strategies that provide chiral “sp3 rich” molecular scaffolds are highly desirable to medicinal chemists.1 To address this, numerous research groups have focused on the development of protocols to activate and functionalise “traditionally inert” C(sp3)-H bonds.2 While progress in this area has been rapid, the realisation of complementary processes that exploit the activation of C-C bonds, which are also found in almost all organic molecules, has proven more challenging.3 However, as highlighted in this article, recent advances in C-C bond activation have led to catalytic protocols that provide direct access to challenging chiral ring systems, thereby illustrating the potential for this activation mode to access underexplored chemical space.
Key challenges for developing methodology based on C-C bond activation are a) the high activation barrier for metal insertion into C-C bonds (cf. C-H bonds)4 and b) control of regioselectivity in the C-C bond oxidative addition step. A common strategy for overcoming the barrier to oxidative addition is to use highly strained 3- and 4-membered cyclic systems for catalysis initiation; here, relief of ring strain drives insertion of the metal catalyst. Alternatively, coordination of the metal catalyst to an appropriate directing group can enhance both the rate of oxidative addition and the regioselectivity of this step.5 Although chelation-assisted metal insertion has been exploited for the activation of both strained and unstrained C-C bonds,3,5,6 protocols involving strained systems are far more extensive.
Rhodacyclopentanones provide a versatile catalysis platform for an emerging range of C-C bond activation triggered methodologies. Although the first report of an isolable rhodacyclopentanone complex dates back to the 1960s,7 the reactivity of these Rh(III)-metallacycles has only been exploited in reaction design within the last 20 years. In this context, rhodacyclopentanones represent an “sp3-rich” class of metallacyclic intermediate that is capable of delivering an array of complex molecular scaffolds. In particular, rhodacyclopentanones participate efficiently in cycloaddition reactions with various π-unsaturated components. Recently, there have been significant and impressive developments in cycloaddition methodologies of this type. Herein, strategies for accessing rhodacyclopentanones (and related species) will be highlighted, and the scope and mechanism of representative synthetic methodologies will be discussed. Note that hydroacylative and oxidative coupling approaches to rhodacyclopentanones are not covered in this Feature Article.8
Synthesis of rhodacyclopentanones
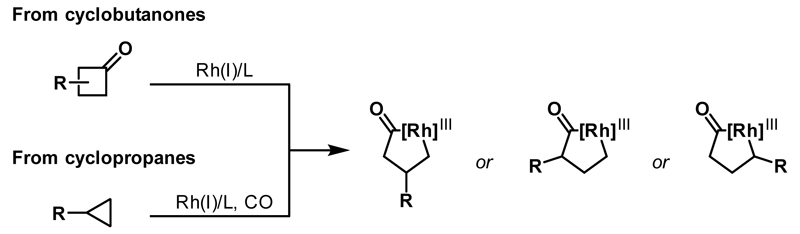
Rhodacyclopentanones can be generated catalytically by C-C bond activation of either cyclobutanone or cyclopropane precursors (Scheme 1). In the former case, Rh(I)-catalysts preferentially undergo oxidative addition into the acyl-C(sp3) bond of cyclobutanones to deliver rhodacyclopentanones. For cyclopropanes, generation of the Rh(III)-metallacycle requires migratory insertion of a CO ligand, thereby necessitating the use of a carbonylative atmosphere for catalytic transformations. In both cases, insertion of the Rh(I)-catalyst into substituted ring systems can potentially deliver a number of regioisomeric rhodacyclopentanones. The selectivity of C-C bond activation can be influenced by both steric and electronic factors, with chelation-assisted metal insertion often enabling precise control of regioselectivity.
Scheme 1.
Generation of rhodacyclopentanones from cyclobutanones or cyclopropanes.
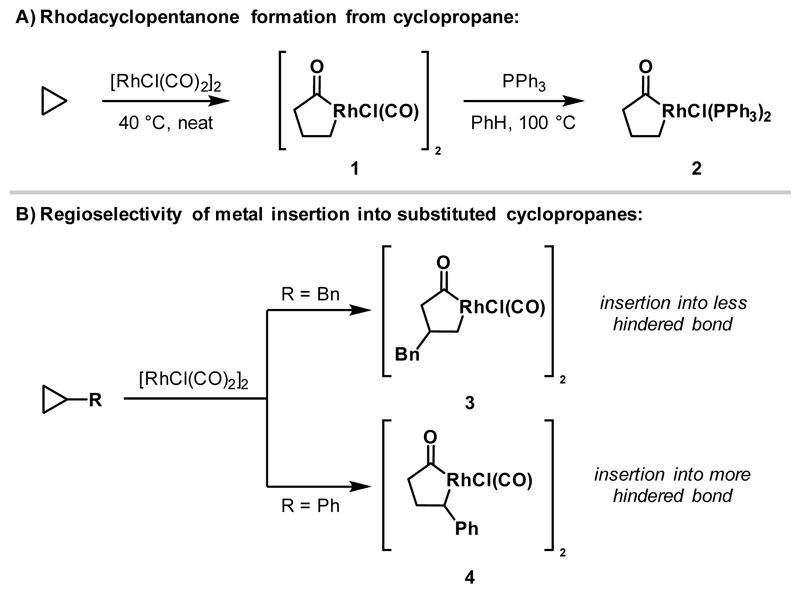
In 1968, Wilkinson and co-workers reported that exposure of cyclopropane to a CO-ligated Rh(I)-catalyst resulted in ring expansion to deliver dimeric rhodacyclopentanone 1 (Scheme 2A).7 Upon treatment with PPh3, the CO and bridging Cl ligands were displaced and monomeric phosphine-bound rhodacyclopentanone 2 was generated. Subsequently, McQuillin and Powell examined the effect of cyclopropane substitution on the regioselectivity of rhodacyclopentanone formation (Scheme 2B).9 Exposure of benzyl cyclopropane to [RhCl(CO)2]2 resulted in Rh-insertion into the less hindered C-C bond to deliver rhodacycle 3. In contrast, phenyl cyclopropane afforded dimeric rhodacycle 4, derived from insertion into the more hindered cyclopropane C-C bond, demonstrating that electronic activation can override steric factors. Stoichiometric carbonylative ring expansions of other cyclopropane-containing structures, such as quadricyclene,10 3,5-dehydronoriceane,11 and dibenzosemibullvalene,12 to rhodacyclopentanones have also been reported.
Scheme 2.
Stoichiometric Rh(I)-mediated ring expansions of cyclopropanes to generate rhodacyclopentanones.
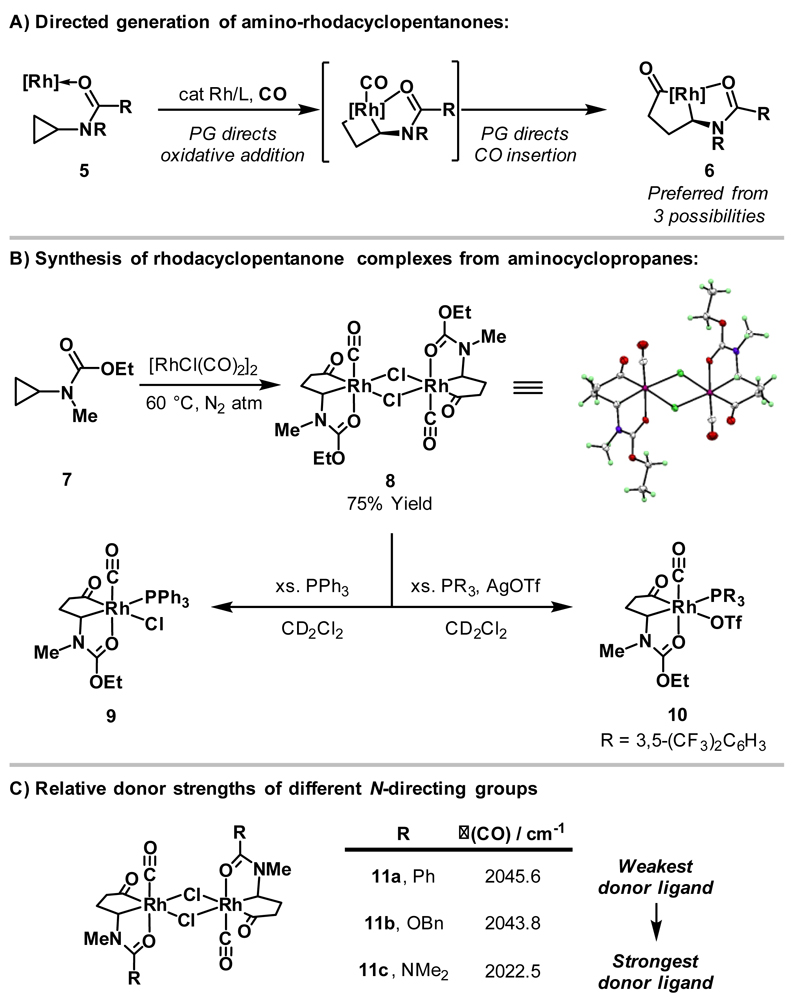
In 2013, our group reported a strategy for accessing amino-rhodacyclopentanones from carbamate-substituted aminocyclopropanes.13 Here, the carbonyl directing/protecting group of 5 directs metal insertion into the more hindered cyclopropane C-C bond and, in addition, directs migratory insertion of the CO ligand to deliver amino-rhodacyclopentanone 6 selectively (Scheme 3A). Exposure of carbamate-substituted cyclopropane 7 to [RhCl(CO)2]2 resulted in regioselective formation of dimeric rhodacycle 8, the structure of which was confirmed by X-ray diffraction; this was the first X-ray crystal structure of a rhodacyclopentanone complex derived from carbonylative cyclopropane ring expansion.14 Note that earlier studies by Chirik and co-workers had shown that strongly coordinating phosphinites can direct Rh(I)-catalysts into the more hindered C-C bond of tethered cyclopropanes, albeit under non-carbonylative conditions.15
Scheme 3.
Synthesis and evaluation of carbonyl-protected amino-rhodacyclopentanone complexes.
