Abstract
Helminth parasite infections are associated with a battery of immunomodulatory mechanisms that affect all facets of the host immune response to ensure their persistence within the host. This broad-spectrum modulation of host immunity has intended and unintended consequences, both advantageous and disadvantageous. Thus the host can benefit from suppression of collateral damage during parasite infection and from reduced allergic, autoimmune, and inflammatory reactions. However, helminth infection can also be detrimental in reducing vaccine responses, increasing susceptibility to coinfection and potentially reducing tumor immunosurveillance. In this review we will summarize the panoply of immunomodulatory mechanisms used by helminths, their potential utility in human disease, and prospective areas of future research.
Key words: Allergy, infection, pathology, therapy, tolerance
Abbreviations used: Breg, Regulatory B; DC, Dendritic cell; Foxp3, Forkhead box protein 3; Treg, Regulatory T
Discuss this article on the JACI Journal Club blog: www.jaci-online.blogspot.com.
Helminths are highly prevalent metazoan worm parasites, which have evolved a spectrum of sophisticated means to regulate and evade the host immune system.1 Helminths appear to act as successful xenotransplants into the mammalian body, neutralizing immune pathways that would otherwise expel them and resetting the thresholds of immune reactivity.2 In so doing, they also dampen responses to unrelated bystander specificities, such as allergens and autoantigens, in a manner that might in fact benefit the host.1, 3
Only a dozen or so species of helminths are widespread in human subjects, but together, they infect some 2 billion persons, nearly one third of the human population.4 Their extraordinary prevalence bears witness to their success at defeating host defenses and suggests we have much to learn from how these parasites modulate our own immune system.
Although helminths establish in a range of tissue and intestinal niches, in nearly all cases they do not multiply within the host but produce eggs or larvae to infect new hosts; hence they tend to establish stable chronic infections that can endure for surprisingly long (up to 20 years) in an individual host. In this setting almost every facet of the immune system is modified or even recalibrated, with infected subjects displaying a state of immune hyporesponsiveness that can be considered a form of immunologic tolerance.5, 6, 7
Immunologic tolerance in human helminth infections
Immunologic hyporesponsiveness in helminth infections was first seen through muted parasite antigen-specific T-cell responses, from patients' PBMC cultures.7, 8, 9, 10 In particular, specific unresponsiveness was seen in asymptomatic carriers rather than those with progressive pathologic manifestations, such as elephantiasis. Furthermore, anthelminthic drug clearance of parasites from hyporesponsive carriers resulted in a recovery of antigen-specific responses, suggesting that they were actively inhibited by the presence of helminths.10, 11 In addition, T cells from helminth-infected asymptomatic human subjects show skewed cytokine profiles, favoring IL-4 over IL-17 and IFN-γ10 and with more conspicuous IL-10 and TGF-β components.12, 13 In contrast, in patients in whom symptomatic disease develops, there is a failure of tolerance, allowing TH1 and TH17 responses to surface and mediate significant pathology in infected tissues.14, 15
In the immune system homeostatic tolerance to self-antigens and harmless environmental antigens (including commensal bacteria and food components) is primarily maintained by an immunosuppressive T-cell subset, the regulatory T (Treg) cell.16 As discussed below, a strong link has emerged between long-term helminth infection and Treg cell activity, particularly in the asymptomatic or hyporesponsive state.17, 18, 19
Immune downregulation by helminths further extends into many local and systemic settings, with modulation of responses to a variety of unrelated bystander specificities.3 One example is that polyclonal immune responses to childhood vaccines can be compromised in heavily infected subjects.20 In addition, helminths can undermine host defenses against other major pathogens, such as Mycobacterium tuberculosis.3, 21 In the case of malaria, however, the consequences of helminth infection are more nuanced, with evidence of increased susceptibility combined with moderated inflammatory responses and hence attenuated disease severity.22, 23
The delicate balance between inflammation and immune regulation is exemplified in cysticercosis, a neurological pathology caused by inflammatory responses to Taenia solium cysts.24 It is well recognized that pathologic inflammation can be dampened by immunoregulatory mechanisms that might underpin the asymptomatic phase of disease.25 However, patients with the most highly disseminated infections actually show the greatest degree of immunoregulation, with increased IL-10 levels, decreased TH1 and TH2 cytokine levels, and a trend for increased Treg cell numbers.26 Thus the immune response to T solium infection is finely poised: strong immunomodulation leads to dissemination of the parasite, whereas failure to regulate inflammation causes seizures and death.
Helminth parasites clearly establish hyporesponsiveness in the naive adult in model systems, but in endemic settings it is common for offspring to be born to infected mothers, become infected at a very early age, or both. Maternal infection boosts tolerance in the newborn, so that offspring of Haitian mothers with the filarial infection Wuchereria bancrofti were 2- to 3-fold more likely to become infected themselves while showing a lower level of T-cell reactivity to parasite antigens than children of uninfected mothers.27 In a remarkable study in the Cook Islands, it was confirmed that even at 17 years of age, subjects born to infected mothers mounted substantially weaker T-cell responses to parasite antigens.28 Hence in the endemic setting it seems likely that many subjects experience in utero tolerization to parasite antigens. Furthermore, prenatal exposure also affects bystander reactivities both in human subjects29 and in experimental models, such as airway allergy.30 As will be discussed further, anti-inflammatory effects of helminth infection are observed not only in the setting of allergy but also in the context of autoimmunity and transplantation reactions.2, 31, 32
Treg cells in helminth infection: from the field to the laboratory
A key association has emerged between helminth infection and expansion of regulatory cell populations, most importantly the Treg cell subset.17, 18, 33 In human subjects Treg cells expressing the transcription factor forkhead box protein 3 (Foxp3) are more numerous and more active in helminth-infected subjects but decrease after anthelmintic chemotherapy.19, 34, 35 In filarial infections patients with pathologies, such as elephantiasis and hyperreactive onchocerciasis, show diminished Treg cell levels compared with those in unresponsive asymptomatic carriers, supporting the argument that the Treg cell compartment both maintains tolerance and prevents pathology in these infections.15, 36, 37 Likewise, in highly prevalent soil-transmitted intestinal nematode infections, a similar profile of increased Treg cell activity, immunosuppressive cytokine production, and antigen hyporesponsiveness is evident.38, 39 Mechanistically, multiple pathways are implicated in the downregulation of human responses to helminths, involving the cytokines IL-10 and TGF-β, and cell-surface interactions through cytotoxic T lymphocyte–associated antigen 4 and programmed death-1.19, 38, 39, 40
In human subjects the activity of Treg cells and production of IL-10 correlate closely with an isotype switch from the proallergic/inflammatory IgE to the noninflammatory IgG441; Foxp3− TR1 cell are the predominant source of IL-10,36 although Foxp3+ Treg cells are also present, and both contribute to driving IgG4 in human subjects.42 Serum IgG4 is largely composed of mixed dimers. Because the heavy chains lack linking disulfide bonds, they exchange with other IgG4 molecules; such mixed molecules are functionally monovalent and noninflammatory.43 Drug treatment of patients resulted in sharp decreases in circulating IgG4 levels, again arguing that parasites press the host immune system to favor this isotype.44
The causal links between helminth infections and Treg cells have now been established in both directions. First, certain helminths directly drive Treg cell responses from the host45 or do so indirectly through inducing host cells to produce TGF-β, a key cytokine that promotes regulatory cell function.46 Hence the expansion of Treg cells is not simply a corollary of the host inflammatory response that must accompany it to prevent overreaction.
