Abstract
Background
Infectious crystalline keratopathy is a rare, progressive infection characterized by the insidious progression of branches and crystalline corneal opacities with minimal or no inflammation. This case report describes the evolution of an infectious crystalline keratopathy caused by Cladosporium sp., which developed after tectonic keratoplasty in a patient with a history of ocular trauma.
Case presentation
A 40-year-old Brazilian male was the victim of firework-induced trauma to the left eye, which resulted in a corneal laceration that could not be sutured as well as a severe traumatic cataract. The patient underwent penetrating keratoplasty and phacoemulsification. During postoperative follow-up, another therapeutic keratoplasty was required because unresponsive infectious keratitis was observed. The infiltrate’s characteristics were suggestive of infectious crystalline keratopathy; in particular, the infiltrate was insidious and progressive, and grayish-white branches appeared in the anterior corneal stroma. As different therapies were administered, inflammatory reactions ranging from mild to severe were observed. The infection was unresponsive to typical antifungal drugs. This lack of response most likely occurred due to steroid treatment and the diffuse corneal spread of an atypical microorganism, which was subsequently identified in culture as Cladosporium sp. After the second therapeutic keratoplasty, the patient’s eye integrity was successfully reestablished.
Conclusion
This study likely provides the first report describing a case of infectious crystalline keratopathy caused by Cladosporium sp. This case emphasizes the clinical characteristics and outcome of this type of keratitis.
Keywords: cornea, keratitis, Cladosporium, penetrating keratoplasty, corneal ulcer
Background
Infectious keratitis is a frequent cause of blindness and ocular morbidity in developing countries.1 Infectious crystalline keratopathy (ICK) is a rare, progressive infection characterized by the insidious progression of branches and crystalline corneal opacities with minimal or no inflammation. Predisposing factors most commonly include topical corticosteroids, previous corneal surgery, herpetic keratitis, neurotrophic keratopathy, and topical anesthetic abuse. The appearance and evolution of ICK are typically the results of clusters of bacterial colonies, particularly streptococcus viridan colonies.2,3 This is the first description of a case of Cladosporium sp.-induced infectious crystalline keratopathy, although other forms of keratitis caused by Cladosporium have been described.
Case presentation
The patient was a 40-year-old previously healthy male, who was referred to a general ophthalmologist 12 hours after experiencing firework-induced ocular trauma to his left eye (oculus sinister [OS]). The right eye was normal at the time of examination. The trauma resulted in a lacerated and perforated cornea that could not be sutured, with an associated severe traumatic cataract. A spontaneous positive result was obtained in the Seidel test. The patient’s injured eye had a shallow anterior chamber and exhibited visual acuity (VA) of hand motion. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
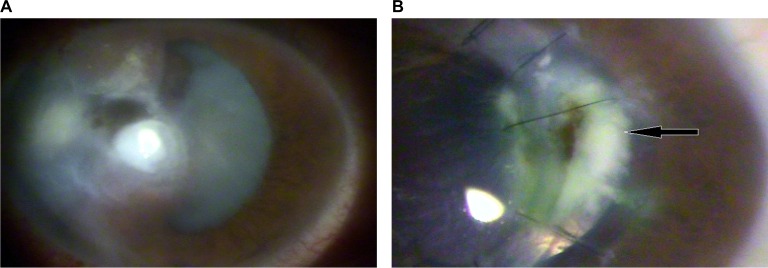
A bandage contact lens (CL) was placed, and tobramycin 0.3% (Tobrex®; Alcon Brazil, São Paulo, SP, Brazil) and moxifloxacin 0.5% (Vigamox®; Alcon Brazil) were prescribed for a period of 4 weeks, until total closure of the cornea injury and negative Seidel test (Figure 1A). The eyedrops prescribed were the free samples available at the general ophthalmologist clinic. The patient had no financial resources for buying eyedrops. After 6 months, the patient underwent optical penetrating keratoplasty without complications (donor: 7.75 mm, recipient: 7.50 mm). On the 40th postoperative (PO) day, the patient returned with an intraocular pressure of 28 mmHg and was prescribed brinzolamide 1% (Azopt®, Alcon Brazil). At 4 months after the operation, the cornea was clear, and two loose sutures were removed.
Figure 1.
(A) Left eye at first evaluation. (B) 50th DOI: the beginning of the infiltrate (black arrow) accumulation.
Abbreviation: DOI, day of infection.