As mentioned previously, Wilkinson and co-workers reported that exposure of dimeric rhodacycle 1 to excess PPh3 delivered complex 2 with two phosphine ligands bound to the Rh-centre (Scheme 2A).7 In contrast, treatment of rhodacycle 8 with excess PPh3 delivered monomeric complex 9 bound to a single phosphine ligand, such that coordination of the directing group is maintained (Scheme 3B).16 Formation of monomeric cationic Rh(III)-complex 10 was accomplished by treatment of 8 with P(3,5-(CF3)2C6H3)3 and AgOTf.17 Subsequently, a series of dimeric rhodacyclopentanone complexes (11a-c) containing different carbonyl protecting/directing groups were synthesised, with the stretching frequency of the CO ligand trans to the directing group allowing quantification of its donor strength (Scheme 3C). The following ranking was determined: urea>>carbamate>amide, and this data was used to guide subsequent methodology development.16,17
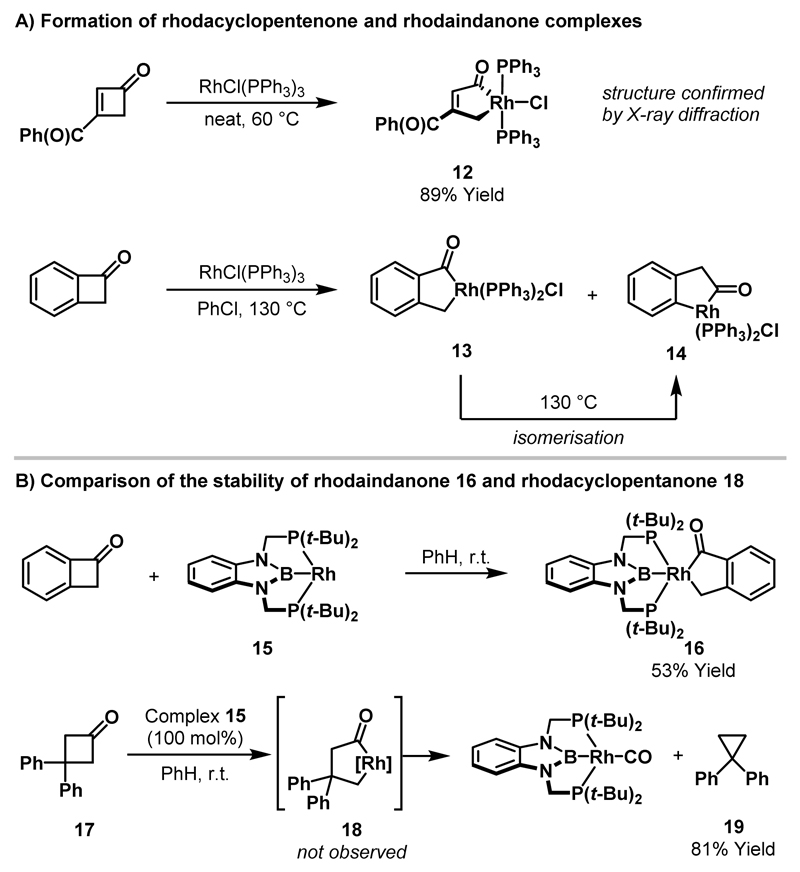
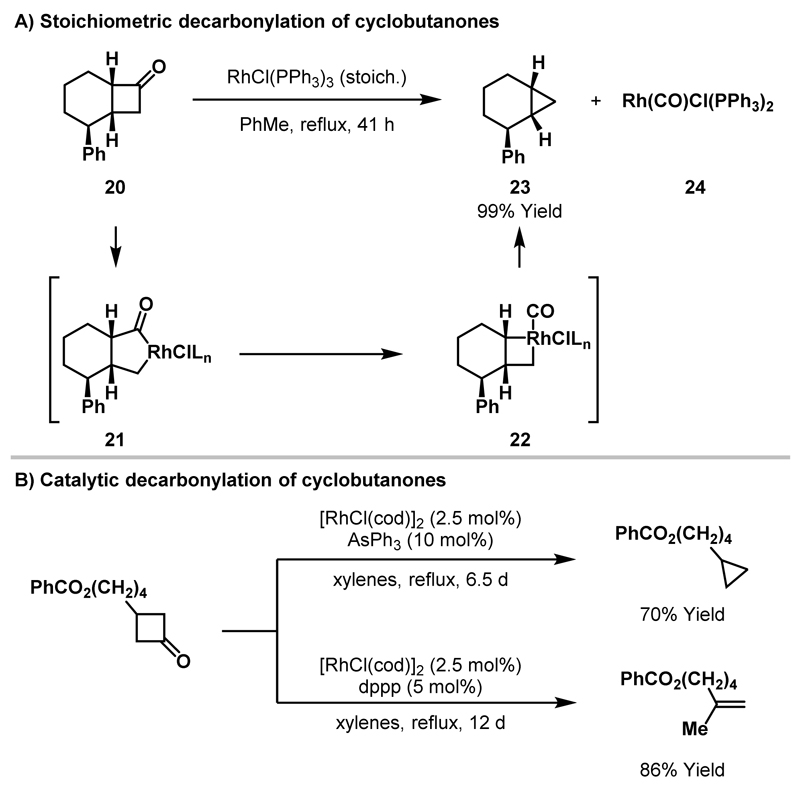
Stoichiometric reactions between cyclobutanones and Rh(I)-complexes to deliver isolable rhodacyclopentanones have not been achieved. However, insertion of Rh(I)-catalysts into cyclobutenones and benzocyclobutenone, to deliver rhodacyclopentenones and rhodaindanones respectively, was reported by Liebeskind and co-workers in 1992 (Scheme 4A).18 In contrast with (η5-C9H7)Co(PPh3)2, which delivered η4-vinylketene complexes, RhCl(PPh3)3 underwent insertion into substituted cyclobutenones to deliver stable rhodacyclopentenone complexes; the structure of rhodacycle 12 was confirmed by X-ray diffraction. Exposure of benzocyclobutenone to Wilkinson’s catalyst delivered a mixture of rhodaindanones 13 and 14, resulting from activation of the acyl-C(sp3) bond and acyl-C(sp2) bond, respectively. Upon heating, conversion of 13 to 14 was observed, which indicates that rhodaindanone 14 arises via isomerisation of kinetically favoured rhodaindanone 13. Subsequent computational studies support isomerisation by way of a retrocarbonylation-recarbonylation mechanism.19 Recently, Murakami and co-workers reported an X-ray crystal structure of rhodaindanone 16 (Scheme 4B).20 By utilising the bulky electron-rich Rh-catalyst 15, insertion into benzocyclobutenone proceeded at room temperature to deliver a single rhodaindanone regioisomer 16. Attempts to isolate an analogous intermediate derived from cyclobutanone 17 were unsuccessful, with the putative rhodacyclopentanone 18 undergoing rapid decarbonylation to cyclopropane 19.
Scheme 4.
Stoichiometric Rh(I)-mediated ring expansions of benzocyclobutenone and cyclopentenones.
Reactivity of rhodacyclopentanones: decarbonylative transformations
In 1994, Murakami and Ito reported the first example of Rh-mediated C-C bond activation of cyclobutanones.21 Here, stoichiometric Wilkinson’s catalyst was employed to effect decarbonylation of cyclobutanone 20 to cyclopropane 23 in 99% yield (Scheme 5A). Oxidative addition of the Rh(I)-catalyst into the less hindered acyl-C(sp3) bond of 20 delivers rhodacyclopentanone 21, which extrudes CO prior to reductive elimination of 22 to yield cyclopropane 23. The requirement for stoichiometric RhCl(PPh3)3 was postulated to result from the formation of catalytically inactive CO-ligated Rh(I)-complex 24. The ring contraction of larger cyclic ketones was also reported, although in these cases the efficiency was significantly reduced (cyclobutanone: 41 h, 99% yield vs. cyclopentanone: 8 days, 57% yield).
Scheme 5.
Rh(I)-mediated decarbonylation of cyclobutanones.
In a subsequent report, Murakami and Ito demonstrated that decarbonylation of cyclobutanones could be rendered catalytic by utilising chelating or electron-deficient monodentate ligands on the Rh-catalyst (Scheme 5B).22 Cyclopropane and alkene decarbonylation products were both observed and the product selectivity could be controlled by judicious choice of ligand. Alkene products, derived from β-hydride elimination and reductive elimination of the intermediate rhodacyclobutane (cf. 22), were favoured by the use of wide bite angle ligands (e.g. dppp and dppb) with a neutral Rh(I)-catalyst. Alternatively, by employing AsPh3, an electron-deficient monodentate ligand, the selectivity of the reaction could be switched to favour formation of the cyclopropane product.
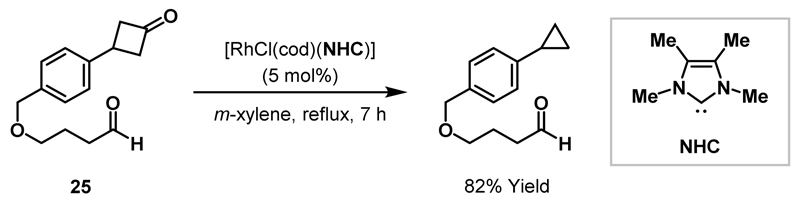
Murakami and co-workers highlighted the importance of ligand choice in Rh-mediated C-C bond activation protocols by demonstrating that an NHC-ligated Rh(I)-catalyst could selectively decarbonylate cyclobutanones in the presence of aldehydes (Scheme 6).23 Transition metal-catalysed activation of aldehydic C-H bonds has been demonstrated to be a relatively facile process24 and other phosphine-ligated Rh(I)-catalysts exhibited either no selectivity or selectivity towards the aldehyde in the decarbonylation of substrate 25. As previously discussed, Murakami and co-workers later reported that Rh-catalyst 15 inserts into cyclobutanones at room temperature to deliver cyclopropane products (see Scheme 4B).20 Related decarbonylation reactions typically require high temperatures (cf. Scheme 5, 110-140 °C), however the highly electron-donating ligand system of 15 promotes C-C bond activation under relatively mild reaction conditions.
Scheme 6.
Selective decarbonylation of a cyclobutanone in the presence of an aldehyde.
Multicomponent decarbonylative transformations involving rhodacyclopentanone intermediates have not been reported, however, protocols involving related rhodacyclopentenones and rhodaindanones have been developed. In 2004, Mitsudo, Kondo and co-workers described direct and decarbonylative cycloadditions of alkyl-substituted cyclobutenones and 2-norbornene upon exposure to [RhCl(CO)2]2 (Scheme 7).25 For both transformations, one feasible mechanistic pathway involves insertion of the Rh(I)-catalyst into the acyl-C(sp3) bond of the cyclobutenone to generate rhodacyclopentenone intermediate 26 directly. An alternate possibility involves rhodacyclopentenone formation via initial ring opening of the cyclobutenone to vinyl ketene intermediate 27.
Scheme 7.
Direct and decarbonylative cycloadditions of cyclobutenones.
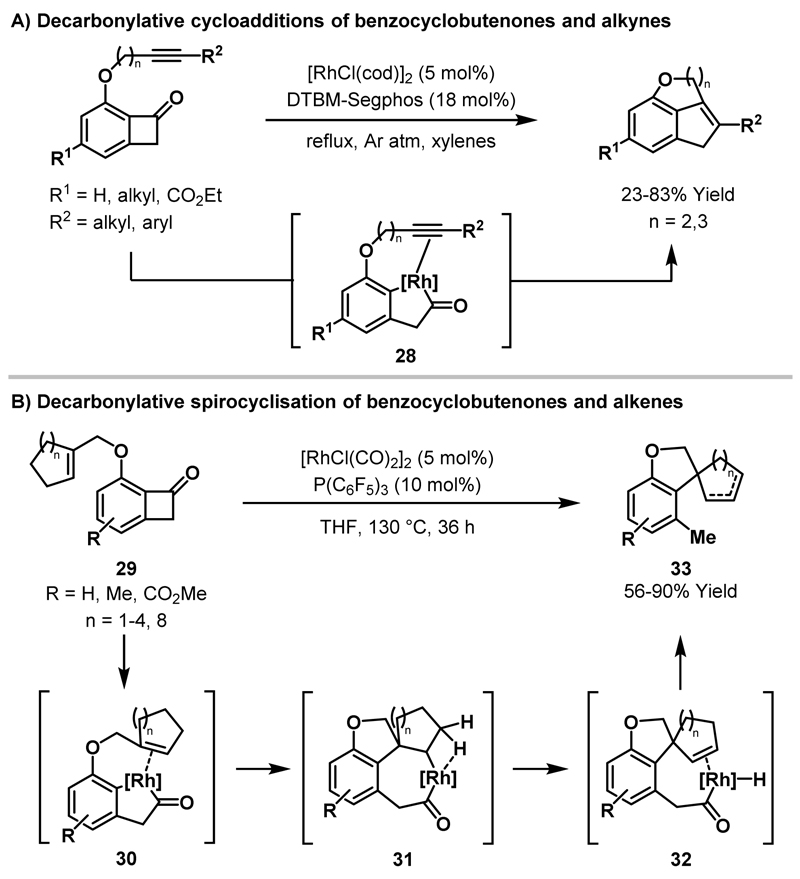
Recently, Dong and co-workers showed that rhodaindanones 28, generated from benzocyclobutenones, undergo intramolecular decarbonylative cycloadditions with tethered alkynes to deliver fused indane ring systems in moderate to good yields (Scheme 8A).26 Subsequent studies revealed that intramolecular decarbonylative couplings of benzocyclobutenones with tethered trisubstituted alkenes (29) proceed via a mechanistically distinct pathway to deliver spirocyclic products 33 (Scheme 8B).27 In the proposed mechanism, initial C-C bond activation of benzocyclobutenone 29 delivers the expected rhodaindanone intermediate 30 (cf. 28), which engages the alkene to deliver rhodacycle 31. At this stage, β-hydride elimination occurs to give acyl-Rh-hydride species 32 and, upon decarbonylation and reductive elimination, spirocyclic product 33 is formed. In some cases, isomerisation of the alkene was observed and experimental mechanistic studies suggested that the two products are generated via independent reaction pathways, rather than by post-reaction isomerisation. Subsequent computational studies implicated acyl-Rh-hydride complex 32 as a key intermediate in the alkene isomerisation pathway.28.
Scheme 8.
Decarbonylative transformations involving rhodaindanones.