Second, Treg cells are essential for parasites to survive in the immunocompetent host because their depletion in mouse model systems results in clearance of the infection,47, 48, 49 whereas expansion of Treg cells through IL-2 administration renders mice more susceptible.49 Interestingly, in the mouse model of filariasis, Treg cells establish hyporesponsiveness in the effector population, so that clearance of tissue-dwelling parasites requires not only ablation of Treg cells but also restimulation of the effector population.50, 51
The effects of Treg cells in murine helminth infections also mirror those in human subjects in other ways. For example, Treg cells are instrumental in attenuation of allergy in mice infected with gastrointestinal nematodes52 or schistosomes.53 They also play a vital role protecting the host from pathology because Treg cell depletion can exacerbate inflammatory responses with lethal results.48, 49, 54 Thus although partial Treg cell depletion can strengthen the TH2 response required for parasite expulsion, in their total absence an inflammatory storm prevails, preventing a coherent protective immune response.49
Treg cells are also implicated in the weakened defenses against other parasites, and in human subjects in vitro T-cell proliferative responses to BCG and malaria are attenuated in helminth-infected patients but recover if Treg cells are removed from the test cultures.55 Similarly, BCG vaccination of helminth-infected subjects elicits poor inflammatory cytokine responses to purified protein derivative antigen in contrast to significant TGF-β production; anthelmintic treatment reverses this scenario, suggesting that interference with vaccine responses might be due to the presence of immunosuppressive cytokines.56 Supporting this, a recent study reported that tuberculosis-infected migrants in the United Kingdom who were coinfected with helminths had higher Treg cell frequencies than those with tuberculosis alone but that anthelmintic treatment decreased Treg cell numbers while increasing TH1 effector populations.57
Regulatory B cells, dendritic cells, and macrophages in helminth infection
Often overshadowed by their T-cell counterparts, regulatory B (Breg) cells are also crucially important in control of the immune response during helminth infection.58 B cells from Heligmosomoides polygyrus–infected mice can suppress experimental autoimmune encephalomyelitis and airway allergy when transferred to recipient mice.59 Similarly, airway allergy can be suppressed by B cells from Schistosoma mansoni–infected mice, directly through their production of IL-10 and indirectly by enhancing Treg cell activity.60 Importantly, the latter study found similar Breg phenotype cells in schistosome-infected human subjects. High numbers of functional IL-10 producing Breg cells were also found in patients with multiple sclerosis protected from relapse after acquiring intestinal helminth infection compared with otherwise comparable uninfected patients.61 Along with Treg cell, Breg cells are strongly implicated in the development of tolerance to allergens,62 and strategies to encourage their expansion could increase the efficacy of allergen-specific immunotherapy.
Dendritic cells (DCs) in patients with helminth infections have been widely investigated for their propensity to induce TH2 responses in distinction to microbially stimulated DCs, which effectively drive TH1 and TH17 outcomes.63, 64 As yet, how DCs recognize the presence of helminths is unresolved, although certain key intracellular signals, such as the Kruppel-like factor 4 (KLF4), are now known to be essential for DCs to adopt the pro-TH2 phenotype65; beyond this stage, the mechanisms through which DCs instruct TH2 development are similarly opaque but are likely to include surface interactions, such as OX40/OX40 ligand costimulation.66
The question of whether DCs in helminth-infected mice are more tolerogenic and contribute to the expansion of Treg cells in vivo is also of great interest. DCs recovered from helminth-infected mice show altered phenotypes with, in the case of H polygyrus infection, expansion of CDllcloCD103− DCs, which are preferential inducers of Foxp3+ Treg cells in vitro. In contrast, CD11chi DCs induced stronger effector responses. In CD11cDTR mice diphtheria toxin administration depleted only the CD11chi subset, greatly diminishing the TH2 response, but spared the CD11clo population and the Treg cell response they induced.67 In other studies intestinal DCs from mice infected with the same parasite were able, when transferred into recombination-activating gene–deficient mice, to protect recipients from T cell–mediated colitis.68
A more reductionist approach has tested DCs differentiated in vitro from bone marrow precursors with various helminth products before appraising their ability to induce regulatory cytokines or cells from T cells or from mice receiving a bolus of pulsed DCs. A recurrent finding in these studies has been that helminth antigens (eg, secreted products or egg extracts) block the Toll-like receptor–stimulated pathway that leads to IL-12 production and TH1 induction.69, 70, 71 In terms of in vivo immunoregulation, DCs pulsed with Hymenolepis diminuta antigens are able to downmodulate dinitrobenzene sulfonic acid colitis in recipient mice, and CD4+ T cells from those recipients can be further transferred to new hosts and protect against colitis, requiring IL-10 production for their effect.72 A substantial range of different helminth molecules have now been reported to modulate DC reactivity and function, as recently reviewed,64 promising a more mechanistic understanding in the near future. In particular, the S mansoni secreted protein ω-1, a glycosylated T2 ribonuclease, is the first helminth-derived molecule in which the mechanism of action of DCs has been characterized. This glycoprotein is taken up by mannose receptor binding to the glycan side chains, and once inside the cell, its ribonuclease activity degrades host mRNA, ablating IL-12 production and encouraging TH2 differentiation.73
Macrophages in patients with helminth infection are profoundly altered in their profile, adopting an alternatively activated phenotype (also termed M2) driven by the type 2 cytokines IL-4 and IL-13 and adopting a pattern of gene expression, metabolism, and function markedly different from that of classically activated (M1) macrophages, which respond to microbial stimulation through Toll-like receptors.74 Signature protein products of the M2 macrophage include arginase-1, RELM-α, and the chitinase-like molecule Ym1.75 M2 macrophages are required for effective immunity to some parasites (including H polygyrus76, 77 in an arginase-1–dependent manner) and are instrumental in repair and resolution of tissue damage caused, for example, by migratory helminths.78 In this context helminth-stimulated macrophages adopt an anti-inflammatory role with immunosuppressive characteristics, for example inhibiting T-cell proliferation,79 in part through expression of programmed death ligand 180 and enhancement of Treg cell differentiation through vitamin A production by retinal dehydrogenase.81
Protection from allergy, autoimmunity, and allograft rejection
To investigate the immunomodulatory pathways used by helminths, it is useful to consider the responses involved in their ejection as the most likely targets for modulation. For example, in the field of virology, the class I MHC presentation pathway is crucial to orchestration of a productive antiviral CD8 T-cell response: for almost every step in this pathway, a viral immunomodulator can be found that interferes in its normal functioning.82 Likewise, as the field of immunoparasitology matures, it is clear that parasites have evolved strategies to modulate, subvert, or evade each component of the immune response which might be capable of eliminating them. Because it is often difficult to assess the importance of immune pathways while under active suppression during parasitic infection, models of immunopathology have been useful tools to assess modulation mediated by parasites or their products.
It has been noted since 1968 that inflammatory disorders, such as arthritis, are much less frequent in low-income countries with high levels of parasite infection.83 Subsequent studies have further established a reciprocal link between endemic helminth infection and reduced prevalence of allergic reactivity and autoimmune antibodies, as well as increases in these immunologic indicators after anthelmintic treatment.84, 85, 86 The downmodulation of immune dysfunction in the presence of helminths depends on the exact parasite species in question, as well as the intensity of infection,87, 88 but has been reported across the spectrum of tropical environments using a range of different approaches (reviewed by McSorley and Maizels1). It is likely that helminths impact at 2 levels: (1) modifying the level of host reactivity during development of the infant immune system and (2) dampening immune responses in mature subjects who might be exposed to helminths for the first time in adult life.89 It is the latter setting that led to the proposal that helminths or their products could be used as therapies for inflammatory diseases in the parasite-free developed world.90
In settings of both allergy and autoimmunity, attenuation of reactivity has been linked to downmodulatory cytokine production, in particular IL-10 responses.84, 86 Among the most remarkable studies has been that of a cohort of patients with multiple sclerosis in Argentina who unintentionally acquired gastrointestinal helminth infections, with subsequent increased TGF-β and IL-10 levels and higher Treg and Breg cell activity. Strikingly, the infected patients enjoyed clinical remission from symptomatic disease,61, 91 but those subjects given anthelmintic treatment experienced loss of regulatory cytokines and relapse of disease.92
Experimental data echo and extend these findings in laboratory models of allergic and autoimmune pathology.1, 33, 93, 94 Helminth-infected mice are less susceptible to airway inflammation after allergen sensitization, and Treg cells from these mice can confer protection against allergy when transferred to naive animals.52, 53 Moreover, Breg cells,59, 95 helminth-stimulated DCs,68 and regulatory macrophages96 are each able, in different settings, to confer protection against pathology in recipient mice.
