Abstract
Purpose
In this work, we propose an in situ precise electrospinning of medical glue fibers onto dural wound for improving sealing capability, avoiding tissue adhesion, and saving time in dural repair.
Methods
N-octyl-2-cyanoacrylate, a commercial tissue adhesive (medical glue), can be electrospun into ultrathin fibrous film with precise and homogeneous deposition by a gas-assisted electrospinning device.
Results
The self-assembled N-octyl-2-cyanoacrylate film shows high compactness and flexibility owing to its fibrous structure. Simulation experiments on egg membranes and goat meninges demonstrated that this technology can repair small membrane defects quickly and efficiently.
Conclusion
This method may have potential application in dural repair, for example, working as an effective supplementary technique for conventional dura suture.
Keywords: cyanoacrylates, electrospun fibers, in situ repair dural, gas-assisted electrospinning apparatus
Introduction
In recent years, technological advancement and technical improvement have lowered morbidity and mortality rates of many neurosurgical procedures.1–3 However, dural repair and reconstruction after major operations remain a challenge for neurosurgeons.4,5 It is well known that intact dura matters cover the brain surface and function as a natural barrier to prevent cerebrospinal fluid (CSF) leakage. Postoperative CSF leakage is a life-threatening complication that can lead to various consequences, such as meningeal pseudocyst and arachnoiditis.6–8 Currently, suture remains the most frequently adopted methods in dural repair regardless of the location of defects. However, suture operation, even dural destretching suture, is very difficult to be carried out, when dural defects are friable or located in relatively inaccessible areas.9 In particular, the prevalence of meningeal tear accompanied with spinal operations urges the discovery of more safe and efficient dural repair techniques.
Cyanoacrylates (CAs),10 fibrin glue,11 and CO2 laser12 have been used to achieve dural closure, to prevent infections, and to provide a surface along which the dura can be regenerated. CAs are the generic name of the CA-based “fast-acting” adhesives, such as methy-2-cyanoacrylate and ethyl-2-cyanoacrylate (commonly sold under trade names like super glue). N-octyl-2-cyanoacrylate (NOCA), an easily biocompatible and bioresorbable polymer glue, provides an alternative to conventional rigid fixation techniques.13 CA medical adhesives have been investigated for use as tissue adhesive in wound closure since 1959.14 Compared to other materials, CAs have several advantages, including convenience of use, relative painlessness, and no need of removal, which provide an acceptable cosmetic effect for external use.15 The CA tissue adhesives are liquid monomers that become self-polymerized in an exothermic reaction upon contact with tissue surfaces and form strong yet flexible film bondings on the apposed wound edges.16 The traditional methods to deliver NOCA adhesive include injection, daubing, and spraying, all of which are problematic. First, it is difficult to control the homogeneity and thickness of the coating layer with those traditional techniques. Second, it is difficult to control the deposition range in adherence to surrounding tissue, which may cause serious tissue adhesion and inflammatory response after dural repair. Furthermore, the tissue adhesions are associated with numerous postsurgical complications, including patient’s pain, functional obstruction, and sometimes difficult reoperative surgery.17
Electrospinning has been recognized as a highly facile and efficient nanotechnology to produce continuous fibers with diameters ranging from a few micrometers to a few nanometers.18 Various polymers major in solvent solution and melt form have been successfully electrospun into ultrathin fibers in recent years.19 A thin charged jet is formed when the electrostatic force generated by a high operating voltage overcomes the surface tension of the polymer droplet during conventional eletrospinning. The jet is accelerated toward the grounded collector and produces fibers in the form of a nonwoven mat.20 With large surface-to-volume ratio and high porosity, electrospun nanofibers from natural and synthetic biomedical polymers can be made for applications in biomedical,21–23 energy storage,24,25 environment engineering,26–28 health care,29,30 biotechnology,31,32 etc.