During the seventh PO month, phacoemulsification was performed, and an “in-the-bag” intraocular lens was implanted. On the first PO day, the eye was calm and exhibited an uncorrected VA of 20/400. However, on the fifth PO day, the patient returned with complaints of mild pain, redness, tearing, and decreased vision. An infiltrate was found around a loose suture on the graft’s temporal interface. The infiltrate was whitish, had well-defined margins, measured 2×1 mm (a slit lamp scale was used to measure the infiltrate’s size), and affected up to 50% of the stromal depth. The patient also presented with a fibrin pupillary membrane and a VA of light perception. The infiltrate was not cultured due to the lack of a laboratory in the region where the patient was being treated. The following regimen of eyedrops was prescribed empirically: Vigamox® (Alcon, Brazil) every hour; moxifloxacin 0.5% + dexamethasone 0.1% (Vigadexa®; Alcon Brazil) four times per day; and nepafenac 0.1% (Nevanac®; Alcon Brazil) and brimonidine tartrate 0.2% + timolol 0.5% (Combigan®; Allergan Brazil, Guarulhos, SP, Brazil) two times per day.
On the fifth day of infection (DOI), the patient reported improvement in symptoms, although the infiltrate remained unchanged. At this time, treatment with Vigadexa®, Nevanac®, and Combigan® was maintained. However, on the 50th DOI, the patient experienced pain and a marked decrease in VA in his OS. Examinations revealed that the infiltrate had increased to a size of 3.6×2.5 mm. In addition, central pigmentation and feathery margins were found, as well as a shallow anterior chamber and a positive Seidel test (Figure 1B).
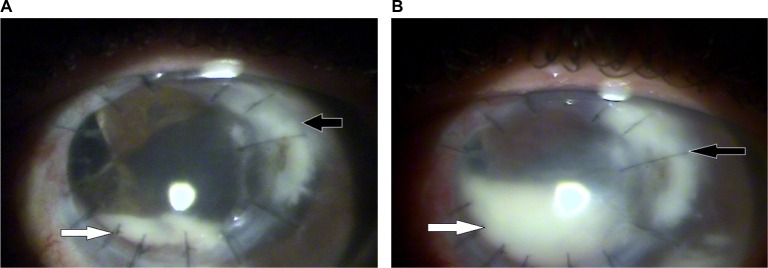
A bandage CL was placed, and Vigadexa® was discontinued because the ophthalmologist wanted to observe the infiltrate’s progression without the use of antibiotics or steroids. A marked increase in temporal limbal injection, a 2 mm hypopyon, and increased density of the infiltrate at the recipient border (Figure 2A) were observed on the 60th DOI. Ultrasonography revealed no changes in the posterior segment. Fungal infection was suspected; thus, the patient was prescribed hourly natamycin 5% (Pimaricina®; Ophthalmos, São Paulo, SP, Brazil), daily fluconazole 150 mg (Medley, Campinas, SP, Brazil), and twice-daily Vigadexa®. On the 70th DOI, the continued persistence of a positive Seidel test and increased infiltrate density and size (up to 5.4×3.8 mm) were observed. In addition, the hypopyon occupied half of the anterior chamber (Figure 2B).
Figure 2.
(A) 60th DOI: the infiltrate’s progression and the hypopyon. (B) 70th DOI. Note: Black arrow: infiltrate; white arrow: hypopyon.
Abbreviation: DOI, day of infection.
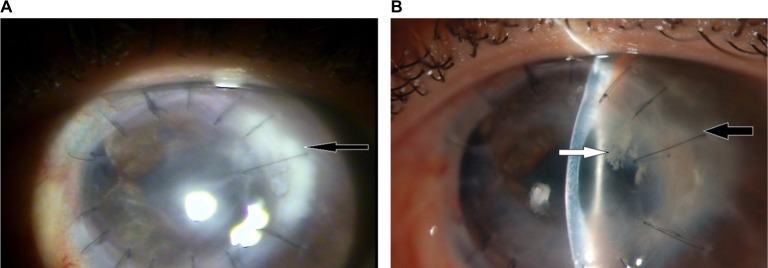
At this time, the treatment regimen was maintained by the ophthalmologist. On the 85th DOI, the patient experienced significant reduction in redness and pain, and there was a decrease in infiltrate density and size (to 4.6×2.5 mm), negative Seidel test results, and no hypopyon (Figure 3A). VA of light perception persisted. The bandage CL was removed, and the eyedrops were maintained.
Figure 3.
(A) 85th DOI: improvement in the infiltrate size and the hypopyon. (B), 140th DOI. Note: (A, B) black arrow: infiltrate; (B) white arrow: new infiltrate.