Reactivity of rhodacyclopentanones: carbonylative ring expansions of cyclopropanes
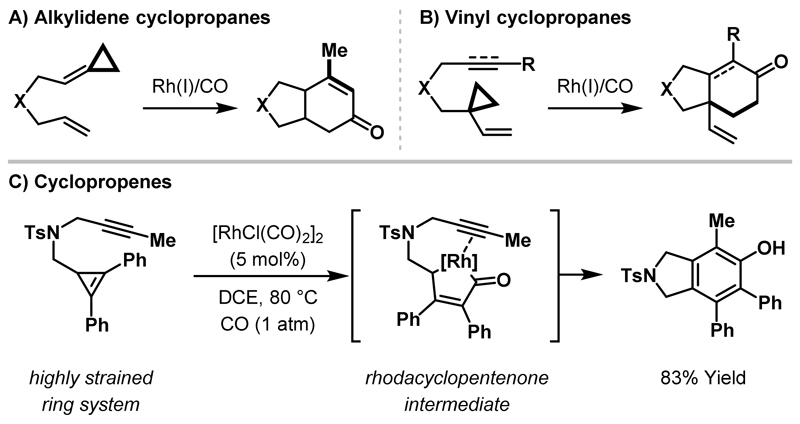
Carbonylative ring expansions of simple cyclopropanes to rhodacyclopentanones using stoichiometric Rh(I)-complexes were reported over 40 years ago,7,9 however, until recently, there were few reports of catalytic ring expansions based on this activation mode. In contrast, there have been numerous reports of cycloadditions involving cyclopropane derivatives activated by internal or adjacent unsaturation, such as alkylidene cyclopropanes, vinyl cyclopropanes and cyclopropenes (Scheme 9). For transformations utilising alkylidene cyclopropanes as a three-carbon component, the alkylidene carbon generally contributes to the three-carbon unit in the new ring system (vs. the cyclopropane delivering all three carbons).29 Accordingly, carbonylative cycloadditions of alkylidene cyclopropanes are not proposed to proceed via the formation of a rhodacyclopentanone intermediate (Scheme 9A).30 Vinyl cyclopropanes have been shown to undergo carbonylative couplings with tethered π-unsaturates, however, the vinyl cyclopropane is typically incorporated as a five-carbon unit.31 Yu et al. reported (3+1+2) carbonylative cycloadditions that utilise the vinyl cyclopropane moiety as a three-carbon unit but a rhodacyclopentanone intermediate was not implicated in the mechanistic pathway (Scheme 9B).32 In 2010, Wang and co-workers demonstrated that cyclopropenes were competent coupling partners for carbonylative (3+1+2) cycloadditions with tethered alkenes and alkynes (Scheme 9C).33 The increased ring strain of cyclopropenes (vs. cyclopropanes) renders them highly reactive towards metal insertion34,35 and the ring expansion protocol was proposed to proceed via a rhodacyclopentenone intermediate.
Scheme 9.
Reactivity of activated cyclopropane derivatives in Rh-catalysed cycloadditions.
Two component ring expansions of cyclopropanes
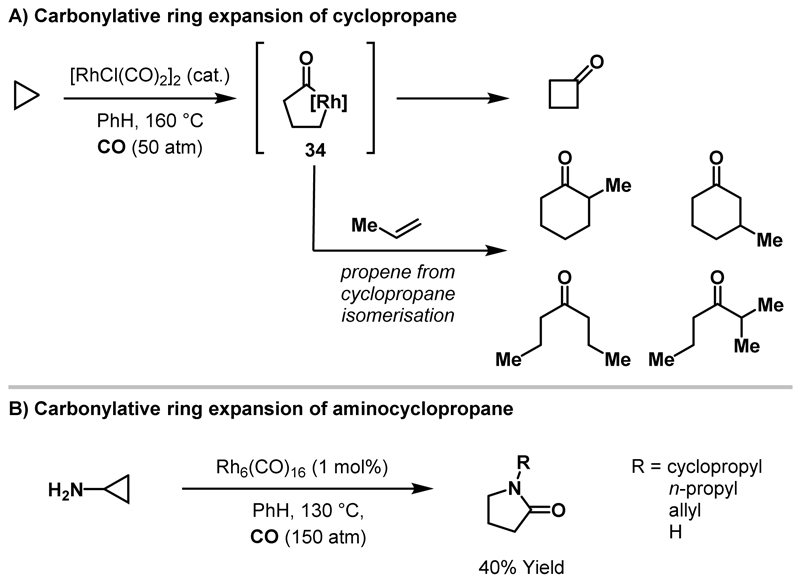
The catalytic carbonylative ring expansion of unsubstituted cyclopropane was reported by Uchida and co-workers in 1980 (Scheme 10A).36 Under forcing reaction conditions, exposure of cyclopropane to [RhCl(CO)2]2 afforded a mixture of cyclic and acyclic ketones in low yield (<5% yield for each product). In addition, formation of propene was observed and this likely derives from β-hydride elimination of a rhodacyclobutane intermediate (cf. Scheme 5B). The simplest ring expansion product, cyclobutanone, results from reductive elimination of rhodacyclopentanone 34. The higher order ketone products are proposed to form via migratory insertion of propene into rhodacyclopentanone 34, however, the source of reductant for the acyclic ketone products was not identified. Under similarly forcing reaction conditions, Iqbal reported a Rh-catalysed C-C bond activation of aminocyclopropane to deliver a mixture of N-substituted γ-lactams in moderate yield.37 Although not proposed, it is not unreasonable to implicate the intermediacy of a rhodacyclopentanone in this process.
Scheme 10.
Carbonylative ring expansions of cyclopropane and aminocyclopropane.
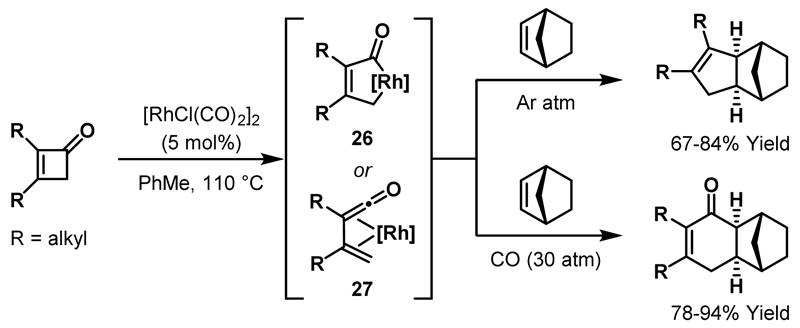
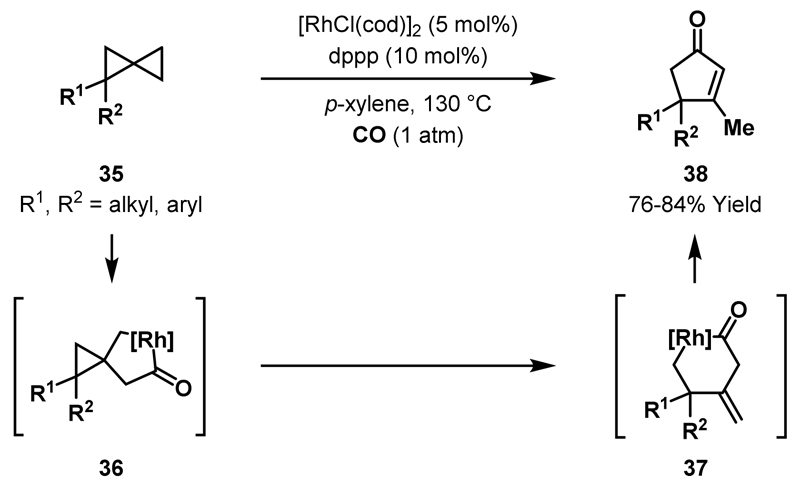
Murakami and co-workers demonstrated that spiropentanes exhibit enhanced reactivity towards C-C bond activation, presumably due to the increased ring strain of the spirocyclic system. Rh-catalysed carbonylative ring expansions of spiropentanes 35 delivered cyclopentenones 38 in high yields (Scheme 11).38 The proposed mechanism involves oxidative addition of a Rh(I)-catalyst into the least hindered C-C bond of 35, followed by migratory insertion of a CO ligand to furnish rhodacyclopentanone 36. Regioselective β-carbon elimination affords 6-membered rhodacycle 37, which can undergo reductive elimination and isomerisation to provide cyclopentenone 38. The regioselectivity of β-carbon elimination was diminished when mono-substituted spiropentanes were employed and, consequently, mixtures of isomeric cyclopentenone products were obtained (see Scheme 23B). Notably, C-C bond activation of alkyl-substituted cyclopropanes was not observed under the same reaction conditions.
Scheme 11.
Carbonylative rearrangements of spiropentanes.
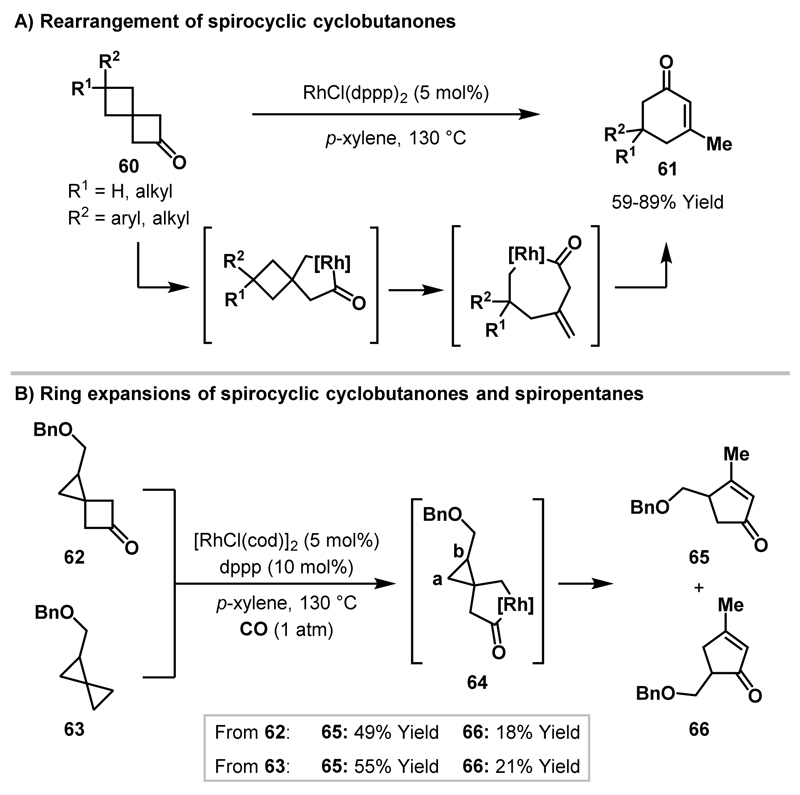
Scheme 23.
Ring expansions of spirocyclic cyclobutanones and spiropentanes.
Three component ring expansions of cyclopropanes
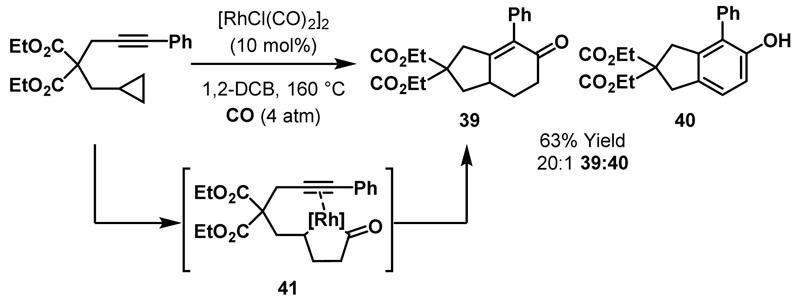
Intramolecular cycloadditions between catalytically generated rhodacyclopentanones and π-unsaturates have enabled access to complex carbocyclic and heterocyclic scaffolds. Within this area, processes involving the carbonylative generation of rhodacyclopentanones (i.e. Rh + CO + cyclopropane) remain challenging. Narasaka and Koga reported the formation of bicyclic cyclohexenones (e.g. 39) and phenols (e.g. 40) from cyclopropanes bearing a tethered alkyne (Scheme 12).39 This carbonylative cycloaddition was proposed to proceed via rhodacyclopentanone intermediate 41, which derives from insertion of the Rh(I)-catalyst into the more hindered C-C bond of the cyclopropane. In this process, oxidative addition of the Rh(I)-catalyst may be directed by the alkyne. The yield of carbocyclic products 39 and 40 improved under elevated pressures of CO (43% yield at 1 atm vs. 63% yield at 4 atm) as the formation of by-products (e.g. alkenes) derived from non-carbonylative pathways was disfavoured. Substitution on the alkyne tether and cyclopropane ring was tolerated and the substituted cyclohexenones were obtained in moderate yield.
Scheme 12.
(3+1+2) cycloaddition involving a cyclopropane, carbon monoxide and an alkyne.