In this study, we attempt to develop NOCA ultrathin fibers by the solvent-free electrospinning technique, in which the NOCA monomers undergo self-assembly into micro/nanofibers in the presence of hydroxide ions. This study is expected to introduce for the first time a nonsuture dural repair technique via electrospun NOCA fibers. A novel gas-assisted electrospinning apparatus was self-designed to in situ electrospin NOCA fibers on the dura defects. After electrospinning, we characterized the morphology and structure of the NOCA fibers. In vitro experiment revealed that the self-assembled electrospun NOCA membrane has properties of high strength, good flexibility, and waterproofness without resulting in leakage even under a pressure of 78 mmHg. Furthermore, we showed that the gas-assisted electrospinning apparatus could precisely deposit ultrafine fibers on the desired site to form a continuous and compact protective film and avoid tissue adhesion in the simulation experiment.
Methods
Medical glue (Otologic and Craniocerebral Glue series), mainly composed of NOCA and a little medical poly(methyl methacrylate) as an additive (in order to increase viscosity), was purchased from Guangzhou Baiyun Medical Adhesive Co., Ltd (Guangzhou, People’s Republic of China) and used directly without further treatments.
Gas-assisted electrospinning apparatus
A gas-assisted electrospinning apparatus is self-designed to electrospin NOCA fibers and achieve precise deposition for dural repair (Figure 1). The apparatus consists of the following four parts: high-voltage power supply, syringe pump, air pump, and a three-way handle spinneret.33 The handle spinneret is connected to the positive electrode of the high-voltage power supply (DW-P303-1ACFO; Tianjin Dongwen, Tianjin, People’s Republic of China), which could provide 6–10 kV voltage to produce fibers. A syringe pump is applied to maintain a stable and precise feeding rate of the electrospun solution. In addition, to facilitate the electrospinning process and control the fiber deposition scope, we connected a gas pump to the auxiliary spinneret, which blows and exerts a stretching force on the electrospinning solution.
Figure 1.
A schematic illustration of the in situ electrospinning of NOCA nanofibers for dural repair.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
Sample characterization and evaluation
The morphologies of the NOCA ultrathin fibers were characterized by optical microscope (BX51; Olympus Corporation, Tokyo, Japan) and scanning electron microscopy (SEM; TM-1000; Hitachi Ltd., Tokyo, Japan). The intermolecular structure was determined by Fourier transform infrared (FTIR) spectrometer (Nicolet iN10; Thermo Fisher Scientific, Waltham, MA, USA). In vitro experiments were carried out on fresh bovine pericardium purchased from the market to study the flexibility and integrity of the NOCA nanofibrous membranes.
In vitro and in vivo surgical simulation of dural repair
In vitro simulation experiments were first carried out on eggs to determine the sealing property of the electrospun NOCA nanofibrous membranes. After removal of the outer shell, two kinds of common operative incisions were cut on egg membranes. The incisions were treated with NOCA film via the gas-assisted electrospinning apparatus. Furthermore, we carried out in vivo modeling experiment on goat brain in order to determine the efficacy of this nonsuture technique. All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the guidelines of the Animal Welfare Act. All the procedures involving animal use were approved by the Chancellor’s Animal Research Committee at Qingdao University.
Results and discussion
In vitro characterization and evaluation of NOCA nanofibrous membrane
Figure 2 shows the morphologies of the NOCA fibers prepared by the gas-assisted electrospinning apparatus. Different from the conventional electrospinning and spraying processes, the electrospun NOCA fibers were rapidly polymerized into a solid self-assembled membrane on the surface of wound when a high voltage was applied. In our present electrospinning process, the applied high voltage was 10 kV, and the distance from the spinneret to the collector was 6–8 cm. The solution feeding rate was 15 µL min−1 with the airflow velocity of 12 L min−1. Figure 2B shows the SEM image of the electrospun NOCA coating on the goat dural, which was fabricated with a feeding rate of 25 µL min−1 in 30 seconds. The self-assembled NOCA nanofibers formed a very compact film and barrier preventing leakage.