Abbreviation: DOI, day of infection.
After 4 months of infection, the patient had no complaints of pain, although examination revealed that the infiltrate remained unchanged. Natamycin was decreased to three times per day, and fluconazole was maintained at 150 mg/d. The lack of improvement in the infection motivated the referral of the patient to an ophthalmic reference hospital. At this hospital, the possibility of a bacterial etiology was reconsidered. Therefore, empirical therapy with topical vancomycin 5% (Ophthalmos) every hour, Vigamox® four times per day, and fluorometholone acetate 0.1% (Flutinol®; Latinofarma, Cotia, SP, Brazil) once per day was initiated, and the antifungal medication was discontinued. When the patient returned 19 days later, the size of the initial infiltrate had decreased to 3.8×2.5 mm2. However, a new infiltrate measuring 2.5×1.7 mm2, forming a white opacity with the aspect of small needles and without surrounding inflammation or edema, was observed in the anterior stromal third of the graft, which made the assistant ophthalmologist think on the possibility of ICK (Figure 3B).
Antibacterial treatment was held due to the fact that bacteria are the most common etiology of ICK. In addition, edema was present in the inferior third of the graft. At 6 months after the onset of the infection, an increase in the size and density of the infiltrate was observed, with the infiltrate measuring 6.8×4.5 mm. At this point, the medications were discontinued. Due to the clinical appearance of the lesion and the worsening of the patient’s condition after the use of antibiotics, an antifungal regimen of natamycin 5% every hour and fluconazole 150 mg/d was restarted.
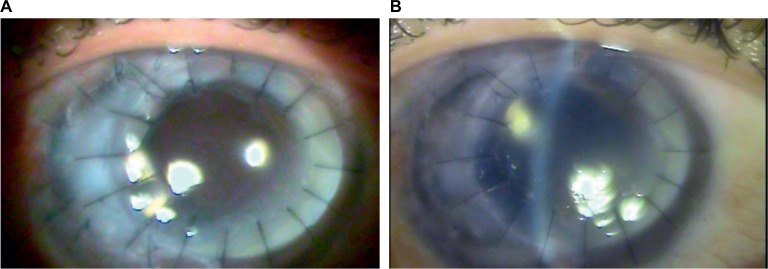
After 8 months of infection, the injury remained unchanged, and a positive Seidel test and a shallow anterior chamber were observed again. Thus, another therapeutic keratoplasty was indicated. To remove the peripheral corneal infiltrate, this keratoplasty (donor: 7.75 mm, recipient: 7.50 mm) was performed in an off-center manner, with a deviation toward the temporal limbus. The excised cornea was sent for culture. The patient continued to be treated with Flutinol® once per day, Vigamox® four times per day, Combigan® two times per day, natamycin 5% every 3 hours, and fluconazole 150 mg/d. On the sixth PO day, the patient’s uncorrected VA was 20/400. The graft was clear and exhibited no signs of active inflammation or infection (Figure 4A). At the first PO month, the graft remained clear, and no signs of recurrence were observed. At the second PO month, natamycin and fluconazole were discontinued, although treatment with Flutinol® once per day and Combigan® two times per day was maintained. The culture results, which were obtained after 3 months, were positive for Cladosporium sp. The final follow-up, which occurred at the sixth PO month (Figure 4B), revealed a clear graft, no signs of recurrence, and an uncorrected VA value of 20/200. The patient was subsequently maintained without medications.
Figure 4.
(A) Regraft at the sixth PO day. (B) Regraft at the sixth PO month.
Abbreviation: PO, postoperative.
Discussion
ICK is a unique corneal infection characterized by the slowly progressive development of needle-like opacities in the corneal stroma, most commonly caused by streptococcal species. This condition is often difficult to eradicate due to the presence of a biofilm produced by the infecting organism, which causes increased resistance to treatment. Some species of fungi, such as Candida sp., have also been identified as etiological pathogens for this condition.4 Occasionally, Cladosporium sp. is found to be responsible for fungal keratopathy, but to our best knowledge, this is the first report of ICK caused by Cladosporium sp. In the described case, a fungal etiology for the patient’s condition was not initially suspected because ICK is generally caused by bacteria and is only rarely induced by fungi.