Reports such as these have fueled interest in the administration of live helminths as therapies for a range of inflammatory conditions from allergy, autism, autoimmunity, and colitis.90 After some promising early studies,97 more recent trials have not proved significant benefit.98, 99 A number of reasons might underpin the perceived lack of efficacy of live helminth therapy.94 For example, the human response to helminths is spectral, and only a subset might gain benefit from live infection; each helminth species inhabits a particular anatomic niche that might or might not affect the site of inflammatory disease; the dynamics of any protective effect in terms of parasite dose and duration of infection are unknown; and therapy of an established inflammatory disorder might require a particularly high parasite load or long-term infection. For each of these reasons, a more analytic approach of identifying immunomodulatory mechanisms and molecular mediators from helminths is advocated as the best strategy for developing new therapies inspired by the immunosuppressive capacities of parasites.100, 101, 102 By this means, individual molecular products can be assessed, validated, and developed as defined pharmaceuticals, which can then be delivered in a manner most consistent with the indication in question. Most significantly, this approach separates benefit from harm and removes the need to introduce a potentially pathogenic parasite in the treatment of disease.
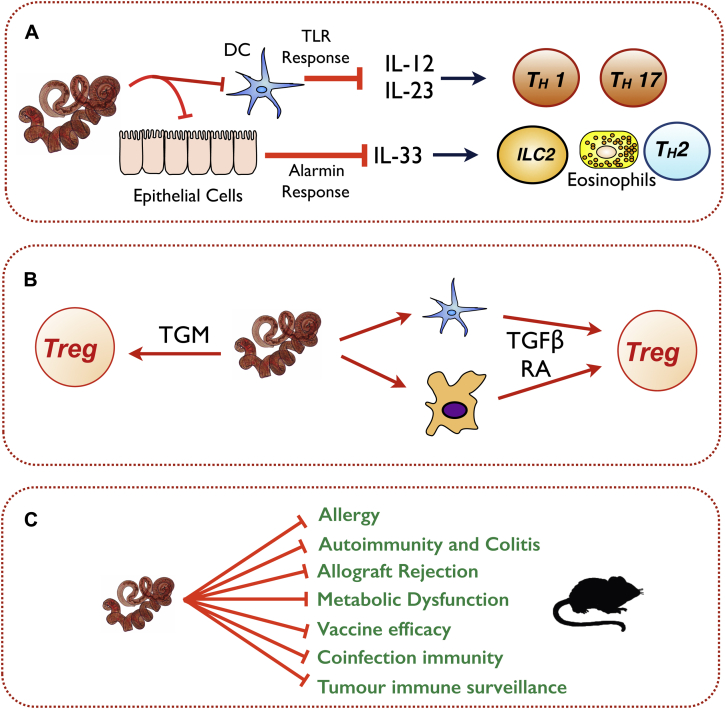
A schematic summary of some known helminth modulatory pathways and targets for immune modulation is presented in Fig 1, while a more detailed list of immunomodulatory effects of parasite products with potential for use in immune-mediated disease is shown in Table I.45, 46, 73, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115
Fig 1.
Immune system–parasite interactions during helminth infections. A, Blockade of innate sensing and alarmin production, such as inhibiting Toll-like receptor (TLR) responses of dendritic cells, thereby impairing inflammatory TH1/TH17 development, and abrogating epithelial cell production of IL-33, thereby pre-empting the type 2 response. ILC2, Type 2 innate lymphoid cell. B, Modulation of the adaptive immune response, promoting Treg cell differentiation either directly through production of TGF-β–like mimics (TGM) or indirectly by inducing host TGF-β and retinoic acid (RA) from DCs and macrophages. C, Modification of bystander immune responses in the infected host.
Table I.
Selected parasite-derived molecules with activity in immune-mediated diseases
| Immune modulatory effect | Example parasite product | Mechanism of action | Disease models in which efficacy is shown | References |
|---|---|---|---|---|
| Suppression of innate and adaptive immune cell activation | A viteae ES-62 | Nonconventional signaling through TLR4, leading to sequestration of PKC-α | Asthma, atopic dermatitis, SLE, and arthritis | 106, 107, 108 |
| Suppression of antigen presentation | S mansoni ω-1 | Degradation of DC mRNA, preventing IL-12 secretion | NOD diabetes, metabolic homeostasis | 73, 105, 109 |
| F hepatica FhHDM-1 | Inhibition of vacuolar ATPase resulting in reduced endolysosomal acidification | Sepsis | 110, 111 | |
| Cystatins: A viteae Av17 (AvCystatin), B malayi Bm-CPI-2, N brasiliensis Nippocystatin |
Inhibition of cysteine proteases required for antigen presentation; induction of IL-10 through signaling events downstream of an unknown receptor (Av17) | Asthma, colitis | 104, 112, 113, 114, 115 | |
| Suppression of ILC2 responses | H polygyrus HES | Suppression of ILC2-inducing IL-33 responses | Asthma | 103 |
| Induction of Treg cells | H polygyrus HES | Secreted TGF-β mimic ligates host TGF-β receptor | Asthma | 45 |
| S mansoni SEA/ω-1 | Induction of tolerogenic DCs, which produce TGF-β and RA | Type I diabetes | 46, 109 |
Bm-CPI-2, Brugia malayi cysteine protease inhibitor 2; FhHDM-1, Fasciola hepatica helminth defense molecule 1; HES, H polygyrus excretory secretory products; Hp-CPI, H polygyrus cysteine protease inhibitor; ILC2, type 2 innate lymphoid cell; PKC, protein kinase C; RA, retinoic acid; SEA, Schistosoma mansoni soluble egg antigen; SLE, systemic lupus erythematosus; TLR, Toll-like receptor.
As we elucidate mechanisms of immune-mediated parasite ejection, we might appreciate new targets for immunomodulation. For instance, eosinophils are required for ejection of many parasites,116 and eosinophil accumulation is potently suppressed by many parasite products in models of allergy.103, 104, 117, 118, 119 However, as yet, no parasite products have been identified that act directly on this population. In subsequent sections we will propose other likely targets of parasite immunomodulation.
Interactions between helminths and microorganisms
Intestinal helminths and those that occupy other mucosal sites, such as the lung, cohabit with a spectrum of microbial organisms.120, 121, 122 The entry of helminth parasites, such as H polygyrus, Trichinella spiralis, or Trichuris muris, into the intestinal tracts of mice significantly perturbs the commensal bacterial populations, with important immunologic and metabolic consequences.123, 124, 125, 126 In several studies helminth-infected mice show increase in Lactobacillus species colonization, which in the case of H polygyrus correlates with increased numbers of Treg cells; moreover, prior administration of lactobacilli to mice renders them more susceptible to H polygyrus infection, demonstrating a reciprocally beneficial interaction between the metazoan and microbial species.124 Moreover, the transfer of intestinal contents from infected to uninfected mice conferred immunomodulatory effects that reduced allergic reactivity in naive recipients.127 A mechanistic insight into how the microbiota might favor parasite establishment was gained recently in studies of retinoic acid–related orphan receptor γt–dependent T cells in the gut, which in response to microbial stimulation differentiate to both TH17 effectors and retinoic acid–related orphan receptor γt–positive Treg cells, which together repress TH2 immunity to H polygyrus.128 It is presently unknown whether these changes in the commensal population represent an adaptation of commensals to the environment in helminth infection or are due to active modulation by helminth-secreted factors (eg, secreted lysozymes).120
Helminths and homeostasis
Increasingly, regulation of metabolism and weight control is recognized as an immunologic process,129, 130 and hence it is fascinating that helminth infections can protect against metabolic disorders.131 In a seminal study, mice infected with Nippostrongylus brasiliensis and fed a high-fat diet were protected against glucose intolerance through activation of adipose tissue eosinophils, which induced alternatively activated M2 macrophages.132 Similarly, not only S mansoni infection of mice but also administration of soluble antigens from schistosome eggs, expanded adipose eosinophils and M2 macrophages.133 Interestingly, one of the major molecular components of soluble antigens from schistosome eggs, ω-1, can itself protect against metabolic disorders when administered to mice,105 opening up a biochemical pathway that helminths can activate during infection that proves beneficial to the host.
