Abstract
Purpose
Exercise (Ex) increases reactive oxygen species and impairs antioxidant defense systems. Recent data suggest that curcumin (CW) possesses peroxisome proliferator-activated receptor gamma activity and anti-inflammatory properties. Therefore, this study was designed to investigate the effects of CW supplementation on Ex performance, endurance, and changes in serum and muscle proteins in rats after exhaustive Ex.
Materials and methods
Twenty-eight (28) male Wistar rats (age: 8 weeks and body weight: 180±20 g) were divided into four treatment groups: 1) control (C; no Ex), 2) C + CW (no Ex + CW), 3) C + Ex, and 4) C + Ex + CW (Ex + CW). CW was administered as 100 mg/kg CurcuWin®, providing 20 mg of curcuminoids daily for 6 weeks. A motor-driven rodent treadmill was used to carry out the Ex protocols. During a 5-day period, animals in chronic Ex groups were put through different regimens: day 1, 10 m/min for 10 minutes; day 2, 20 m/min for 10 minutes; day 3, 25 m/min for 10 minutes; day 4, 25 m/min for 20 minutes; and day 5, 25 m/min for 30 minutes. Animals were exercised at 25 m/min for 45 min/d for 5 d/wk for 6 weeks. Blood and muscle samples were analyzed for muscle markers, oxidative stress, and antioxidant markers.
Results
Lactate and muscle malondialdehyde levels decreased in the CW-treated groups (P<0.0001). However, activities of antioxidant enzyme levels increased in the CW-treated groups. Run to exhaustion (minutes) improved in the CW-treated groups. Muscle nuclear factor-κB (P<0.05) and heat shock protein 70 (P<0.05) levels were much lowered in the CW treated group followed by Ex group. In addition, muscle inhibitors of kappa B, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, thioredoxin-1, sirtuin 1, nuclear factor (erythroid-derived 2)-like 2, and glucose transporter 4 protein levels in the Ex + CW group were higher than those in the control and Ex groups (P<0.05).
Conclusion
This study suggests that novel CW has the potential to help prevent muscle damage by regulating the nuclear factor-κB and nuclear factor (erythroid-derived 2)-like 2 pathways and improve the performance and nutritional values of CW.
Keywords: exercise, curcumin, oxidative stress, NF-kB, Nrf2, muscle
Introduction
Exercise (Ex) induces inflammation, increases reactive oxygen species (ROS), and impairs antioxidant defense systems in the skeletal muscle and blood.1 Antioxidant enzymes and vitamins enhance antioxidant defense systems to protect cells from ROS.1,2 Muscle fatigue during downhill running may lead to impaired strength and muscle damage.1 The severity of muscle damage is influenced by muscle strain and muscle contractions.2 Downhill running elicits a number of cellular adaptive changes in the skeletal muscle. Muscle mitochondrial biogenesis, fusion, and metabolism during Ex induce stress and changes in transcriptional genes.3 Skeletal mitochondrial biogenesis and function are stimulated by stress signals.
Curcumin (CW; 1,7-bis(4-hydroxy 3-methoxy phenyl)-1,6-heptadiene-3,5-dione), a natural polyphenolic compound isolated from the plant turmeric (Curcuma longa L.), has been studied for over 3 decades, and its potential benefits have been reported for oxidative stress, cancer, diabetes, inflammatory diseases, neurodegenerative diseases, and cardiometabolic health.4–8
CW’s low absorption from the gut, rapid metabolism, and rapid systemic elimination have been reported, which are due to its poor water solubility.9 In a recent study,9 a novel formulation of CW that was made water soluble by dispersing it and antioxidants in a water-soluble carrier such as polyvinylpyrrolidone (PVP) resulted in an increased relative absorption by 46 times (CurcuWIN®) of the total curcuminoids over the unformulated standard CW form. Several studies have also indicated that antioxidants prevent oxidative stress during strenuous Ex in humans and rats.10,11,16
Recent pilot studies have reported that CW can attenuate oxidative stress due to Ex by increasing blood’s antioxidant capacity.12,13 In this current study, a water-soluble CW formulation (20% curcuminoids) consisting of turmeric extract, a hydrophilic carrier, cellulosic derivatives, and natural antioxidants9 was administered to test the efficacy in a chronic Ex animal model. Therefore, the present study was undertaken in an animal model to investigate the effects of the water-soluble CW formulation on oxidative stress markers, Ex time of exhaustion, and the antioxidant status in muscles. Furthermore, we investigated the effects of CW on oxidative stress and antioxidant gene proteins such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/hemeoxygenase-1 (HO-1), and sirtuin 1 (SIRT1) pathways in the skeletal muscle of chronically exercised and sedentary rats (control diet group and no Ex).