Figure 2.
Morphologies of the NOCA fiber prepared by the gas-assisted electrospinning apparatus.
Notes: SEM images of (A) NOCA nanofibers and (B) surface morphology of in situ electrospun NOCA fiber coating on the goat dural.
Abbreviations: NOCA, N-octyl-2-cyanoacrylate; SEM, scanning electron microscopy.
The structure of the electrospun NOCA polymer was characterized with FTIR spectrum analysis (Figure 3). The wave ranging from 4,000 cm−1 to 480 cm−1 was obtained. Table 1 shows the main band assignments. The peaks at 2,250 cm−1 and 1,750 cm−1 are characteristic of the –C≡N and –C=O groups of the polymer, respectively. The significant decrease in =CH–stretching vibration (3,000 cm−1) could be ascribed to the opened C=C bond of the vinyl structure in NOCA monomer unit. The presence of peak corresponding to the –OH bond at 3,400 cm−1 proved the hydroxyl group as an anionic initiator inducing polymerization. In addition, the peak corresponding to a great number of CH2 units in the main carbon chain at 724 cm−1 was also found. The presence of –C≡N stretching mode in the spectrum demonstrated the existence of NOCA in the electrospun fibers (Table 1).
Figure 3.
Fourier transform infrared spectrum of the electrospun NOCA medical glue membrane.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
Table 1.
Major mid-IR vibrational modes and wave numbers exhibited by electrospun NOCA fibers
| Description of vibrations | Wave numbers (cm−1) |
|---|---|
| –O–H stretching | 3,400 |
| C–H stretching vibrations (symmetric and asymmetric) of –CH2– and CH3–groups | 3,080–2,800 |
| –C≡N stretching vibration | 2,250 |
| –C=O stretching vibration | 1,750 |
| CH2 and CH3 scissoring and bending region | 1,500–1,350 |
| –(CH2)n– plane bending vibration | 724 |
Abbreviations: NOCA, N-octyl-2-cyanoacrylate; IR, infrared.
Due to the fragility and ossification of the dura, the suppleness of the dural closure was considered in the study. Therefore, we evaluated the flexibility of the electrospun NOCA membrane. Figure 4A–E represents several states of the bending process. First, the bovine pericardium was chosen as a substitute of the dura, and a defect of 2 cm in length was made. Then, the defect was covered by an electrospun NOCA layer using the gas-assisted electrospinning apparatus. One minute later, the membrane was bent to multiangle and recovered back to its original state without any breakage, indicating that the electrospun NOCA film had good strength, elasticity, and flexibility and thus met the requirements of dural closure and reconstruction.
Figure 4.
Optical images during the bending process to test the flexibility of the electrospun NOCA film.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
Making watertight and meticulous dural closure is an essential step after intradural neurosurgical operations. It has been reported that fibrous membrane of NOCA glue has high strength and strong tissue-bonding ability.31 We first evaluated the bonding strength and watertightness of the electrospun NOCA nanofibrous membranes in water leakage tests (Figure 5). After mounting the bovine pericardium securely on the bottom of poly(methyl methacrylate) pipe, we produced a defect with a scalpel. The defect was then covered by a layer of NOCA nanofiber film in 30 seconds using the gas-assisted electrospinning apparatus. Strikingly, we found no water leakage for 12 hours under the pressure of 78 mmHg, indicating that the electrospun NOCA fiber membrane is compact and strong. Further experiment showed that the electrospun NOCA nanofibrous membrane could stand as high as 125 mmHg pressure without water seeping, while the traditional sprayed NOCA film could only sustain a relatively low pressure of 38 mmHg.
Figure 5.