The Cladosporium genus is a group of fungi that are extremely common and that exhibit the potential to inhabit multiple environments. These fungi characteristically form punctate colonies that are dark olive or grayish in color. The ability of these fungi to grow on various substrates, such as plants, wood, paper, leather, and food, can be attributed to their abundance of airborne conidia. Cladosporium spores are found in outdoor aerosols, and the quantity of these spores increases in the summer and warmer months.5,6
In the described case, the evolution of the infection was insidious and progressive, involving the appearance of grayish-white branches in the anterior corneal stroma. The inflammatory reaction varied, with different presentations as various treatments were administered. Clinical observation revealed that the Cladosporium sp. exhibited only a weak response to treatment with topical natamycin and oral fluconazole.
Antifungal susceptibility testing in the literature indicates that the Cladosporium genus is sensitive to amphotericin B (minimum inhibitory concentration [MIC] =0.03–8 µg/mL), itraconazole (MIC =0.03–32 µg/mL), and voriconazole (MIC =0.06–1 µg/mL).7 Our first-choice drug for fungal keratitis is topical natamycin 5% because of its broad antifungal spectrum; therefore, this drug was the proposed treatment when fungal keratitis was suspected.
In general, treatment for ICK includes antibiotics that combat the most common bacterial agents, especially viridans streptococci. In particular, penicillin, cefazolin and vancomycin are administered via fortified eyedrops.8 This practice justifies the fact that in the described case, vancomycin 5% was prescribed when ICK was suspected at the reference hospital, based on this condition’s frequent bacterial etiology.
A consensus in the literature is that steroid use for keratitis cases should usually be discontinued as quickly as possible when a fungal etiology is suspected. In the described case, this fact is relevant because steroids were used consistently throughout the treatment by the general ophthalmologist; thus, steroid treatment probably acted as a predisposing factor for the infection’s progression. Because of a lack of response to the prescribed therapy, the reported patient had to undergo a second therapeutic keratoplasty. The acquisition of a positive culture early in the keratitis course could have greatly improved this patient’s outcome. If available, antifungal susceptibility tests could also have helped to select the most effective antifungal drug for the case.
Another interesting observation was the infiltrate’s pigmentation on the 50th DOI. This rare finding indicated that the pathogen produced pigments. Cladosporium sp., a dematiaceous fungus, is characterized by its pigmented hyphae and conidia. Thus, if an examiner notices this finding in similar cases, it could be a clue toward a diagnosis of fungal keratitis.
Conclusion
In the authors’ view, this case has scientific relevance not only for being the first report of ICK caused by Cladosporium sp. but also for emphasizing the importance of caution when prescribing topical steroids to patients with corneal infiltrates. In addition, the use of topical cyclosporin A instead of steroids is a suitable option for patients undergoing keratoplasty, particularly in cases of suspected or confirmed fungal keratitis. Cyclosporin A, which belongs to the class of drugs known as calcineurin inhibitors, not only lacks the deleterious effects of steroids in cases involving infections but also exhibits fungistatic activity and synergizes with most other antifungal drugs.1,9,10
Footnotes
Author contributions
RAS examined and treated the patient. EC performed the acquisition of the data and the literature review. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Miller WL, Giannoni AG, Perrigin J. A case of fungal keratitis: a clinical and in vivo confocal microscopy assessment. Cont Lens Anterior Eye. 2008;31(4):201–206. doi: 10.1016/j.clae.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell BM, Wilhelmus KR. Inflammatory response to fungal keratitis. Ocul Surf. 2005;3(4 suppl):S152–S153. doi: 10.1016/s1542-0124(12)70243-4. [DOI] [PubMed] [Google Scholar]
- 4.Ainbinder DJ, Parmley VC, Mader TH, Nelson ML. Infectious crystalline keratopathy caused by Candida guiliermondii. Am J Ophthalmol. 1998;125(5):723–725. doi: 10.1016/s0002-9394(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 5.Zeng QY, Westermark SO, Rasmuson-Lestander A, Wang XR. Detection and quantification of Cladosporium in aerosols by real-time PCR. J Environ Monit. 2006;8(1):153–160. doi: 10.1039/b509515h. [DOI] [PubMed] [Google Scholar]
- 6.Dixon DM, Polak-Wyss A. The medically important dematiaceous fungi and their identification. Mycoses. 1991;34(1–2):1–18. doi: 10.1111/j.1439-0507.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A, Boyle K, Sheehan DJ. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia. 2001;150(3):101–115. doi: 10.1023/a:1010954803886. [DOI] [PubMed] [Google Scholar]
- 8.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother. 2000;44(9):2373–2381. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbach WJ, Reedy JL, Cramer RA, Jr, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5(6):418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]