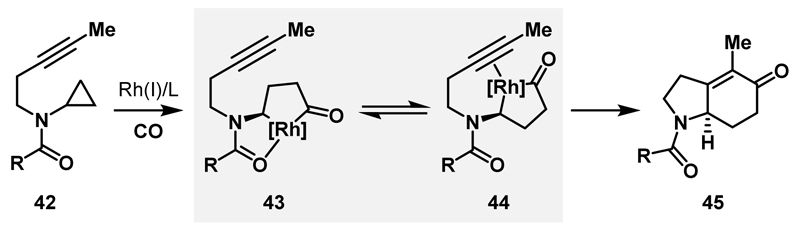
In 2013, our group demonstrated that regioselective generation of amino-rhodacyclopentanones from aminocyclopropanes can be achieved by utilising an N-protecting group directed C-C bond activation strategy (see Scheme 3).13 The ability to access these metallacyclic intermediates in an efficient and selective manner provides a catalytic platform for the synthesis of chiral N-heterocyclic scaffolds. Carbonylative (3+1+2) cycloadditions involving aminocyclopropanes, CO and alkynes to deliver N-heterobicyclic enones were investigated as a proof-of-principle transformation. As shown in Scheme 13, the proposed mechanism involves the directed carbonylative ring expansion of 42 to deliver rhodacyclopentanone 43. Directing group dissociation (to 44), migratory insertion of the alkyne and C-C bond reductive elimination then affords the desired N-heterocycle 45.
Scheme 13.
Multicomponent carbonylative ring expansions of aminocyclopropanes
Preliminary studies highlighted the importance of selecting an appropriate N-directing group as it must a) compete with the alkyne for coordination of the catalyst at the stage of 42 but b) be labile enough to allow alkyne coordination at the stage of rhodacycle 43. As previously discussed, stoichiometric studies demonstrated that amide, carbamate and urea directing groups were all capable of delivering the desired rhodacycle, however, for the outlined cycloaddition, considerably higher efficiencies were observed when the most strongly donating dimethylurea directing group was employed (vs. amide or carbamate variants, see Scheme 3C). These results indicate that a strong directing group is required to outcompete the alkyne for coordination of the Rh(I)-catalyst prior to oxidative addition.
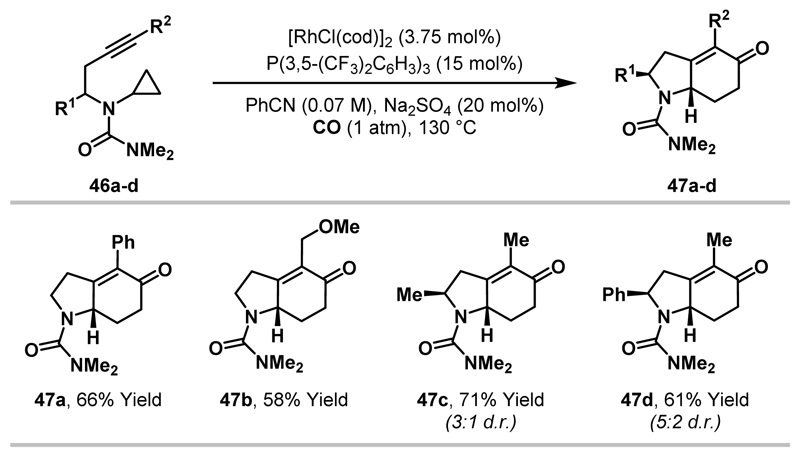
Under optimised reaction conditions, employing a neutral Rh(I)-catalyst, urea-substituted aminocyclopropanes 46 were converted to N-heterobicyclic enones 47 in moderate to good yield (Scheme 14).13 Aryl- and alkyl-substituted alkynes participated to deliver the desired N-heterocycles (47a-d). In addition, products of greater stereochemical complexity were accessed by employing substrates with substitution on the alkyne tether. In these cases, increased cyclisation efficiencies were observed to deliver 47c-d with moderate levels of diastereoselectivity.
Scheme 14.
Urea-directed (3+1+2) cycloadditions between aminocyclopropanes, CO and tethered alkynes.
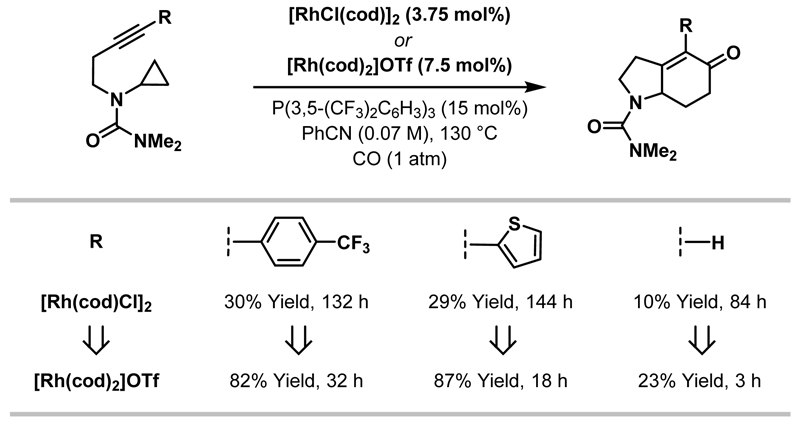
Further investigations revealed that cationic Rh(I)-systems provide higher yields and enhanced reaction rates for challenging cycloadditions.16 However, in order to achieve this beneficial effect coordinating solvents are required to stabilise the Rh-catalyst, with benzonitrile identified as the optimal choice.40 As shown in Scheme 15, by using a cationic Rh(I)-system, significantly improved reaction efficiencies were observed for terminal alkynes and those with electron-deficient or heteroaromatic substituents.
Scheme 15.
Comparison of “neutral” and “cationic” conditions for (3+1+2) cycloadditions involving functionalised alkynes.
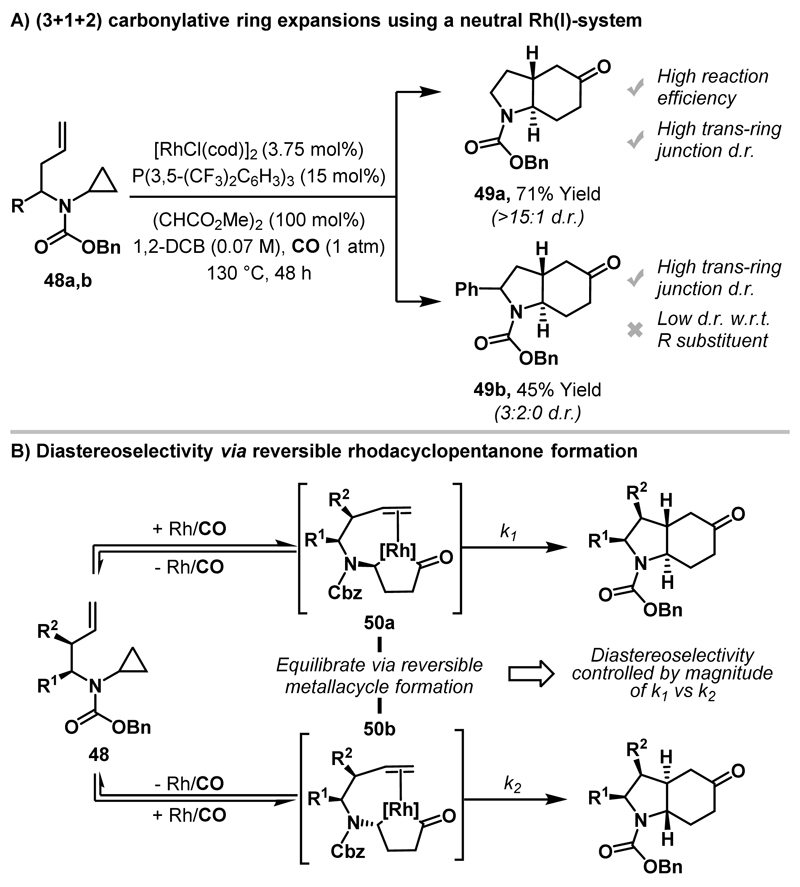
Subsequent studies established that synthetically flexible directing groups could be used to generate heterocycles with higher “sp3-content”. Indeed, Cbz-directed (3+1+2) cycloadditions involving aminocyclopropanes, CO and alkenes afforded stereochemically complex N-heterocycles (i.e. 49) in an efficient manner (Cbz = carboxybenzyl).17 In preliminary work, it was found that a neutral Rh(I)-system provided high yield and diastereoselectivity for the cyclisation of 48a to 49a (Scheme 16A). However, for substrates with substitution on the alkene tether (e.g. 48b, R = Ph) little diastereocontrol with respect to the R substituent was observed (3:2 d.r.), even though high trans-diastereoselectivity at the ring junction was maintained. This result was not surprising because if carbonyl-directed oxidative addition is the diastereo-determining step, then high diastereocontrol requires the R substituent to bias Rh-insertion into one of the two proximal diastereotopic cyclopropane C-C bonds, an aspect which at the outset appeared challenging. To circumvent this, a strategy that achieves high levels of diastereocontrol via reversible rhodacyclopentanone formation was established, as depicted in Scheme 16B. Here, non-diastereoselective oxidative addition into cyclopropane 48, leads to diastereomeric rhodacyclic π-complexes 50a and 50b. At this stage, because these structures are bicyclic, the steric effects of the R1 and R2 substituents are magnified such that the rate of alkene insertion (k1 and k2) from 50a and 50b is different. Consequently, in a scenario where rhodacyclopentanone formation is rapid and reversible, product diastereoselectivity should be principally dependent on the relative rates of alkene insertion (k1 vs. k2).
Scheme 16.
(3+1+2) carbonylative cycloadditions between aminocyclopropanes and tethered alkenes, and a strategy for achieving high diastereoselectivity.
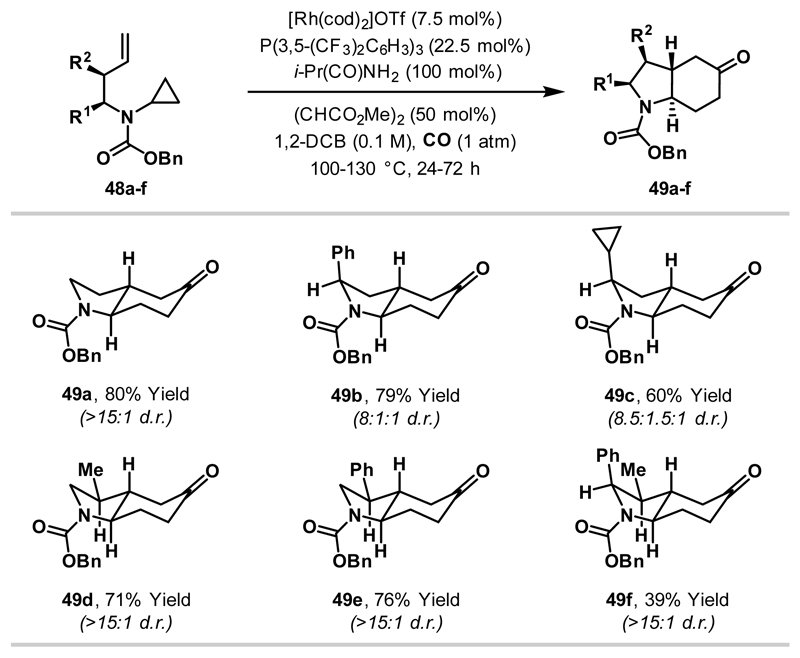
The realisation of the strategy outlined in Scheme 16B was achieved by switching to a cationic Rh(I)-system. Here, an additional vacant coordination site on the catalyst may enhance reversibility by facilitating retro-carbonylation from 50a/b. Due to the instability of cationic Rh(I)-systems, optimisation was highly challenging. However, it was eventually found that the use of [Rh(cod)2]OTf, in conjunction with a coordinating additive (isobutyramide), enabled highly diastereoselective cyclisation of substrate 48b (79% yield, 8:1:1 d.r.) (Scheme 17). Under these reaction conditions, a broad array of substrates with alkyl and aryl substituents at R1 and/or R2 cyclised in good yield and with excellent diastereoselectivity. Note that even cyclopropane substituents are tolerated (as in 49c).
Scheme 17.
Diastereoselective (3+1+2) cycloadditions between aminocyclopropanes, CO and tethered alkenes.