Water leakage test to evaluate watertight property of NOCA membrane.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
Different from the conventional electrospinning and spraying, one remarkable characteristic of our self-designed gas-assisted electrospinning apparatus is precise deposition on a desired site, namely, the deposition range and deposition location can be precisely controlled under the assistance of gas. As shown in Figure 6, the deposition width was investigated with different distances. With an increase in electrospinning distance, the deposition width increases gradually. When the spinning distance is 4–5 cm, the deposition width is ~8 mm.
Figure 6.
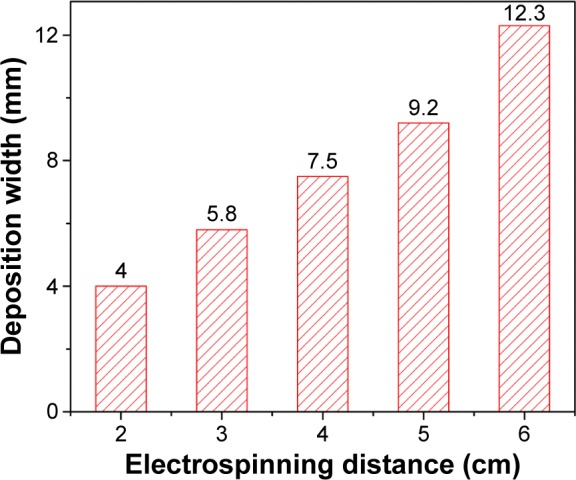
Deposition width under different electrospinning distance of NOCA fibers.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
Surgical simulation of dural repair in vitro and in vivo
The efficacy of electrospun NOCA membrane was first determined in the modeling experiment on eggs (Figure 7). The outer shell of fresh eggs was removed, and two kinds of common wounds were created on the egg membranes: one wound with a width of 2–4 cm and the other with an area of 4–9 cm2. The air pump was turned on before electrospinning, which could not only precisely direct electrospinning and deposition but also help to clean the collector surface. When a high voltage was applied, the electrospun NOCA fibers were deposited and rapidly polymerized on the wound surface to form a solid self-assembled membrane. After electrospun for 20 seconds, the dural wound was rapidly covered by a layer of NOCA fibrous film. No tissue fluid was observed when eggs were inverted 1 minute after wound closure, demonstrating that the eletrospun NOCA membrane is leakage proof.
Figure 7.
Dural repair simulation experiment in egg membrane via NOCA ultrathin fibers.
Notes: (A) Two types of defects: (i) a narrow wound with a length of 2–4 cm, a solid self-assembled membrane was formed to cover the dural wound (dotted oval; ii) and (iii) a large wound with area of 4–9 cm2, covered with a layer of mater and sprayed with NOCA to avoid tissue fluid (dotted square; iv). (B) Schematic illustration of the membrane repair of two types of wounds via electrospun NOCA fibers.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
If an unintended dural rent or pinhole tear occurs, it is tamponaded to prevent CSF loss. Figure 8 presents the operation procedure of dural repair in a goat brain using the gas-assisted electrospinning apparatus. Gross appearance of every stage in the simulation experiment was observed (as shown in Figure 8 and Video S1). Figure 8A shows that a defect of 7 cm in length was produced by the scalpel. Remarkably, considering the fragility of the large defect, the dura was primarily sutured to avert rupture (Figure 8B). Then, the NOCA fibers were precisely electrospun onto the defect by the gas-assisted electrospinning apparatus (Figure 8C). Consistently, we found that the dural defect was repaired by the NOCA membrane in 30 seconds. As shown in Figure 8D, the in situ electrospun NOCA fibers can not only produce good sealing but also have precise deposition effectively avoiding tissue adhesion, which makes the procedure more secure and simple. The above operation was repeated in ten goat brains with similar result, indicating that this technique is reliable and replicable.
Figure 8.
Dural repair in a goat brain model via the in situ electrospinning of NOCA fibers.
Notes: Optical images show the procedure of in vivo simulation experiment. (A) A defect of 7 cm in length was produced. (B) The dura was primarily sutured to avert rupture. (C) The NOCA fibers were precisely electrospun onto the defect. (D) The dural defect was repaired by the NOCA membrane. Scale bar represents 20 mm.