Helminths and cancer
There are many parallels between immune responses that result in the progression of tumors and maintenance of parasite infection. By better understanding immunosuppressed responses to parasites and how these could be abrogated to expel the pathogen, we can better understand anticancer responses and how to bolster them for immunity. Parasitic infection could increase carcinogenesis through associated low-grade chronic inflammatory responses (in the absence of parasite ejection), secretion of directly procarcinogenic factors, or suppression of immune surveillance.134, 135
Schistosoma haematobium infection results in deposition of eggs in the bladder wall and is strongly linked to the development of bladder cancer.136 Mouse models using egg injection into the bladder wall have shown that egg deposition results in an inflammatory environment, leading to a preneoplastic environment136 and predisposing to tumorigenesis. In contrast, the trematode Opisthorchis viverrini resides in the bile duct and secretes a granulin-like growth factor (Ov-GRN-1) that directly causes proliferation of host cells and, with cofactors such as dietary carcinogens, leads to transformation of bile duct cells and ultimately cholangiocarcinoma.137, 138 Likewise, the closely-related parasite Clonorchis sinensis also encodes a granulin-like molecule that is hypothesized to carry out the same function.139 Because these parasites feed on bile duct cells, it has been proposed that by encouraging cell proliferation, they are decreasing the damage caused by their feeding (while increasing their food source), with carcinogenesis being an unintended byproduct of this pathway.140
The least well-studied mechanism of carcinogenesis by parasite infection is suppression of immune surveillance, leading to escape of mutated host cells, which would normally be eliminated by the immune system. Myeloid-derived suppressor cells accumulate during parasitic infections and are either involved in parasite ejection141, 142 or suppress antiparasite immune responses,143 depending on the parasite species and chronicity of infection. In antitumor responses myeloid-derived suppressor cells are a well-characterized suppressive population.144 Likewise, the expansion of Treg cells and alternative activation of macrophages during parasitic infection is associated with suppression of antitumor immune responses.145 Together, parasitic infection appears to result in a protumorigenic immune milieu. Epidemiologic data in this area are presently lacking, and the effects of parasitic infection in cancer progression requires further attention.
Epithelial responses
The importance of epithelial barriers in initiation of immune responses is now widely appreciated.146 In response to parasitic infection or allergen administration, epithelial cell damage results in release of damage-associated molecular patterns, such as ATP, high mobility group box 1 (HMGB1), uric acid, and S100, as well as proallergic alarmin cytokines, such as IL-33, IL-25, and thymic stromal lymphopoietin, together with more generally inflammatory cytokines, such as GM-CSF and IL-1α.147 The critical nature of these responses can be seen in systems in which radioresistant stromal cells (including epithelial cells) are specifically targeted for knockdown of pattern recognition or cytokine receptors in which allergic responses do not develop.148, 149 Furthermore, because the gut epithelium is critically involved in ejection of parasitic infections (through increased mucus production and epithelial cell turnover), the importance of the epithelium to the antiparasite immune response cannot be overstated.150 Combined with the intimate association between many helminths and the epithelial barrier, this makes the epithelium a prime site for helminth modulation.
Release of the alarmin cytokine IL-33 is a potent signal for type 2 response initiation. Mice lacking the IL-33 signaling pathway have abrogated type 2 responses to allergens and parasites and are more susceptible to infection with Litomosoides sigmodontis,151 N brasiliensis,152 and T spiralis,153 whereas administration of exogenous IL-33 leads to ejection of H polygyrus,154 N brasiliensis,155 Strongyloides venezuelensis,156 and T muris.157 Thus the IL-33 pathway appears to be an ideal target for parasite immunomodulation to allow persistence of infection. Indeed, the excretory/secretory products of H polygyrus potently inhibit the IL-33 pathway, both by suppressing IL-33 release103 and by suppressing expression of the IL-33 receptor,158 resulting in reduced type 2 responses and abrogated inflammation in a mouse model of asthma. Whether these pathways of immunomodulation are common to many intestinal helminths or unique to the chronically infective H polygyrus remains to be investigated.
In parallel to IL-33, IL-25 activates type 2 innate lymphoid cells, potentiates type 2 immune responses, and is crucial for ejection of parasites. Mice deficient in IL-25 or its receptor show increased susceptibility to H polygyrus159, 160 and N brasiliensis primary or secondary infections.161 Chemosensory tuft cells of the intestinal epithelium were recently identified as the major source of IL-25 in the intestine during parasite infection.162, 163, 164 Remarkably, tuft cell–deficient mice show extremely abrogated immunity to N brasiliensis infection, with all tuft cell–deficient mice retaining productive infections up to 42 days after infection,162 whereas IL-25–deficient mice show only a slightly delayed response.161 Thus although tuft cells clearly have an important role in producing IL-25, their antiparasite functions must extend beyond this. As an emerging crucial element of the antiparasite response, it appears likely that some parasites will have developed mechanisms for modulating tuft cell responses to allow their persistence in the host.
Helminth vaccines
As we have gained greater understanding of the complex response required for ejection of parasites, we have attempted to apply this to development of vaccines against helminth infections. However, to date, no vaccines have been developed for human helminth infections. The reasons for this include the subtle and complex interplay of factors required for ejection,159 the potential for collateral damage to the host, the risk of anaphylaxis in infected populations,165 and the lack of defined single immunodominant antigens.166 Finally, the competing immune regulatory response, which is known to suppress bystander vaccinations,20 means that vaccination may only be a successful strategy in populations cured of helminth infections by anthelmintics or in previously helminth-naive children.
Conclusion
Parasites are subtle but powerful regulators of host immune responses, suppressing some pathways of immune activation (eg, DC antigen presentation, T-cell cytokine and B-cell antibody production, and epithelial cell alarmin release), modulating other pathways (eg, TH cell subset differentiation and B-cell isotype switching), and inducing still others (eg, Treg and Breg cell differentiation and tolerogenic DC responses). The immune pathways required for induction, expansion, and maintenance of antiparasite responses are still being elucidated. As we discover more about how productive antiparasite responses are produced, we are also discovering new pathways for immunomodulation of these pathways by helminth infections and exploring new possibilities for exploiting parasite molecules as therapies for inflammatory diseases.
What is unknown?
-
•
What are the molecules secreted by parasites to induce Treg and Breg cells?
-
•
Do multiple parasite products suppress DC responses through ω-1–like ribonuclease activity? Is this unique to schistosomes? Which other mechanisms are co-opted by other parasites?
-
•
Do parasite infections lead to reduced tumor immune surveillance and increased cancer diagnoses?
-
•
Do parasites interfere in epithelial cell responses beyond IL-33?
-
•
How can parasite-derived molecules be used to treat immunopathologies?
Footnotes
Disclosure of potential conflict of interest: The authors have received grants from the Wellcome Trust (Ref 106122), the Kenneth Rainin Foundation (2015-964), and Asthma UK (SPD-2012-172).