Materials and methods
Animals and feeding protocols
Male Wistar rats (N=28; n=7 per arm; age: 8 weeks and body weight: 180±20 g) were housed in a controlled standard laboratory environment (12/12-hour light/dark cycle at 22 °C) and fed with rat chow and water ad libitum. All experiments were conducted according to the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Firat University. Table 1 provides the composition of the basal (control) diet. Animals were divided randomly into the following four groups: 1) control (C; no Ex), 2) Control + CurcuWIN, 3) Ex + C and 4) Ex + C + Ex + CW (Ex + CW). CW was administered as 100 mg/kg CurcuWIN, providing 20 mg of CW daily for 6 weeks. A novel water-soluble CW formulation (lot number CU20DNS1-008/009) was provided by OmniActive Health Technologies, Ltd. (Pune, India). CW dose at 100 mg/kg was chosen based on previously reported value for effective antioxidant activity in rodents.14,15
Table 1.
Composition of basal diet
| Description | % |
|---|---|
| Barley | 30.2 |
| Soybean meal | 10.0 |
| Sunflower meal | 38.0 |
| Wheat bran | 6.0 |
| Molasses | 10.0 |
| Limestone | 3.0 |
| Salt | 1.5 |
| dl-Methionine | 0.8 |
| Dicalcium phosphate | 0.3 |
| Vitamin and mineral premixa | 0.2 |
| Analysis | |
| Crude protein | 24.3 |
| Ether extract | 3.4 |
| Crude cellulose | 6.9 |
| Ash | 8.1 |
| Ca | 1.3 |
| P | 0.9 |
Note:
The vitamin–mineral premix provides the following (per kilogram): all-trans-retinyl acetate, 1.8 mg; cholecalciferol, 0.025 mg; all-rac-a-tocopherol acetate, 12.5 mg; menadione (menadione sodium bisulfate), 1.1 mg; riboflavin, 4.4 mg; thiamine (thiamine mononitrate), 1.1 mg; vitamin B6, 2.2 mg; niacin, 35 mg; calcium pantothenate, 10 mg; vitamin B12, 0.02 mg; folic acid, 0.55 mg; d-biotin, 0.1 mg; manganese (from manganese oxide), 40 mg; iron (from iron sulfate), 12.5 mg; zinc (from zinc oxide), 25 mg; copper (from copper sulfate), 3.5 mg; iodine (from potassium iodide), 0.3 mg; selenium (from sodium selenite), 0.15 mg; choline chloride, 175 mg.
Exercise protocol
The Ex protocols were performed on a motor-driven rodent treadmill (MAY-TME; Commat Ltd., Ankara, Turkey). Ex based on the treadmill protocol and tests was performed over a 5-day period. All rats were pre-trained for a week and subjected to the treadmill Ex. The Ex protocol was as follows: 1) day 1, 10 m/min for 10 minutes; 2) day 2, 20 m/min for 10 minutes; 3) day 3, 25 m/min for 10 minutes; 4) day 4, 25 m/min for 20 minutes; and 5) day 5, 25 m/min for 30 minutes. All animals were subjected to the treadmill Ex for 25 m/min for 45 min/d for 5 d/wk for 6 weeks.
Sample collection
Animals were sacrificed after the last Ex by cardiac puncture. To minimize diurnal effects, all animals were killed at the same hour. Blood muscle, and tissue samples were stored at −80° C till further analyses.
Laboratory analyses
Serum glucose, lipid profile, aspartate transaminase, alanine transaminase, urea, and creatinine levels were measured. The malondialdehyde (MDA) level in muscle tissue was measured by high-performance liquid chromatography (Shimadzu, Tokyo, Japan) using a Shimadzu UV-vis SPD-10 AVP detector and C18 ODS-3, 5 μm, 4.6 mm ×250 mm column. Superoxide dismutase (SOD), glutathione (GSH), and GSH peroxidase (GPx) were measured using commercial kits (Cayman Chemical, Ann Arbor, MI, USA).