Abbreviation: NOCA, N-octyl-2-cyanoacrylate.
Conclusion
NOCAs were successfully electrospun into ultrathin fibrous film via the solvent-free electrospinning technique. The morphology and structure of the NOCA membrane were characterized by the SEM and FTIR. A home-made gas-assisted electrospinning apparatus was designed to realize precise and homogeneous deposit of NOCA ultrathin fibers onto the dural defects. The in vitro simulation experiment on egg membranes and in vivo experiment on goat brains showed that this flexible and compact self-assembled membrane could not only rapidly suture dural defects but also avoid tissue adhesion. Compared with typical conventional spraying, the dosage of electrospinning method is much less and thus has lower heat dissipation. This technology may be very promising in surgery especially on dural repair with high sealing capacity and low tissue adhesion. However, further studies are needed to test the practicability of this technology and the applicability of other adhesive agents as well.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (51373082), the Taishan Scholars Programme of Shandong Province, People’s Republic of China (ts20120528), and the Postdoctoral Scientific Research Foundation of Qingdao.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chowdhry SA, Spetzler RF. Genealogy of training in vascular neurosurgery. Neurosurgery. 2014;74(Suppl 1):S198–S203. doi: 10.1227/NEU.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 2.Bydon M, Abt NB, De la Garza-Ramos R, et al. Impact of resident participation on morbidity and mortality in neurosurgical procedures: an analysis of 16,098 patients. J Neurosurg. 2015;122(4):955–961. doi: 10.3171/2014.11.JNS14890. [DOI] [PubMed] [Google Scholar]
- 3.Moosa S, Chen CJ, Ding D, et al. Volume-staged versus dose-staged radiosurgery outcomes for large intracranial arteriovenous malformations. Neurosurg Focus. 2014;37(3):E18. doi: 10.3171/2014.5.FOCUS14205. [DOI] [PubMed] [Google Scholar]
- 4.Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122(2):452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 5.Velasco-Torres HS, Amador JLG, Feinholz SR. Mass effect due to hypertrophic pericranial flap in the reconstruction of dural defect. World Neurosurg. 2015;84(6):e11–e2077. doi: 10.1016/j.wneu.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 6.Westworth DR, Sturges BK. Congenital spinal malformations in small animals. Vet Clin N Am Small. 2010;40(5):951–981. doi: 10.1016/j.cvsm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Hutter G, von Felten S, Sailer MH, et al. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomized controlled trial: Clinical article. J Neurosurg. 2014;121(3):735–744. doi: 10.3171/2014.6.JNS131917. [DOI] [PubMed] [Google Scholar]
- 8.McCoul ED, Anand VK, Singh A, Nyquist GG, Schaberg MR, Schwartz TH. Long-term effectiveness of a reconstructive protocol using the nasoseptal flap after endoscopic skull base surgery. World Neurosurg. 2014;81(1):136–143. doi: 10.1016/j.wneu.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Ozisik PA, Inci S, Soylemezoglu F, Orhan H, Ozgen T. Comparative dural closure techniques: a safety study in rats. Surg Neurol. 2006;65(1):42–47. doi: 10.1016/j.surneu.2005.04.047. discussion 47. [DOI] [PubMed] [Google Scholar]
- 10.Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg. 2001;182(Suppl 2):S40–S44. doi: 10.1016/s0002-9610(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 11.Green AL, Arnaud A, Batiller J, et al. A multicentre, prospective, randomized, controlled study to evaluate the use of a fibrin sealant as an adjunct to sutured dural repair. Brit J Neurosurg. 2015;29(1):11–17. doi: 10.3109/02688697.2014.948808. [DOI] [PubMed] [Google Scholar]