References
- 1.McSorley H.J., Maizels R.M. Helminth infections and host immune regulation. Clin Microbial Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston C.J., McSorley H.J., Anderton S.M., Wigmore S.J., Maizels R.M. Helminths and immunological tolerance. Transplantation. 2014;97:127–132. doi: 10.1097/TP.0b013e3182a53f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra P.K., Palma M., Bleich D., Loke P., Gause W.C. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014;7:753–762. doi: 10.1038/mi.2014.23. [DOI] [PubMed] [Google Scholar]
- 4.Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutman T.B., Kumaraswami V., Ottesen E.A. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79:1516–1523. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maizels R.M., Lawrence R.A. Immunological tolerance: the key feature in human filariasis? Parasitol Today. 1991;7:271–276. doi: 10.1016/0169-4758(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 7.King C.L., Kumaraswami V., Poindexter R.W., Kumari S., Jayaraman K., Alling D.W. Immunologic tolerance in lymphatic filariasis. Diminished parasite-specific T and B cell lymphocyte precursor frequency in the microfilaremic state. J Clin Invest. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piessens W.F., McGreevy P.B., Piessens P.W., McGreevy M., Koiman I., Saroso J.S. Immune responses in human infections with Brugia malayi. Specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980;65:172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colley D.G., Garcia A.A., Lambertucci J.R., Parra J.C., Katz N., Rocha R.S. Immune responses during human schistosomiasis. XII. Differential responsiveness in patients with hepatosplenic disease. Am J Trop Med Hyg. 1986;35:793–802. [PubMed] [Google Scholar]
- 10.Sartono E., Kruize Y.C.M., Kurniawan A., van der Meide P.H., Partono F., Maizels R.M. Elevated cellular responses and interferon-γ release after long-term diethylcarbamazine treatment of patients with human lymphatic filariasis. J Infect Dis. 1995;171:1683–1687. doi: 10.1093/infdis/171.6.1683. [DOI] [PubMed] [Google Scholar]
- 11.Grogan J.L., Kremsner P.G., Deelder A.M., Yazdanbakhsh M. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobium infection. Eur J Immunol. 1996;26:1365–1370. doi: 10.1002/eji.1830260628. [DOI] [PubMed] [Google Scholar]
- 12.Mahanty S., Mollis S.N., Ravichandran M., Abrams J.S., Kumaraswami V., Jayaraman K. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J Infect Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- 13.Doetze A., Satoguina J., Burchard G., Rau T., Loliger C., Fleischer B. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-b but not by a Th1 to Th2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 14.Mbow M., Larkin B.M., Meurs L., Wammes L.J., de Jong S.E., Labuda L.A. T-helper 17 cells are associated with pathology in human schistosomiasis. J Infect Dis. 2013;207:186–195. doi: 10.1093/infdis/jis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babu S., Bhat S.Q., Pavan Kumar N., Lipira A.B., Kumar S., Karthik C. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 17.Maizels R.M., Smith K.A. Regulatory T cells in infection. Adv Immunol. 2011;112:73–136. doi: 10.1016/B978-0-12-387827-4.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig-Portugall I., Layland L.E. TLRs, Treg, and B cells, an interplay of regulation during helminth infection. Front Immunol. 2012;3:8. doi: 10.3389/fimmu.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metenou S., Nutman T. Regulatory T cell subsets in filarial infection and their function. Front Immunol. 2013;4:305. doi: 10.3389/fimmu.2013.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labeaud A.D., Malhotra I., King M.J., King C.L., King C.H. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salgame P., Yap G.S., Gause W.C. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–1126. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartgers F.C., Obeng B.B., Kruize Y.C., Dijkhuis A., McCall M., Sauerwein R.W. Responses to malarial antigens are altered in helminth-infected children. J Infect Dis. 2009;199:1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 23.Dolo H., Coulibaly Y.I., Dembele B., Konate S., Coulibaly S.Y., Doumbia S.S. Filariasis attenuates anemia and proinflammatory responses associated with clinical malaria: a matched prospective study in children and young adults. PLoS Negl Trop Dis. 2012;6:e1890. doi: 10.1371/journal.pntd.0001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash T.E., Garcia H.H. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol. 2011;7:584–594. doi: 10.1038/nrneurol.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma A., Prasad K.N., Cheekatla S.S., Nyati K.K., Paliwal V.K., Gupta R.K. Immune response in symptomatic and asymptomatic neurocysticercosis. Med Microbiol Immunol. 2011;200:255–261. doi: 10.1007/s00430-011-0198-x. [DOI] [PubMed] [Google Scholar]
- 26.Tuero I., Palma S., Cabeza F., Saleemi S., Rodriguez S., Gonzales I. A comparative study of peripheral immune responses to Taenia solium in individuals with parenchymal and subarachnoid neurocysticercosis. PLoS Negl Trop Dis. 2015;9:e0004143. doi: 10.1371/journal.pntd.0004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammie P.J., Hitch W.L., Walker Allen E.M., Hightower W., Eberhard M.L. Maternal filarial infection as risk factor for infection in children. Lancet. 1991;337:1005–1006. doi: 10.1016/0140-6736(91)92661-k. [DOI] [PubMed] [Google Scholar]
- 28.Steel C., Guinea A., McCarthy J.S., Ottesen E.A. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial antigens. Lancet. 1994;343:890–893. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 29.Mpairwe H., Tweyongyere R., Elliott A. Pregnancy and helminth infections. Parasite Immunol. 2014;36:328–337. doi: 10.1111/pim.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straubinger K., Paul S., Prazeres da Costa O., Ritter M., Buch T., Busch D.H. Maternal immune response to helminth infection during pregnancy determines offspring susceptibility to allergic airway inflammation. J Allergy Clin Immunol. 2014;134:1271–1279.e10. doi: 10.1016/j.jaci.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Weinstock J.V., Elliott D.E. Helminth infections decrease host susceptibility to immune-mediated diseases. J Immunol. 2014;193:3239–3247. doi: 10.4049/jimmunol.1400927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Chen H.L., Bannick N., Henry M., Holm A.N., Metwali A. Intestinal helminths regulate lethal acute graft-versus-host disease and preserve the graft-versus-tumor effect in mice. J Immunol. 2015;194:1011–1020. doi: 10.4049/jimmunol.1303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finlay C.M., Walsh K.P., Mills K.H. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206–230. doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K., Mwinzi P.N., Black C.L., Muok E.M., Karanja D.M., Secor W.E. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77:676–682. [PMC free article] [PubMed] [Google Scholar]
- 35.Wammes L.J., Hamid F., Wiria A.E., Wibowo H., Sartono E., Maizels R.M. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Negl Trop Dis. 2012;6:e1655. doi: 10.1371/journal.pntd.0001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metenou S., Dembele B., Konate S., Dolo H., Coulibaly S.Y., Coulibaly Y.I. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katawa G., Layland L.E., Debrah A.Y., von Horn C., Batsa L., Kwarteng A. Hyperreactive onchocerciasis is characterized by a combination of Th17-Th2 immune responses and reduced regulatory T cells. PLoS Negl Trop Dis. 2015;9:e3414. doi: 10.1371/journal.pntd.0003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner J.D., Jackson J.A., Faulkner H., Behnke J., Else K., Kamgno J. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis. 2008;197:1204–1212. doi: 10.1086/586717. [DOI] [PubMed] [Google Scholar]
- 39.Figueiredo C.A., Barreto M.L., Rodrigues L.C., Cooper P.J., Silva N.B., Amorim L.D. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78:3160–3167. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoguina J., Mempel M., Larbi J., Badusche M., Löliger C., Adjei O. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes Infect. 2002;4:1291–1300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 41.Satoguina J.S., Weyand E., Larbi J., Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–4726. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 42.Satoguina J.S., Adjobimey T., Arndts K., Hoch J., Oldenburg J., Layland L.E. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-β. Eur J Immunol. 2008;38:3101–3113. doi: 10.1002/eji.200838193. [DOI] [PubMed] [Google Scholar]