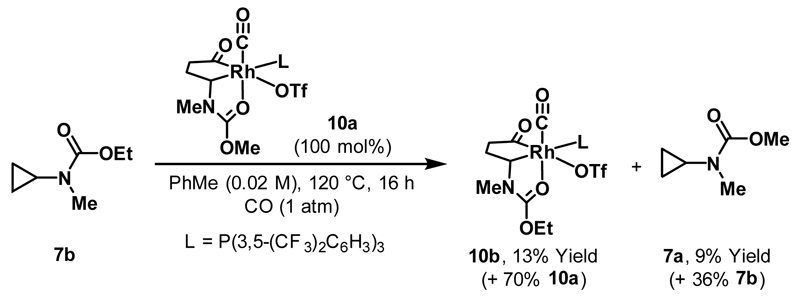
Exchange studies supported the “reversibility” proposition outlined in Scheme 16B (Scheme 18).17 Model cationic rhodacyclopentanone complex 10a, which possesses a methyl carbamate directing group, was exposed to stoichiometric quantities of ethyl carbamate protected cyclopropane 7b under a CO atmosphere. This resulted in partial exchange to deliver rhodacycle 10b and cyclopropane 7a. An inverse experiment, utilising complex 10b, delivered analogous results. Complex 10a was also shown to be catalytically competent for the conversion of 48a to 49a. These studies demonstrated that reversible rhodacyclopentanone formation can occur under carbonylative conditions.
Scheme 18.
Stoichiometric reversibility studies.
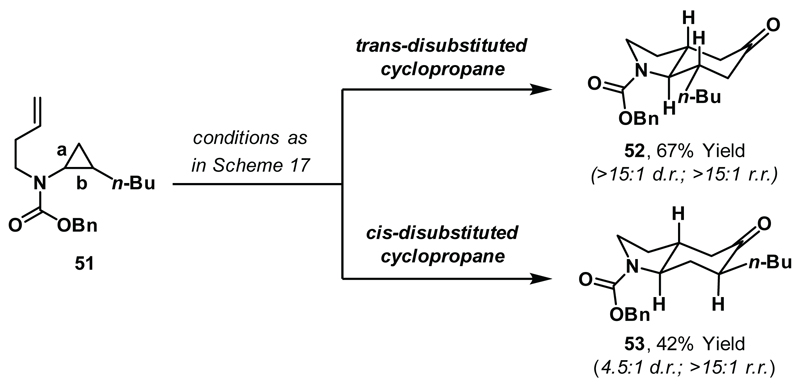
Catalytic carbonylative ring expansions of cyclopropanes are particularly attractive as substituted (and enantioenriched) cyclopropanes can be readily synthesised using existing technologies.41 Exposure of trans-1,2-disubstituted cyclopropane 51 to optimised reaction conditions afforded adduct 52 in good yield. 52 derives from regioselective Rh-insertion into the less hindered proximal C-C bond of the cyclopropane (bond a) and complete transfer of the cyclopropane stereochemistry was observed (Scheme 19). In contrast, cis-1,2-disubstituted cyclopropane 51 generated solely regioisomer 53, which derives from Rh-insertion into the more hindered proximal C-C bond (bond b). Stoichiometric experiments confirmed, in both cases, that product regioselectivity reflects the regiochemical preference of rhodacyclopentanone formation.17,42
Scheme 19.
Cycloadditions of trans- and cis-1,2-disubstituted cyclopropanes.
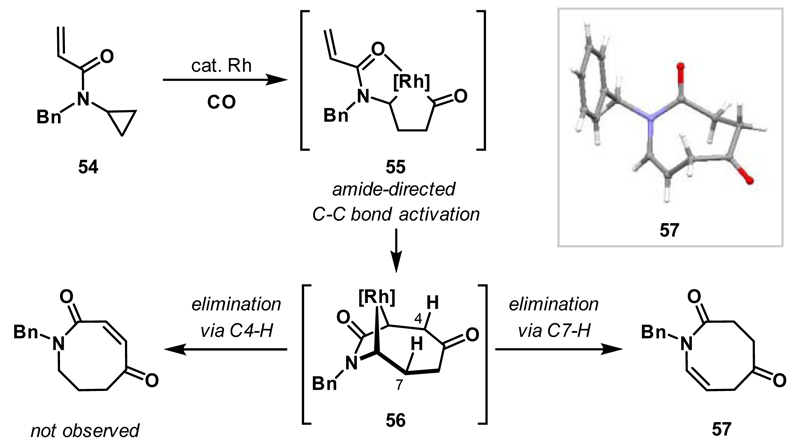
Amino-rhodacyclopentanones can also be utilised as intermediates in mechanistically distinct cycloaddition-fragmentation processes. Using this approach, a direct and modular entry to azocanes 57 from N-cyclopropylacrylamides 54 was developed, via a formal (7+1) cycloaddition-tautomerisation sequence (Scheme 20).42 Amide-directed C-C bond activation of 54 delivers rhodacyclopentanone 55, and, from here, migratory insertion of the alkene affords rhodabicycle 56. At this point, instead of C-C reductive elimination (cf. Scheme 13), β-hydride elimination of C7-H and C-H reductive elimination affords azocane 57. This latter step is highly regioselective and products derived from β-hydride elimination via C4-H are not generated. Deuterium labelling studies support the proposed mechanism.
Scheme 20.
Rh-catalysed cycloaddition-fragmentation for the synthesis of azocanes.
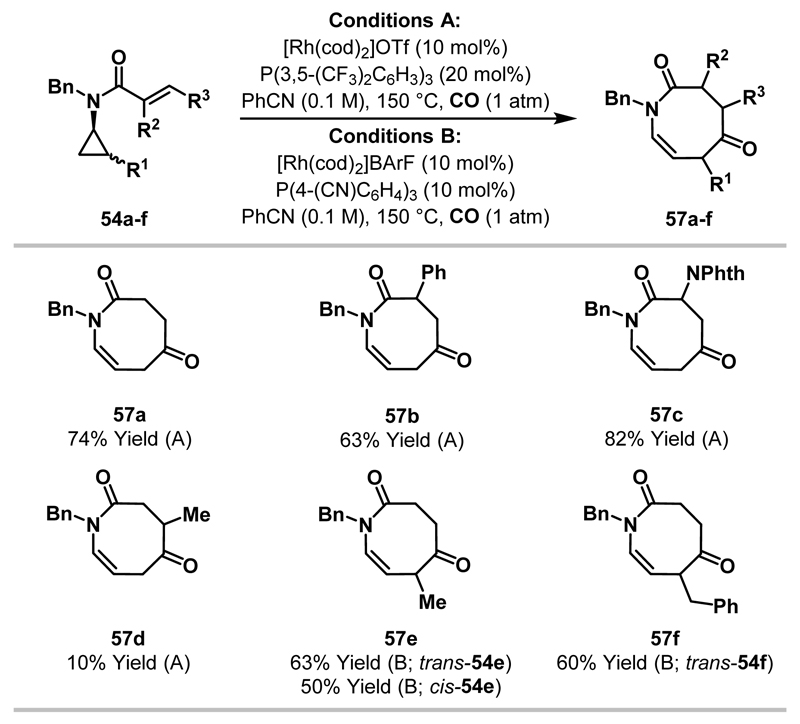
The scope of the azocane methodology is outlined in Scheme 21. Under “conditions A”, 1,1-disubstituted alkenes cyclised efficiently to afford R2-substituted azocanes, however, 1,2-disubstituted alkenes were not well tolerated (e.g. 57d, 10% yield). Note that the azocane products are highly strained, possessing an unusual twisted enamide (see X-ray crystal structure, Scheme 20). Cycloadditions involving 1,2-disubstituted aminocyclopropanes required alternate conditions (“conditions B”), but proceeded smoothly. Interestingly, both cis-54e and trans-54e afforded the same Me-substituted azocane 57e. Adduct 57e arises from insertion of Rh and CO into the more hindered proximal C-C bond of 54e. This result was expected for cis-54e as it mirrored C-C bond activation regioselectivities observed in earlier work (see Scheme 19). However, the conversion of trans-54e to 57e was inconsistent with stoichiometric studies and earlier work where trans-1,2-disubstituted cyclopropanes delivered products derived solely from Rh-insertion into the less hindered proximal C-C bond.
Scheme 21.
Rh-catalysed carbonylative cycloadditions of N-cyclopropylacrylamides to deliver substituted azocanes.
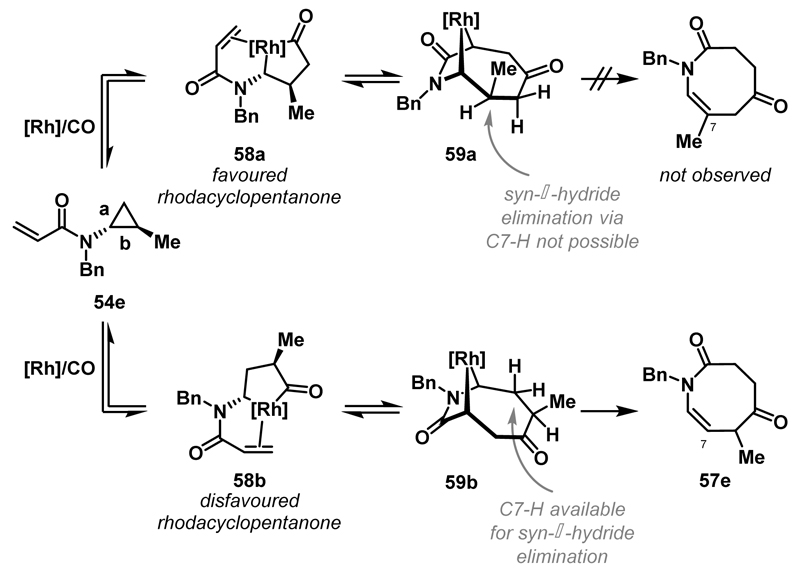
The exchange studies in Scheme 18 show that rhodacyclopentanone formation is reversible using cationic Rh(I)-systems under a CO atmosphere. This observation underpins a mechanism that accounts for the regioselectivity of cyclisations of trans-1,2-disubstituted cyclopropanes to azocanes (Scheme 22). Insertion of Rh and CO can occur into bond a or b of 54e to generate either rhodacyclopentanone 58a or 58b. The former pathway is favoured on steric grounds, but, following migratory insertion of the alkene into the acyl-Rh bond, syn-β-hydride elimination via C7-H of 59a is not possible. If the alkene insertion step is also reversible, then cyclopropane 54e can be regenerated, and equilibration to regioisomeric metallacycle 59b is possible. Here, syn-β-hydride elimination via C7-H can occur to deliver the observed regioisomer of the product. An alternate possibility is that alkene insertion into 58a is slower than into 58b, such that it is the facility of this step (rather than the β-hydride elimination step) that controls product regiochemistry.
Scheme 22.
Mechanistic rationale for the regioselectivity of cycloadditions involving trans-disubstituted aminocyclopropanes.
Reactivity of rhodacyclopentanones: ring expansions of cyclobutanones
Catalytic activation of cyclobutanone C-C bonds was first reported by Murakami and co-workers in 1994.21 Since this seminal publication, a variety of synthetic transformations have been developed that involve rhodacyclopentanones generated via Rh-insertion into cyclobutanones. In particular, cycloadditions with 2π-unsaturates have gained considerable attention as complex molecular scaffolds can be readily accessed using this approach. Additionally, protocols have been developed involving subsequent nucleophilic trapping or β-carbon/β-oxygen elimination steps. Oxidative addition of Rh(I)-catalysts into cyclobutanones generally occurs at the weaker acyl-C(sp3) bond rather than the comparatively stronger C(sp3)-C(sp3) bond (see Scheme 26 for an exception to this rule). In the absence of coordinating functionality, Rh-insertion into unsymmetrical cyclobutanones occurs at the less hindered acyl-C(sp3) bond.22 However, directed oxidative addition of Rh(I)-catalysts into the more hindered acyl-C(sp3) bond has been demonstrated in a number of multicomponent ring expansion protocols.
Scheme 26.
Ligand-dependent insertion of Rh(I)-complexes into cyclobutanone C-C bonds.