- 12.Zhong H, Wang Z, Yang Z, et al. Laser soldering for the reconstruction of dural defects in the minipig model. Turk Neurosurg. 2016;26(2):240–245. doi: 10.5137/1019-5149.JTN.5579-11.1. [DOI] [PubMed] [Google Scholar]
- 13.Singer AJ, Thode HC. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187(2):238–248. doi: 10.1016/j.amjsurg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Coover HW, Joyner FB, Shearer NH, Wicker TH. Chemistry and performance of cyanoacrylate adhesives. Special Technical Papers. 1959;5:413–417. [Google Scholar]
- 15.Montanaro L, Arciola CR, Cenni E, et al. Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials. 2000;22(1):59–66. doi: 10.1016/s0142-9612(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Son HS, Jung GB, et al. Enhanced biocompatibility and wound healing properties of biodegradable polymer-modified allyl 2-cyanoacrylate tissue adhesive. Mater Sci Eng C. 2015;51:43–50. doi: 10.1016/j.msec.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Falk K, Lindman B, Bengmark S, Larsson K, Holmdahl L. Sodium polyacrylate potentiates the anti-adhesion effect of a cellulose-derived polymer. Biomaterials. 2001;22(16):2185. doi: 10.1016/s0142-9612(00)00360-4. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–2253. [Google Scholar]
- 20.Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew Chem Int Ed. 2007;46(30):5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 21.Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 2016;11(1):01418. doi: 10.1088/1468-6996/11/1/014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan A, Memic A, Annabi N, et al. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10(1):11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rim NG, Shin CS, Shin H. Current approaches to electrospun nanofibers for tissue engineering. Biomed Mater. 2013;8(1):014102. doi: 10.1088/1748-6041/8/1/014102. [DOI] [PubMed] [Google Scholar]
- 24.Jost K, Dion G, Gogotsi Y. Textile energy storage in perspective. J Mater Chem A. 2014;2(28):10776–10787. [Google Scholar]
- 25.Chen X, Xu SY, Yao N, Shi Y. 1.6 V nanogenerator for mechanical energy harvesting using PZT nanofibers. Nano Lett. 2010;10(6):2133–2137. doi: 10.1021/nl100812k. [DOI] [PubMed] [Google Scholar]
- 26.Gopal R, Kaur S, Ma Z, Chan C, Ramakrishna S, Matsuura T. Electrospun nanofibrous filtration membrane. J Membr Sci. 2006;28:581–586. [Google Scholar]
- 27.Yoon K, Hsiao BS, Chu B. Functional nanofibers for environmental applications. J Mater Chem. 2008;18:5326–5334. [Google Scholar]
- 28.Ramakrishna S, Jose R, Archana PS, et al. Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine. J Mater Sci. 2010;45:6283–6312. [Google Scholar]
- 29.Xue J, Niu Y, Gong M, et al. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano. 2015;9(2):1600–1612. doi: 10.1021/nn506255e. [DOI] [PubMed] [Google Scholar]
- 30.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF) Biomaterials. 2008;29(5):587–596. doi: 10.1016/j.biomaterials.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 31.López-Rubio A, Sanchez E, Sanz Y, Lagaron JM. Encapsulation of living bifidobacteria in ultrathin PVOH electrospun fibers. Biomacromolecules. 2009;10(10):2823–2829. doi: 10.1021/bm900660b. [DOI] [PubMed] [Google Scholar]
- 32.Sampson SL, Saraiva L, Gustafsson K, Jayasinghe SN, Robertson BD. Cell electrospinning: an in vitro and in vivo study. Small. 2014;10(1):78–82. doi: 10.1002/smll.201300804. [DOI] [PubMed] [Google Scholar]
- 33.Jiang K, Long YZ, Chen ZJ, et al. Airflow-directed in situ electrospinning of a medical glue of cyanoacrylate for rapid hemostasis in liver resection. Nanoscale. 2014;6(14):7792. doi: 10.1039/c4nr01412j. [DOI] [PubMed] [Google Scholar]