- 43.van der Neut Kolfschoten M., Schuurman J., Losen M., Bleeker W.K., Martinez-Martinez P., Vermeulen E. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 44.Grogan J.L., Kremsner P.G., van Dam G.J., Metzger W., Mordmüller B., Deelder A.M. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J Infect Dis. 1996;173:1242–1247. doi: 10.1093/infdis/173.5.1242. [DOI] [PubMed] [Google Scholar]
- 45.Grainger J.R., Smith K.A., Hewitson J.P., McSorley H.J., Harcus Y., Filbey K.J. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaccone P., Burton O., Miller N., Jones F.M., Dunne D.W., Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39:1098–1107. doi: 10.1002/eji.200838871. [DOI] [PubMed] [Google Scholar]
- 47.Blankenhaus B., Klemm U., Eschbach M.L., Sparwasser T., Huehn J., Kuhl A.A. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol. 2011;186:4295–4305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- 48.Sawant D.V., Gravano D.M., Vogel P., Giacomin P., Artis D., Vignali D.A.A. Regulatory T cells limit induction of protective immunity and promote immune pathology following intestinal helminth infection. J Immunol. 2014;192:2904–2912. doi: 10.4049/jimmunol.1202502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith K.A., Filbey K.J., Reynolds L.A., Hewitson J.P., Harcus Y., Boon L. Low level regulatory T cell activity is essential for functional type-2 effector immunity to expel gastrointestinal helminths. Mucosal Immunol. 2016;9:428–443. doi: 10.1038/mi.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor M., Le Goff L., Harris A., Malone E., Allen J.E., Maizels R.M. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 51.Taylor M.D., van der Werf N., Maizels R.M. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Wilson M.S., Taylor M., Balic A., Finney C.A.M., Lamb J.R., Maizels R.M. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Layland L.E., Straubinger K., Ritter M., Loffredo-Verde E., Garn H., Sparwasser T. Schistosoma mansoni-mediated suppression of allergic airway inflammation requires patency and Foxp3+ Treg cells. PLoS Negl Trop Dis. 2013;7:e2379. doi: 10.1371/journal.pntd.0002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Elia R., Behnke J.M., Bradley J.E., Else K.J. Regulatory T cells: a role in the control of helminth driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wammes L.J., Hamid F., Wiria A.E., de Gier B., Sartono E., Maizels R.M. Regulatory T cell in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur J Immunol. 2010;40:437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- 56.Elias D., Britton S., Aseffa A., Engers H., Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 57.Toulza F., Tsang L., Ottenhoff T.H., Brown M., Dockrell H.M. Mycobacterium tuberculosis-specific CD4+ T-cell response is increased, and Treg cells decreased, in anthelmintic-treated patients with latent TB. Eur J Immunol. 2016;46:752–761. doi: 10.1002/eji.201545843. [DOI] [PubMed] [Google Scholar]
- 58.Hussaarts L., van der Vlugt L.E., Yazdanbakhsh M., Smits H.H. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011;128:733–739. doi: 10.1016/j.jaci.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Wilson M.S., Taylor M.D., O'Gorman M.T., Balic A., Barr T.A., Filbey K. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Vlugt L.E., Labuda L.A., Ozir-Fazalalikhan A., Lievers E., Gloudemans A.K., Liu K.Y. Schistosomes induce regulatory features in human and mouse CD1d B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS One. 2012;7:e30883. doi: 10.1371/journal.pone.0030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Correale J., Farez M., Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008;64:187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- 62.Akdis M., Akdis C.A. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133:621–631. doi: 10.1016/j.jaci.2013.12.1088. [DOI] [PubMed] [Google Scholar]
- 63.Perona-Wright G., Jenkins S.J., MacDonald A.S. Dendritic cell activation and function in response to Schistosoma mansoni. Int J Parasitol. 2006;36:711–721. doi: 10.1016/j.ijpara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Everts B., Smits H.H., Hokke C.H., Yazdankbakhsh M. Sensing of helminth infections by dendritic cells via pattern recognition receptors and beyond: consequences for T helper 2 and regulatory T cell polarization. Eur J Immunol. 2010;40:1525–1537. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 65.Tussiwand R., Everts B., Grajales-Reyes G.E., Kretzer N.M., Iwata A., Bagaitkar J. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015;42:916–928. doi: 10.1016/j.immuni.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jenkins S.J., Perona-Wright G., Worsley A.G., Ishii N., MacDonald A.S. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–3523. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 67.Smith K.A., Hochweller K., Hämmerling G.J., Boon L., Macdonald A.S., Maizels R.M. Chronic helminth infection mediates tolerance in vivo through dominance of CD11clo CD103− DC population. J Immunol. 2011;186:7098–7109. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blum A.M., Hang L., Setiawan T., Urban J.P., Jr., Stoyanoff K.M., Leung J. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J Immunol. 2012;189:2512–2520. doi: 10.4049/jimmunol.1102892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balic A., Harcus Y., Holland M.J., Maizels R.M. Selective maturation of dendritic cells by Nippostrongylus brasiliensis secreted proteins drives T helper type 2 immune responses. Eur J Immunol. 2004;34:3047–3059. doi: 10.1002/eji.200425167. [DOI] [PubMed] [Google Scholar]
- 70.Cervi L., MacDonald A.S., Kane C., Dzierszinski F., Pearce E.J. Dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 71.Segura M., Su Z., Piccirillo C., Stevenson M.M. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 72.Matisz C.E., Leung G., Reyes J.L., Wang A., Sharkey K.A., McKay D.M. Adoptive transfer of helminth antigen-pulsed dendritic cells protects against the development of experimental colitis in mice. Eur J Immunol. 2015;45:3126–3139. doi: 10.1002/eji.201545579. [DOI] [PubMed] [Google Scholar]
- 73.Everts B., Hussaarts L., Driessen N.N., Meevissen M.H., Schramm G., van der Ham A.J. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209:1753–1767. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez F.O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 75.Nair M.G., Gallagher I.J., Taylor M.D., Loke P., Coulson P.S., Wilson R.A. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anthony R.M., Urban J.F., Jr., Alem F., Hamed H.A., Rozo C.T., Boucher J.L. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Filbey K.J., Grainger J.R., Smith K.A., Boon L., van Rooijen N., Harcus Y. Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunol Cell Biol. 2014;92:436–448. doi: 10.1038/icb.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gause W.C., Wynn T.A., Allen J.E. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacDonald A.S., Maizels R.M., Lawrence R.A., Dransfield I., Allen J.E. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J Immunol. 1998;160:4124–4132. [PubMed] [Google Scholar]
- 80.Terrazas L.I., Montero D., Terrazas C.A., Reyes J.L., Rodriguez-Sosa M. Role of the programmed death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol. 2005;35:1349–1358. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Broadhurst M.J., Leung J., Lim K.C., Girgis N., Gundra U.M., Fallon P. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent Type-2 immunity to helminth infection in mice. PLoS Pathog. 2012;8:e1002883. doi: 10.1371/journal.ppat.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hewitt E.W. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110:163–169. doi: 10.1046/j.1365-2567.2003.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenwood B.M. Autoimmune disease and parasitic infections in Nigerians. Lancet. 1968;2:380–382. doi: 10.1016/s0140-6736(68)90595-3. [DOI] [PubMed] [Google Scholar]