In their initial report, Murakami and Ito showed that activation of the cyclobutanone acyl-C(sp3) bond could be combined with hydrogenolysis of the resultant rhodacyclopentanone to deliver alcohols.21,22 In subsequent studies, two different modes of C-C bond activation – cyclobutanone C-C bond oxidative addition and β-carbon elimination – were used in sequence to generate cyclohexenones 61 from spirocyclic cyclobutanones 60 (Scheme 23A).43 Notably, upon exposure to carbonylative conditions, spirocyclic cyclobutanone 62 and spiropentane 63 delivered identical mixtures of regioisomeric cyclopentenone products (65 and 66), thereby indicating that a common rhodacyclopentanone intermediate (64) can be generated from either starting material.38 The formation of two isomeric cyclopentenone products was observed because, upon generation of rhodacyclopentanone 64, subsequent β-carbon elimination can proceed through migration of either cyclopropane carbon a or b. The major product derives from migration of the less hindered cyclopropane carbon a (cf. Scheme 11). Prior to these studies, Liebeskind and co-workers reported a Rh-catalysed ring expansion of cyclobutenones with a pendant cyclopropane ring to deliver 7-membered cyclic ketones and this rearrangement likely proceeds via a similar oxidative addition/β-carbon elimination mechanism.44
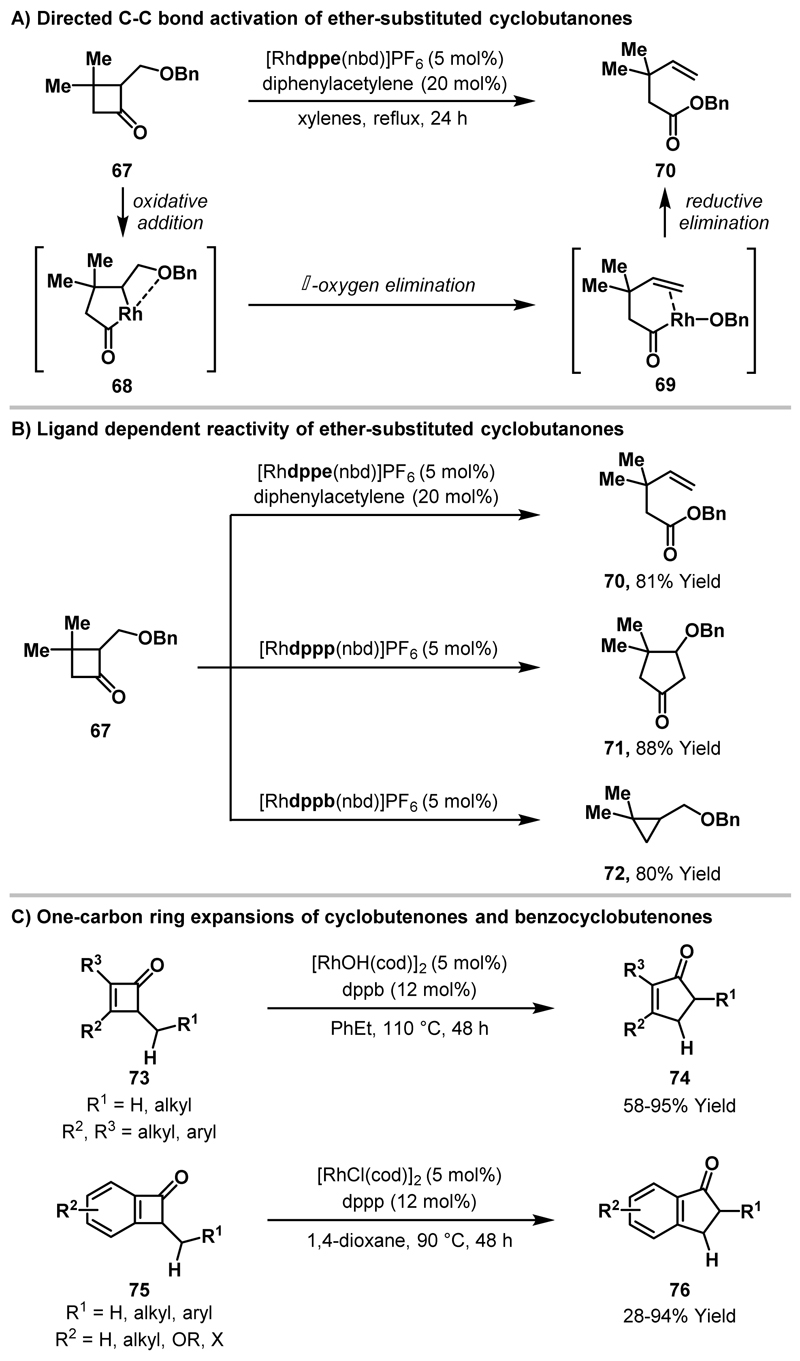
Murakami and Ito demonstrated that cyclobutanone C-C bond activation could also be used in conjunction with β-oxygen elimination to provide access to novel reactivity manifolds.45 When ether-substituted cyclobutanone 67 was exposed to the cationic Rh(I)-catalyst system outlined in Scheme 24A, formation of acyclic ester 70 was observed. 70 derives from Rh-insertion into the more hindered cyclobutanone C-C bond, which indicates that the pendant ether can coordinate and direct oxidative addition. In the proposed mechanism, β-oxygen elimination of rhodacyclopentanone 68 delivers alkene 69 and subsequent acyl-O reductive elimination can then proceed to afford the observed ester 70. Interestingly, the bite angle of the bidentate phosphine ligand had a significant impact on the reaction pathway (Scheme 24B). Selective formation of cyclopentanone 71 was observed when a wider bite angle ligand, dppp (91°), was employed in place of dppe (85°).46 Increasing the bite angle of the ligand further, to dppb (98°), resulted in selective decarbonylation of rhodacyclopentanone 68 to afford cyclopropane 72. For the dppp catalyst system it was postulated that, instead of reductive elimination at the stage of complex 69, 6-endo migratory insertion of the alkene into the Rh-O bond occurs to deliver cyclopentanone 71 upon reductive elimination. Recent studies by Dong and co-workers demonstrated that related one-carbon ring expansions of cyclobutenones 73 and benzocyclobutenones 75 afford the homologated cyclopentenone and 1-indanone products (74 and 76) in moderate to good yield.47 Mechanistic studies supported a similar reaction pathway to that proposed for the formation of 71, wherein β-hydride elimination occurs (rather than β-oxygen elimination, cf. Scheme 24A) prior to alkene migratory insertion and reductive elimination.
Scheme 24.
Rh-catalysed reactions of ether-substituted cyclobutanone 67 and one-carbon ring expansions of cyclobutenones/benzocyclobutenones.
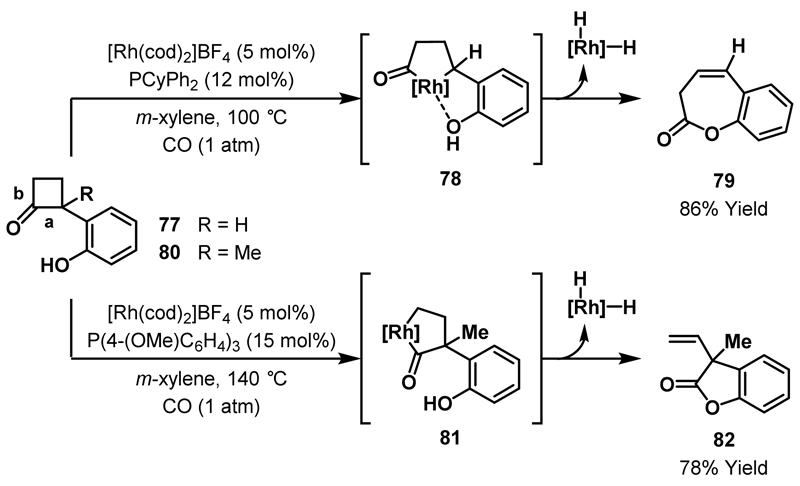
In a subsequent study, Murakami and Ito established that pendant phenols could also direct insertion of Rh(I)-catalysts into the more hindered C-C bond of cyclobutanones (Scheme 25).48 Distinct reactivity was observed in the oxidative ring expansions of phenol-substituted cyclobutanones 77 and 80. When R = H, directed oxidative addition of the Rh(I)-catalyst into the more hindered cyclobutanone C-C bond a delivers rhodacycle 78. Subsequent nucleophilic trapping with the phenol OH, followed by β-hydride elimination and reductive elimination, affords 7-membered cyclic lactone 79. However, when R = Me, the additional steric hindrance around the proximal acyl-C(sp3) bond prevents directed Rh-insertion from occurring. Consequently, insertion takes places at the less hindered bond b to afford ultimately 5-membered cyclic lactone 82 (via 81). Directed ring expansion of 77 proceeded at 100 °C but, at this temperature, no reaction was observed for cyclobutanone 80 and the reaction temperature had to be raised to 140 °C to enable efficient formation of lactone 82. The difference in reactivity between cyclobutanones 77 and 80 highlights the powerful impact directing group assistance can have on C-C bond activation protocols.
Scheme 25.
Synthesis of cyclic lactones via ring expansion of phenol-substituted cyclobutanones.
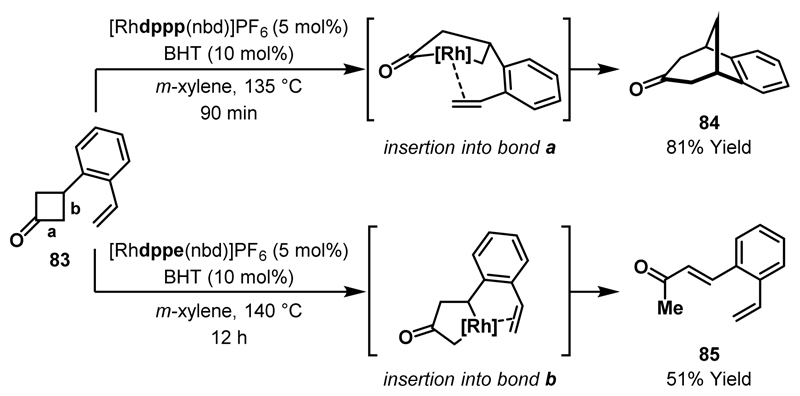
In 2002, Murakami reported the first examples of alkene insertion into cyclobutanone-derived rhodacyclopentanones (Scheme 26).49 Cyclobutanone 83, substituted with an appropriately positioned alkene, underwent an intramolecular (4+2) cycloaddition to afford bicyclic ketone 84. The ring expansion is proposed to proceed via Rh(I)-insertion into the acyl-C(sp3) bond a, followed by alkene insertion and reductive elimination. As previously observed (see Scheme 24), the bite angle of the bidentate phosphine ligand had a substantial impact on the outcome of the reaction. Employing dppe (in place of dppp) resulted in formation of α,β-unsaturated ketone 85, which derives from insertion of the Rh(I)-catalyst into the normally unreactive C(sp3)-C(sp3) bond b of the cyclobutanone. It was postulated that alkene-directed oxidative addition facilitated this unusual reactivity and, in support of this hypothesis, it was observed that extension of the alkene tether prevented Rh-insertion into bond b.
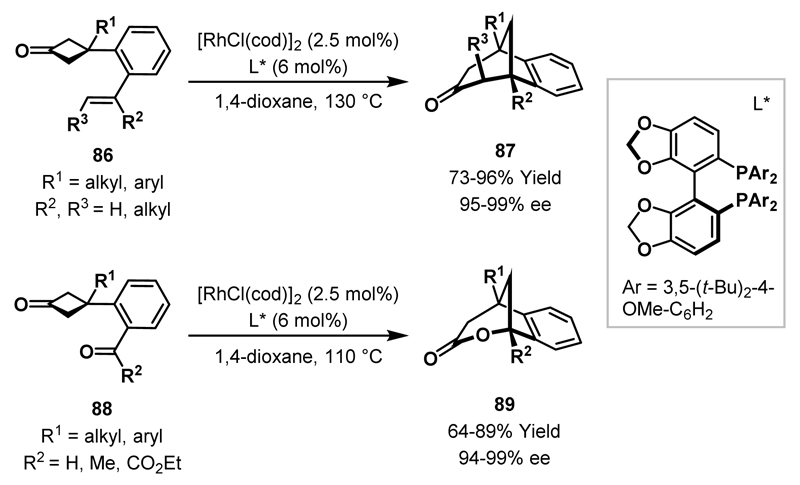
Recently, Cramer and co-workers reported enantioselective Rh-catalysed ring expansions of cyclobutanones, utilising substrates similar to those reported by Murakami (cf. 83, see Scheme 26).50,51 An additional substituent (R1) was incorporated at the 3-position of the cyclobutanone ring (86) and, consequently, cyclisation delivered complex unsymmetrical bicyclic ketones 87. Cramer demonstrated that chiral bisphosphine-ligated zwitterionic Rh(I)-complexes provided significantly enhanced efficiencies in comparison with phosphine-ligated cationic Rh(I)-complexes, however, only moderate enantioselectivities were obtained (42-87% ee).50 In a subsequent publication, a neutral DTBM-Segphos-ligated Rh(I)-catalyst system was disclosed which delivered bicyclic ketones 87 in good to excellent yields and with high levels of enantiocontrol (Scheme 27).51 In this transformation, oxidative addition of the Rh(I)-catalyst into the cyclobutanone acyl-C(sp3) bond is the enantiodetermining step. Bicyclic lactones 89 were synthesised in a highly enantioselective manner by extending the strategy to cycloadditions of cyclobutanones bearing tethered carbonyl groups.52 High yields were obtained when aldehyde substrates (88, R2 = H) were exposed to the optimised reaction conditions and, interestingly, this Rh(I)-catalyst system exhibited complete selectivity for C-C bond activation over insertion into the aldehydic C-H bond. Only activated or relatively unhindered ketones participated and, when sterically bulky ketones were employed, decarbonylation of the cyclobutanone starting material was observed.
Scheme 27.
Enantioselective cycloadditions of rhodacyclopentanones with pendant alkenes or aldehydes/ketones.
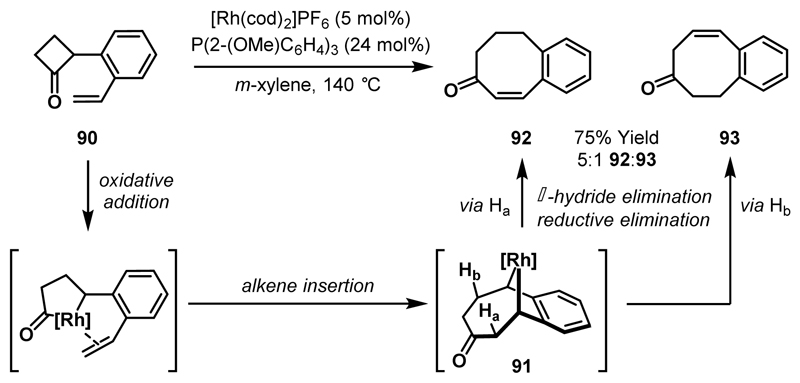
Murakami and co-workers reported that placement of a styryl group at the 2-position of cyclobutanone, rather than the 3-position (cf. Scheme 26), resulted in formation of eight-membered carbocycles 92 and 93 (Scheme 28).53 In the proposed mechanism, the pendant alkene directs Rh-insertion to the more hindered acyl-C(sp3) bond of cyclobutanone 90 and migratory insertion of the alkene into the acyl-Rh bond delivers 91. At this point, there are two possible pathways for β-hydride elimination, from Ha or Hb, with the major product arising from β-hydride elimination via Ha to give conjugated enone 92.
Scheme 28.
Rh-catalysed cycloaddition-fragmentation to deliver eight-membered carbocycles.
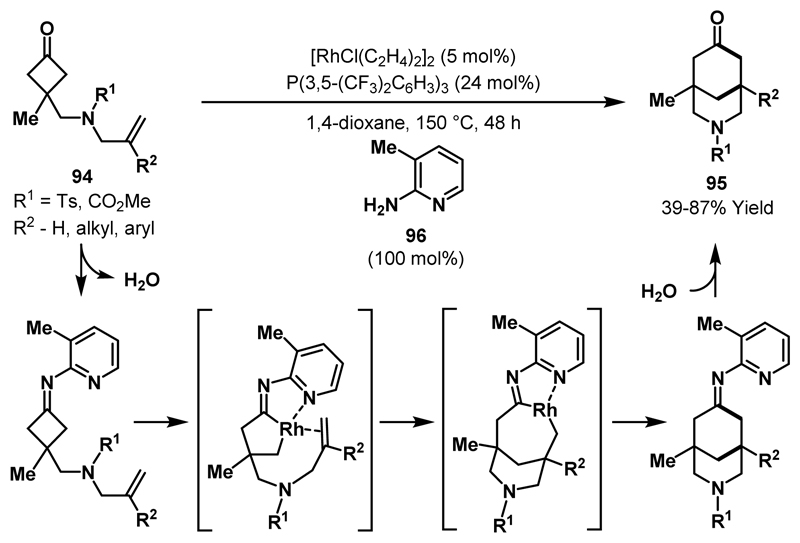
In 2014, the Dong group reported Rh(I)-catalysed (4+2) cycloadditions of cyclobutanones 94 to N-heterobicyclic ketones 95 by utilising a temporary directing group strategy (Scheme 29).54 Previous reports by Jun and co-workers demonstrated that 2-amino-3-methylpyridine 96 can be used to facilitate Rh-mediated C-C bond activation of unstrained ketones.5c,d In these protocols, pyridine 96 is postulated to condense with the ketone starting material and direct insertion into the proximal C-C bond through coordination of the pyridyl moiety to the Rh-catalyst. For the transformation outlined in Scheme 29, it was proposed that 96 a) directs Rh-insertion into the α-bond of the generated imine and b) acts as a protecting group to prevent detrimental decarbonylative side reactions from occurring. Under optimised reaction conditions, a variety of cyclobutanone substrates containing mono- and 1,1-disubstituted alkenes cyclised in moderate to good yield. In support of the proposed role of pyridine 96 as a protecting group, it was observed that, in the absence of 96, only decarbonylated side products were formed.
Scheme 29.
(4+2) cycloadditions of cyclobutanones via a temporary directing group strategy.
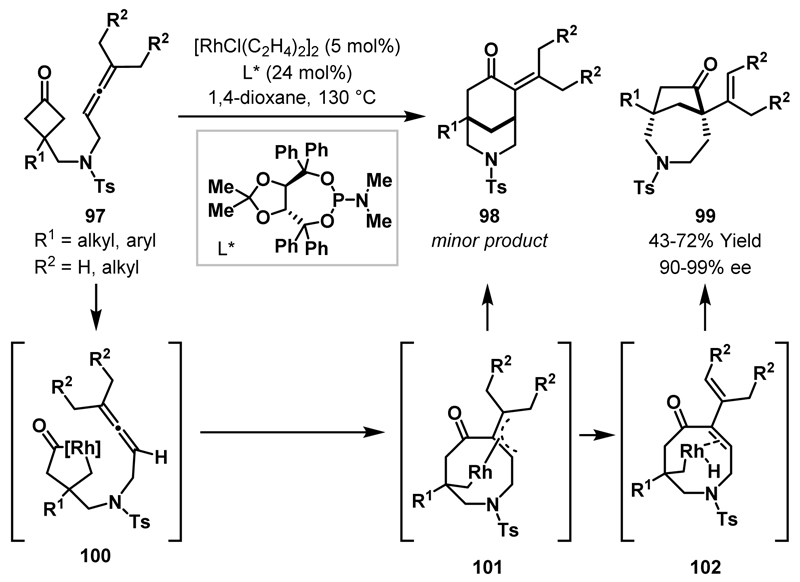
In subsequent work, the Dong group endeavoured to expand this (4+2) cycloaddition protocol to cyclobutanones 97 containing a tethered allene.55 However, interestingly, the (4+2) cycloaddition product 98 was only formed in small quantities and N-heterobicyclic ketone 99 was the major product isolated (Scheme 30). Evaluation of the reaction scope, utilising an electron-deficient achiral phosphine ligand, showed generally high selectivity for the [4.2.1] bicycle 99 over the [3.3.1] adduct 98. Subsequently, an enantioselective variant of the reaction was developed and, using a TADDOL-derived phosphoramidite ligand (L*), high enantioselectivities and moderate to good yields were obtained for bicyclic products 99. The proposed mechanism for this transformation starts with generation of rhodacyclopentanone 100, followed by migratory insertion of the allene to give π-allyl complex 101. From intermediate 101, reductive elimination would result in formation of the minor product 98. Alternatively, β-hydride elimination delivers enone 102 and, after re-insertion of the alkene and reductive elimination, this pathway affords the observed product 99. Deuterium labelling studies supported the proposed mechanism.
Scheme 30.
(4+1) cycloadditions of cyclobutanones with tethered allenes.
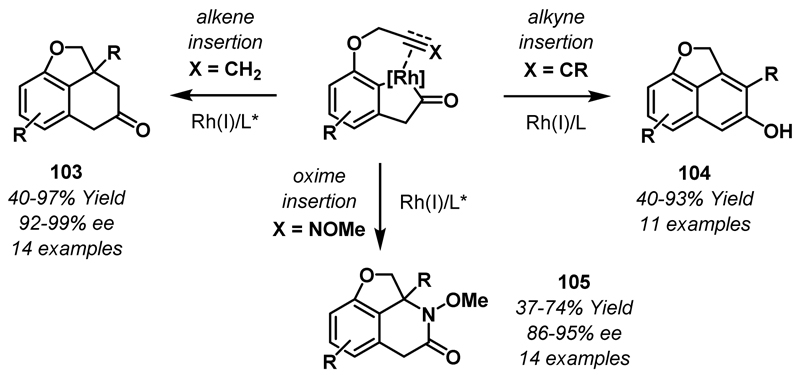
As previously mentioned, seminal work by Liebeskind and co-workers demonstrated that related rhodaindanone complexes could be generated via C-C bond activation of benzocyclobutenones (see Scheme 4A).18 Recently, the Dong group has developed new methodologies that enable rapid access to complex chiral tricyclic scaffolds via the catalytic generation and trapping of rhodaindanones with various 2π-unsaturates (Scheme 31). The first example of this “cut and sew” strategy involved intramolecular coupling of rhodaindanones with mono- or di-substituted alkenes to deliver tricyclic products 103.56 An enantioselective variant of this reaction was developed and, subsequently, this methodology was extended to cycloadditions involving trisubstituted alkenes.57,58 Intramolecular cycloadditions with alkynes and oximes have also been developed and these protocols provide rapid access to phenols 104 and cyclic lactams 105, respectively.26,59
Scheme 31.
Rh-catalysed cycloadditions involving rhodaindanones.
Conclusions
This Feature Article highlights the synthesis and reactivity of rhodacyclopentanones (and related species) derived from C-C bond activation. Predominant methodologies access these reactive intermediates by oxidative addition of Rh(I)-catalysts into cyclobutanones or cyclopropanes. The former approach avoids the requirement of a CO atmosphere, whereas the latter method takes advantage of readily available and stereodefined cyclopropane precursors. For both strategies, C-C oxidative addition regioselectivity can often be controlled using directing groups. In several methodologies the directing group (e.g. phenol, alkyne) inserts into the incipient metallacycle to provide the target product. An alternate, and perhaps more powerful approach, uses N-protecting groups or temporary directing groups to control the C-C activation step. The rhodacyclopentanone intermediates can engage a wide range of π-unsaturates (e.g. alkynes, alkenes, aldehydes) or nucleophiles (e.g. phenols) to provide the target product.
Future development of the area will require an expansion of the range of components (e.g. π-unsaturates, nucleophiles) that rhodacyclopentanones can react with. Additionally, intermolecular rhodacyclopentanone-based cycloaddition methodologies remain a challenging yet tantalising prospect. Here, fine tuning of the Rh-centre provides an avenue to control enantio-, diastereo- and/or regioselectivity. This versatility will likely underpin a flexible framework for developing methodologies that generate “sp3-rich” scaffolds in a direct and atom-economical manner. Such processes would be highly attractive to medicinal chemists for the generation of skeletally diverse compound libraries.
Acknowledgements
We thank the European Research Council for financial support via the European Union’s Horizon 2020 Programme (ERC grant no. 639594 CatHet). M.H.S. thanks the Bristol Chemical Synthesis Centre for Doctoral Training, funded by the EPSRC (EP/G036764/1), and Syngenta for the provision of a Ph.D. studentship. J.F.B. is indebted to the Royal Society for a University Research Fellowship.
Notes and references
- 1.(a) Lovering F, Bikker J, Humblet C. J Med Chem. 2009;52:6752. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; (b) Walters WP, Green J, Weiss JR, Murcko MA. J Med Chem. 2011;54:6405. doi: 10.1021/jm200504p. [DOI] [PubMed] [Google Scholar]; (c) Nadin A, Hattotuwagama C, Churcher I. Angew Chem Int Ed. 2012;51:1114. doi: 10.1002/anie.201105840. [DOI] [PubMed] [Google Scholar]
- 2.For reviews on C(sp3)-H bond activation, see: Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654. doi: 10.1002/chem.200902374. Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem Soc Rev. 2009;38:3242. doi: 10.1039/b816707a. Dastbaravardeh N, Christakakou M, Haider M, Schnürch M. Synthesis. 2014;46:1421. Hartwig JF. J Am Chem Soc. 2016;138:2. doi: 10.1021/jacs.5b08707.