- 84.van den Biggelaar A.H., Rodrigues L.C., van Ree R., van der Zee J.S., Hoeksma-Kruize Y.C., Souverijn J.H. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004;189:892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 85.Feary J., Britton J., Leonardi-Bee J. Atopy and current intestinal parasite infection: a systematic review and meta-analysis. Allergy. 2011;66:569–578. doi: 10.1111/j.1398-9995.2010.02512.x. [DOI] [PubMed] [Google Scholar]
- 86.Mutapi F., Imai N., Nausch N., Bourke C.D., Rujeni N., Mitchell K.M. Schistosome infection intensity is inversely related to auto-reactive antibody levels. PLoS One. 2011;6:e19149. doi: 10.1371/journal.pone.0019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McKay D.M. Not all parasites are protective. Parasite Immunol. 2015;37:324–332. doi: 10.1111/pim.12160. [DOI] [PubMed] [Google Scholar]
- 88.Smits H.H., Hartgers F.C., Yazdanbakhsh M. Helminth infections: protection from atopic disorders. Curr Allergy Asthma Rep. 2005;5:42–50. doi: 10.1007/s11882-005-0053-5. [DOI] [PubMed] [Google Scholar]
- 89.Maizels R.M., McSorley H.J., Smyth D.J. Helminths in the hygiene hypothesis—sooner or later? Clin Exp Immunol. 2014;177:38–46. doi: 10.1111/cei.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weinstock J.V., Elliott D.E. Translatability of helminth therapy in inflammatory bowel diseases. Int J Parasitol. 2013;43:245–251. doi: 10.1016/j.ijpara.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Correale J., Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 92.Correale J., Farez M.F. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233:6–11. doi: 10.1016/j.jneuroim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Smits H.H., Everts B., Hartgers F.C., Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10:3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Evans H., Mitre E. Worms as therapeutics for allergy: understanding why benefits in animal studies have not translated into clinical success. J Allergy Clin Immunol. 2015;135:343–353. doi: 10.1016/j.jaci.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 95.Amu S., Saunders S.P., Kronenberg M., Mangan N.E., Atzberger A., Fallon P.G. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 96.Smith P., Mangan N.E., Walsh C.M., Fallon R.E., McKenzie A.N.J., van Rooijen N. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 97.Summers R.W., Elliott D.E., Urban J.F., Jr., Thompson R.A., Weinstock J.V. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Bager P., Arnved J., Rønborg S., Wohlfahrt J., Poulsen L.K., Westergaard T. Trichuris suis ova therapy for allergic rhinitis: A randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Garg S.K., Croft A.M., Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2014;(1):CD009400. doi: 10.1002/14651858.CD009400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daniłowicz-Luebert E., O'Regan N.L., Steinfelder S., Hartmann S. Modulation of specific and allergy-related immune responses by helminths. J Biomed Biotechnol. 2011;2011:821578. doi: 10.1155/2011/821578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McSorley H.J., Hewitson J.P., Maizels R.M. Immunomodulation by helminth parasites: Defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 102.Shepherd C., Navarro S., Wangchuk P., Wilson D., Daly N.L., Loukas A. Identifying the immunomodulatory components of helminths. Parasite Immunol. 2015;37:293–303. doi: 10.1111/pim.12192. [DOI] [PubMed] [Google Scholar]
- 103.McSorley H.J., Blair N.F., Smith K.A., McKenzie A.N.J., Maizels R.M. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7:1068–1078. doi: 10.1038/mi.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schnoeller C., Rausch S., Pillai S., Avagyan A., Wittig B.M., Loddenkemper C. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180:4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 105.Hams E., Bermingham R., Wurlod F.A., Hogan A.E., O'Shea D., Preston R.J. The helminth T2 RNase omega1 promotes metabolic homeostasis in an IL-33- and group 2 innate lymphoid cell-dependent mechanism. FASEB J. 2016;30:824–835. doi: 10.1096/fj.15-277822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marshall F.A., Watson K.A., Garside P., Harnett M.M., Harnett W. Effect of activated antigen-specific B cells on ES-62-mediated modulation of effector function of heterologous antigen-specific T cells in vivo. Immunology. 2008;123:411–425. doi: 10.1111/j.1365-2567.2007.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harnett W., Goodridge H.S., Allen J.M., Harnett M. Receptor usage by the Acanthocheilonema viteae-derived immunomodulator, ES-62. Exp Parasitol. 2012;132:97–102. doi: 10.1016/j.exppara.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Aprahamian T.R., Zhong X., Amir S., Binder C.J., Chiang L.K., Al-Riyami L. The immunomodulatory parasitic worm product ES-62 reduces lupus-associated accelerated atherosclerosis in a mouse model. Int J Parasitol. 2015;45:203–207. doi: 10.1016/j.ijpara.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zaccone P., Burton O.T., Gibbs S.E., Miller N., Jones F.M., Schramm G. The S. mansoni glycoprotein ω-1 induces Foxp3 expression in NOD mouse CD4+ T cells. Eur J Immunol. 2011;41:2709–2718. doi: 10.1002/eji.201141429. [DOI] [PubMed] [Google Scholar]
- 110.Robinson M.W., Donnelly S., Hutchinson A.T., To J., Taylor N.L., Norton R.S. A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLOS Pathogens. 2011;7:e1002042. doi: 10.1371/journal.ppat.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinson M.W., Alvarado R., To J., Hutchinson A.T., Dowdell S.N., Lund M. A helminth cathelicidin-like protein suppresses antigen processing and presentation in macrophages via inhibition of lysosomal vATPase. FASEB J. 2012;26:4614–4627. doi: 10.1096/fj.12-213876. [DOI] [PubMed] [Google Scholar]
- 112.Manoury B., Gregory W.F., Maizels R.M., Watts C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr Biol. 2001;11:447–451. doi: 10.1016/s0960-9822(01)00118-x. [DOI] [PubMed] [Google Scholar]
- 113.Dainichi T., Maekawa Y., Ishii K., Zhang T., Nashed B.F., Sakai T. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen- specific immune response. Infect Immun. 2001;69:7380–7386. doi: 10.1128/IAI.69.12.7380-7386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klotz C., Ziegler T., Figueiredo A.S., Rausch S., Hepworth M.R., Obsivac N. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011;7:e1001248. doi: 10.1371/journal.ppat.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ziegler T., Rausch S., Steinfelder S., Klotz C., Hepworth M.R., Kuhl A.A. A novel regulatory macrophage Induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J Immunol. 2015;194:1555–1564. doi: 10.4049/jimmunol.1401217. [DOI] [PubMed] [Google Scholar]
- 116.Huang L., Appleton J.A. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol. 2016 doi: 10.1016/j.pt.2016.05.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McSorley H.J., O'Gorman M.T., Blair N., Sutherland T.E., Filbey K.J., Maizels R.M. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. 2012;42:2667–2682. doi: 10.1002/eji.201142161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trujillo-Vargas C.M., Werner-Klein M., Wohlleben G., Polte T., Hansen G., Ehlers S. Helminth derived products inhibit the development of allergic responses in mice. Am J Respir Cell Mol Biol. 2007;175:336–344. doi: 10.1164/rccm.200601-054OC. [DOI] [PubMed] [Google Scholar]