- 3.For selected recent reviews on C-C bond activation, see: Murakami M, Matsuda T. Chem Commun. 2011;47:1100. doi: 10.1039/c0cc02566f. Seiser T, Saget T, Tran DN, Cramer N. Angew Chem Int Ed. 2011;50:7740. doi: 10.1002/anie.201101053. Ruhland K. Eur J Org Chem. 2012:2683. Chen F, Wang T, Jiao N. Chem Rev. 2014;114:8613. doi: 10.1021/cr400628s. Souillart L, Cramer N. Chem Rev. 2015;115:9410. doi: 10.1021/acs.chemrev.5b00138.
- 4.For a discussion on C-H vs. C-C bond activation, see: Rybtchinski B, Milstein D. Angew Chem Int Ed. 1999;38:870. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3.
- 5.(a) Suggs JW, Cox SD. J Organomet Chem. 1981;221:199. [Google Scholar]; (b) Suggs JW, Jun C-H. J Am Chem Soc. 1984;106:3054. [Google Scholar]; (c) Jun C-H, Lee H. J Am Chem Soc. 1999;121:880. [Google Scholar]; (d) Jun C-H, Lee H, Lim S-G. J Am Chem Soc. 2001;123:751. doi: 10.1021/ja0033537. [DOI] [PubMed] [Google Scholar]
- 6.Selected methodologies based on activation of unstrained C-C bonds: Blum J, Oppenheimer E, Bergmann ED. J Am Chem Soc. 1967;89:2338. Chatani N, Ie Y, Kakiuchi F, Murai S. J Am Chem Soc. 1999;121:8645. Nakao Y, Oda S, Hiyama T. J Am Chem Soc. 2004;126:13904. doi: 10.1021/ja0448723. Dreis AM, Douglas CJ. J Am Chem Soc. 2009;131:412. doi: 10.1021/ja8066308. Hirata Y, Yukawa T, Kashihara N, Nakao Y, Hiyama T. J Am Chem Soc. 2009;131:10964. doi: 10.1021/ja901374v. Hirata Y, Yada A, Morita E, Nakao Y, Hiyama T, Ohashi M, Ogoshi S. J Am Chem Soc. 2010;132:10070. doi: 10.1021/ja102346v. Wang J, Chen W, Zuo S, Liu L, Zhang X, Wang J. Angew Chem Int Ed. 2012;51:12334. doi: 10.1002/anie.201206693. Lei Z-Q, Li H, Li Y, Zhang X-S, Chen K, Wang X, Sun J, Shi Z-J. Angew Chem Int Ed. 2012;51:2690. doi: 10.1002/anie.201107136. Dermenci A, Whittaker RE, Dong G. Org Lett. 2013;15:2242. doi: 10.1021/ol400815y. See also reference 5.
- 7.Roundhill DM, Lawson DN, Wilkinson G. J Chem Soc A. 1968:845. [Google Scholar]
- 8.Leading references. Hydroacylation: Park J-W, Kou KGM, Kim DK, Dong VM. Chem Sci. 2015;6:4479. doi: 10.1039/c5sc01553g. Yip SYY, Aïssa C. Angew Chem Int Ed. 2015;54:6870. doi: 10.1002/anie.201500596. Park J-W, Chen Z, Dong VM. J Am Chem Soc. 2016;138:3310. doi: 10.1021/jacs.6b01445. Zhou X, Zafar I, Dong G. Tetrahedron. 2015;71:4478. doi: 10.1016/j.tet.2015.02.087. Oxidative coupling:
- 9.(a) Powell KG, McQuillin FJ. J Chem Soc D. 1971:931. [Google Scholar]; (b) McQuillin FJ, Powell KG. J Chem Soc Dalton Trans. 1972:2129. [Google Scholar]
- 10.Cassar L, Halpern J. J Chem Soc D. 1970:1082. [Google Scholar]
- 11.Yamaguchi R, Kawanisi M. J Org Chem. 1984;49:4460. [Google Scholar]
- 12.Johnson BFG, Lewis J, Tam SW. J Organomet Chem. 1976;105:271. [Google Scholar]
- 13.Shaw MH, Melikhova EY, Kloer DP, Whittingham WG, Bower JF. J Am Chem Soc. 2013;135:4992. doi: 10.1021/ja401936c. [DOI] [PubMed] [Google Scholar]
- 14.An X-ray crystal structure of a bimetallic rhodacyclopentanone complex, derived from a (2+1+1) coupling of 1,1-dimethylallene, carbon monoxide and a bridging methylene unit, has been reported: Chokshi A, Rowsell BD, Trepanier SJ, Ferguson MJ, Cowie M. Organometallics. 2004;23:4759.
- 15.Bart SC, Chirik PJ. J Am Chem Soc. 2003;125:886. doi: 10.1021/ja028912j. [DOI] [PubMed] [Google Scholar]
- 16.Shaw MH, Whittingham WG, Bower JF. Tetrahedron. 2016;72:2731. [Google Scholar]
- 17.Shaw MH, McCreanor NG, Whittingham WG, Bower JF. J Am Chem Soc. 2015;137:463. doi: 10.1021/ja511335v. [DOI] [PubMed] [Google Scholar]
- 18.Huffman MA, Liebeskind LS, Pennington WT. Organometallics. 1992;11:255. [Google Scholar]
- 19.Lu G, Fang C, Xu T, Dong G, Liu P. J Am Chem Soc. 2015;137:8274. doi: 10.1021/jacs.5b04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda Y, Hasegawa M, Yamashita M, Nozaki K, Ishida N, Murakami M. J Am Chem Soc. 2013;135:7142. doi: 10.1021/ja403461f. [DOI] [PubMed] [Google Scholar]
- 21.Murakami M, Amii H, Ito Y. Nature. 1994;370:540. [Google Scholar]
- 22.Murakami M, Amii H, Shigeto K, Ito Y. J Am Chem Soc. 1996;118:8285. [Google Scholar]
- 23.Matsuda T, Shigeno M, Murakami M. Chem Lett. 2006;35:288. [Google Scholar]
- 24.Garralda MA. Dalton Trans. 2009:3635. doi: 10.1039/b817263c. [DOI] [PubMed] [Google Scholar]
- 25.Kondo T, Taguchi Y, Kaneko Y, Niimi M, Mitsudo T. Angew Chem Int Ed. 2004;43:5369. doi: 10.1002/anie.200461002. [DOI] [PubMed] [Google Scholar]
- 26.Chen P-h, Xu T, Dong G. Angew Chem Int Ed. 2014;53:1674. doi: 10.1002/anie.201310100. [DOI] [PubMed] [Google Scholar]
- 27.Xu T, Savage NA, Dong G. Angew Chem Int Ed. 2014;53:1891. doi: 10.1002/anie.201310149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Q, Wang B, Yu H, Fu Y. ACS Catal. 2015;5:4881. [Google Scholar]
- 29.Brandi A, Cicchi S, Cordero FM, Goti A. Chem Rev. 2014;114:7317. doi: 10.1021/cr400686j. [DOI] [PubMed] [Google Scholar]
- 30.(a) Mazumder S, Shang D, Negru DE, Baik M-H, Evans PA. J Am Chem Soc. 2012;134:20569. doi: 10.1021/ja305467x. [DOI] [PubMed] [Google Scholar]; (b) Inglesby PA, Bacsa J, Negru DE, Evans PA. Angew Chem Int Ed. 2014;53:3952. doi: 10.1002/anie.201310232. [DOI] [PubMed] [Google Scholar]
- 31.(a) Wender PA, Gamber GG, Hubbard RD, Zhang L. J Am Chem Soc. 2002;124:2876. doi: 10.1021/ja0176301. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Wang J, Su J, Huang F, Jiao L, Liang Y, Yang D, Zhang S, Wender PA, Yu Z-X. J Am Chem Soc. 2007;129:10060. doi: 10.1021/ja072505w. [DOI] [PubMed] [Google Scholar]; (c) Wegner HA, de Meijere A, Wender PA. J Am Chem Soc. 2005;127:6530. doi: 10.1021/ja043671w. [DOI] [PubMed] [Google Scholar]
- 32.(a) Jiao L, Lin M, Zhuo L-G, Yu Z-X. Org Lett. 2010;12:2528. doi: 10.1021/ol100625e. [DOI] [PubMed] [Google Scholar]; (b) Feng Y, Yu Z-X. J Org Chem. 2015;80:1952. doi: 10.1021/jo502604p. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Zhang H, Feng J, Zhang Y, Wang J. Org Lett. 2010;12:3082. doi: 10.1021/ol101091r. [DOI] [PubMed] [Google Scholar]
- 34.(a) Johnson WTG, Borden WT. J Am Chem Soc. 1997;119:5930. [Google Scholar]; (b) Bach RD, Dmitrenko O. J Am Chem Soc. 2004;126:4444. doi: 10.1021/ja036309a. [DOI] [PubMed] [Google Scholar]
- 35.Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117. doi: 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- 36.Hidai M, Orisaku M, Uchida Y. Chem Lett. 1980;9:753. [Google Scholar]
- 37.Iqbal AFM. Tetrahedron Lett. 1971;12:3381. [Google Scholar]
- 38.Matsuda T, Tsuboi T, Murakami M. J Am Chem Soc. 2007;129:12596. doi: 10.1021/ja0732779. [DOI] [PubMed] [Google Scholar]
- 39.Koga Y, Narasaka K. Chem Lett. 1999;28:705. [Google Scholar]
- 40.For beneficial effects of Lewis basic additives or solvents in other metal-catalysed C-C bond activation processes, see: Yasui Y, Kamisaki H, Takemoto Y. Org Lett. 2008;10:3303. doi: 10.1021/ol801168j. Rondla NR, Levi SM, Ryss JM, Vanden Berg RA, Douglas CJ. Org Lett. 2011;13:1940. doi: 10.1021/ol200274h.
- 41.Perhaps the most appealing approach is by asymmetric alkene cyclopropanation with diazo compounds. For a review, see: Pellissier H. Tetrahedron. 2008;64:7041.
- 42.Shaw MH, Croft RA, Whittingham WG, Bower JF. J Am Chem Soc. 2015;137:8054. doi: 10.1021/jacs.5b05215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murakami M, Takahashi K, Amii H, Ito Y. J Am Chem Soc. 1997;119:9307. [Google Scholar]
- 44.Huffman MA, Liebeskind LS. J Am Chem Soc. 1993;115:4895. [Google Scholar]
- 45.Murakami M, Itahashi T, Amii H, Takahashi K, Ito Y. J Am Chem Soc. 1998;120:9949. [Google Scholar]
- 46.For bite angle values, see: Dierkes P, van Leeuwen PWNM. J Chem Soc Dalton Trans. 1999:1519.
- 47.Chen P-h, Sieber J, Senanayake CH, Dong G. Chem Sci. 2015;6:5440. doi: 10.1039/c5sc01875g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murakami M, Tsuruta T, Ito Y. Angew Chem Int Ed. 2000;39:2484. [PubMed] [Google Scholar]
- 49.Murakami M, Itahashi T, Ito Y. J Am Chem Soc. 2002;124:13976. doi: 10.1021/ja021062n. [DOI] [PubMed] [Google Scholar]
- 50.Parker E, Cramer N. Organometallics. 2014;33:780. [Google Scholar]
- 51.Souillart L, Parker E, Cramer N. Angew Chem Int Ed. 2014;53:3001. doi: 10.1002/anie.201311009. [DOI] [PubMed] [Google Scholar]
- 52.Souillart L, Cramer N. Angew Chem Int Ed. 2014;53:9640. doi: 10.1002/anie.201405834. [DOI] [PubMed] [Google Scholar]
- 53.Matsuda T, Fujimoto A, Ishibashi M, Murakami M. Chem Lett. 2004;33:876. [Google Scholar]
- 54.Ko HM, Dong G. Nature Chem. 2014;6:739. doi: 10.1038/nchem.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X, Dong G. J Am Chem Soc. 2015;137:13715. doi: 10.1021/jacs.5b09799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu T, Dong G. Angew Chem Int Ed. 2012;51:7567. doi: 10.1002/anie.201202771. [DOI] [PubMed] [Google Scholar]
- 57.Xu T, Ko HM, Savage NA, Dong G. J Am Chem Soc. 2012;134:20005. doi: 10.1021/ja309978c. [DOI] [PubMed] [Google Scholar]
- 58.Xu T, Dong G. Angew Chem Int Ed. 2014;53:10733. doi: 10.1002/anie.201404802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng L, Xu T, Li H, Dong G. J Am Chem Soc. 2016;138:369. doi: 10.1021/jacs.5b11120. [DOI] [PMC free article] [PubMed] [Google Scholar]