- 119.Schabussova I., Ul-Haq O., Hoflehner E., Akgün J., Wagner A., Loupal G. Oesophagostomum dentatum extract modulates T cell-dependent immune responses to bystander antigens and prevents the development of allergy in mice. PLoS One. 2013;8:e67544. doi: 10.1371/journal.pone.0067544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reynolds L.A., Finlay B.B., Maizels R.M. Cohabitation in the intestine: interactions among helminth parasites, bacterial microbiota, and host immunity. J Immunol. 2015;195:4059–4066. doi: 10.4049/jimmunol.1501432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gause W.C., Maizels R.M. Macrobiota—helminths as active participants and partners of the microbiota in host intestinal homeostasis. Curr Opin Microbiol. 2016;32:14–18. doi: 10.1016/j.mib.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaiss M.M., Harris N.L. Interactions between the intestinal microbiome and helminth parasites. Parasite Immunol. 2016;38:5–11. doi: 10.1111/pim.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Walk S.T., Blum A.M., Ewing S.A., Weinstock J.V., Young V.B. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reynolds L.A., Smith K.A., Filbey K.J., Harcus Y., Hewitson J.P., Yebra M. Commensal-pathogen interactions in the intestinal tract: lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes. 2014;5:10–19. doi: 10.4161/gmic.32155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holm J.B., Sorobetea D., Kiilerich P., Ramayo-Caldas Y., Estelle J., Ma T. Chronic Trichuris muris infection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of lactobacilli. PLoS One. 2015;10:e0125495. doi: 10.1371/journal.pone.0125495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Houlden A., Hayes K.S., Bancroft A.J., Worthington J.J., Wang P., Grencis R.K. Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: effects reversed by pathogen clearance. PLoS One. 2015;10:e0125945. doi: 10.1371/journal.pone.0125945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zaiss M.M., Rapin A., Lebon L., Dubey L.K., Mosconi I., Sarter K. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity. 2015;43:998–1010. doi: 10.1016/j.immuni.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohnmacht C., Park J.H., Cording S., Wing J.B., Atarashi K., Obata Y. The microbiota regulates type 2 immunity through RORgammat+ T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 129.Brestoff J.R., Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Odegaard J.I., Chawla A. Type 2 responses at the interface between immunity and fat metabolism. Curr Opin Immunol. 2015;36:67–72. doi: 10.1016/j.coi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wiria A.E., Sartono E., Supali T., Yazdanbakhsh M. Helminth infections, type-2 immune response, and metabolic syndrome. PLoS Pathog. 2014;10:e1004140. doi: 10.1371/journal.ppat.1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu D., Molofsky A.B., Liang H.E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hussaarts L., Garcia-Tardon N., van Beek L., Heemskerk M.M., Haeberlein S., van der Zon G.C. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29:3027–3039. doi: 10.1096/fj.14-266239. [DOI] [PubMed] [Google Scholar]
- 134.Herrera L.A., Ostrosky-Wegman P. Do helminths play a role in carcinogenesis? Trends Parasitol. 2001;17:172–175. doi: 10.1016/s1471-4922(00)01942-5. [DOI] [PubMed] [Google Scholar]
- 135.Mayer D.A., Fried B. The role of helminth infections in carcinogenesis. Adv Parasitol. 2007;65:239–296. doi: 10.1016/S0065-308X(07)65004-0. [DOI] [PubMed] [Google Scholar]
- 136.Honeycutt J., Hammam O., Fu C.L., Hsieh M.H. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol. 2014;30:324–332. doi: 10.1016/j.pt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smout M.J., Laha T., Mulvenna J., Sripa B., Suttiprapa S., Jones A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smout M.J., Sotillo J., Laha T., Papatpremsiri A., Rinaldi G., Pimenta R.N. Carcinogenic parasite secretes growth factor that accelerates wound healing and potentially promotes neoplasia. PLoS Pathog. 2015;11:e1005209. doi: 10.1371/journal.ppat.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X., Chen W., Huang Y., Sun J., Men J., Liu H. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. 2011;12:R107. doi: 10.1186/gb-2011-12-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sripa B., Brindley P.J., Mulvenna J., Laha T., Smout M.J., Mairiang E. The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saleem S.J., Martin R.K., Morales J.K., Sturgill J.L., Gibb D.R., Graham L. Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J Immunol. 2012;189:511–515. doi: 10.4049/jimmunol.1200647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morales J.K., Saleem S.J., Martin R.K., Saunders B.L., Barnstein B.O., Faber T.W. Myeloid-derived suppressor cells enhance IgE-mediated mast cell responses. J Leukoc Biol. 2014;95:643–650. doi: 10.1189/jlb.0913510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Valanparambil R.M., Tam M., Jardim A., Geary T.G., Stevenson M.M. Primary Heligmosomoides polygyrus bakeri infection induces myeloid-derived suppressor cells that suppress CD4+ Th2 responses and promote chronic infection. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.36. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 144.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van der Burg S.H., Arens R., Ossendorp F., van Hall T., Melief C.J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 146.Saenz S.A., Taylor B.C., Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hammad H., Lambrecht B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 148.Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Willart M.A., Deswarte K., Pouliot P., Braun H., Beyaert R., Lambrecht B.N. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maizels R.M., Hewitson J.P., Smith K.A. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;25:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ajendra J., Specht S., Neumann A.L., Gondorf F., Schmidt D., Gentil K. ST2 deficiency does not impair type 2 immune responses during chronic filarial infection but leads to an increased microfilaremia due to an impaired splenic microfilarial clearance. PLoS One. 2014;9:e93072. doi: 10.1371/journal.pone.0093072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hung L.Y., Lewkowich I.P., Dawson L.A., Downey J., Yang Y., Smith D.E. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci U S A. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scalfone L.K., Nel H.J., Gagliardo L.F., Cameron J.L., Al-Shokri S., Leifer C.A. Participation of MyD88 and interleukin-33 as innate drivers of Th2 immunity to Trichinella spiralis. Infect Immun. 2013;81:1354–1363. doi: 10.1128/IAI.01307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Z., Grinchuk V., Urban J.F., Jr., Bohl J., Sun R., Notari L. Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS One. 2013;8:e59441. doi: 10.1371/journal.pone.0059441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bouchery T., Kyle R., Camberis M., Shepherd A., Filbey K., Smith A. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat Commun. 2015;6:6970. doi: 10.1038/ncomms7970. [DOI] [PubMed] [Google Scholar]
- 156.Yasuda K., Muto T., Kawagoe T., Matsumoto M., Sasaki Y., Matsushita K. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Humphreys N.E., Xu D., Hepworth M.R., Liew F.Y., Grencis R.K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 158.Buck A.H., Coakley G., Simbari F., Kumar S., Lear M., Abreu-Goodget C. Exosomes secreted by a nematode parasite transfer RNA to mammalian cells and regulate genes of the innate immune system. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hewitson J.P., Filbey K.J., Esser-von Bieren J., Camberis M., Schwartz C., Murray J. Concerted activity of IgG1 antibodies and IL-4/IL-25-dependent effector cells trap helminth larvae in the tissues following vaccination with defined secreted antigens, providing sterile immunity to challenge infection. PLoS Pathog. 2015;11:e1004676. doi: 10.1371/journal.ppat.1004676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zaiss M.M., Maslowski K.M., Mosconi I., Guenat N., Marsland B.J., Harris N.L. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog. 2013;9:e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mearns H., Forbes-Blom E.E., Camberis M., Tang S.C., Kyle R., Harvie M. IL-25 exhibits disparate roles during Th2-cell differentiation versus effector function. Eur J Immunol. 2014;44:1976–1980. doi: 10.1002/eji.201344400. [DOI] [PubMed] [Google Scholar]
- 162.Gerbe F., Sidot E., Smyth D.J., Ohmoto M., Matsumoto I., Dardalhon V. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.von Moltke J., Ji M., Liang H.E., Locksley R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Howitt M.R., Lavoie S., Michaud M., Blum A.M., Tran S.V., Weinstock J.V. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Diemert D.J., Pinto A.G., Freire J., Jariwala A., Santiago H., Hamilton R.G. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130:169–176.e6. doi: 10.1016/j.jaci.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 166.Hewitson J.P., Filbey K.J., Grainger J.R., Dowle A.A., Pearson M., Murray J. Heligmosomoides polygyrus elicits a dominant nonprotective antibody response directed at restricted glycan and peptide epitopes. J Immunol. 2011;187:4764–4777. doi: 10.4049/jimmunol.1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]