NF-kB, inhibitors of kappa B (I-κB), heat shock protein 70 (HSP70), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), thioredoxin-1 (TRX-1), SIRT1, Nrf2, HO-1, and glucose transporter 4 (GLUT4) levels were analyzed by Western blot.17 Samples were analyzed in quadruplicates for each experimental condition, and protein levels were determined densitometrically using an image analysis system (ImageJ; National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± SD The sample size was based on a power of 85% to obtain a P-value of 0.05. Seven animals per treatment were examined to see the significance of the treatments. ANOVA and Tukey tests for post hoc analyses were conducted between treatments and within treatments; P<0.05 was considered statistically significant.
Results
Body weight, endurance time, and biochemical parameters
No significant difference in body weight (P>0.05; Table 2) was observed. As seen from Table 2, a significant difference in the time of exhaustion between the control (C) and Ex rats was observed (P<0.01). CW supplementation affected the time of exhaustion in the exercised rats (P<0.05). No significant differences in the safety end markers were found in liver and kidney function tests in all treatments (P>0.05; Table 3).
Table 2.
CW+ Ex increased the run to exhaustion time.
| Variable | C | C + CW | Ex | Ex + CW |
|---|---|---|---|---|
| Final weight (g) | 257.21 | 261.62 | 253.46 | 259.37 |
| Distance run, average per day (m) | – | – | 1,032 | 1,068 |
| Run to exhaustion (minutes) | 70.26c | 74.78c | 173.45b | 185.14a |
Note: Superscripts with different alphabets differ significantly at P<0.05.
Abbreviations: C, control; CW, curcumin, Ex, exercise.
Table 3.
Changes in liver and kidney function
| Variable | C | CW | Ex | Ex + CW |
|---|---|---|---|---|
| AST (U/L) | 429.96±72.9 | 427.31±68.5 | 425.69±39.78 | 422.41 ±44.38 |
| ALT (U/L) | 74.99± 17.05 | 75.70± 15.24 | 79.64±16.72 | 75.86±17.35 |
| Urea (mg/dL) | 48.43±4.69 | 48.14±5.58 | 49.86±5.87 | 46.71 ±8.60 |
| Creatinine (mg/dL) | 0.43±0.07 | 0.44±0.03 | 0.42±0.04 | 0.40±0.06 |
Note: Data is presented as mean ± standard deviation.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; C, control; CW, curcumin, Ex, exercise.
Table 4 shows metabolic health parameters including glucose and lipid profile in all treatments. No significant difference was found in blood glucose concentrations, but chronically exercised rats had less serum total cholesterol (P<0.001), high-density lipoprotein (HDL) (P<0.002), and triglyceride (P<0.01) concentrations than controls (P<0.0001). Additionally, the serum total cholesterol, HDL, and triglyceride levels decreased in the E+CW group significantly compared with other groups. Serum low-density lipoprotein cholesterol (LDL-C) levels were reduced in the Ex + CW treatment groups compared to those in the untreated rats (P<0.0001). The serum lactate levels in the Ex + CW group was decreased compared to those in the control and Ex groups (P<0.0001); additionally, serum lactate levels in the Ex group were much higher than those in the control group (P<0.01).
Table 4.
Effect of E+CW on cardio-metabolic health markers and lactate
| Variable | C | C + CW | Ex | Ex + CW |
|---|---|---|---|---|
| Glucose (mg/dL) | 86.71 ±24.98 | 83.29±10.11 | 78.00±5.45 | 79.14±3.12 |
| Total-C (mg/dL) | 62.00 ±11.22a | 54.86±6.12ab | 61.43±9.73a | 42.29±7.57b |
| HDL-C (mg/dL) | 46.71±7.06a | 37.29±2.75b | 37.00±4.83b | 38.14±3.18b |
| LDL-C (mg/dL) | 11.43±2.64a | 9.29±1.1ab | 11.29±1.11a | 7.00±1.55b |
| Triglycerides (mg/dL) | 84.14±8.07a | 71.57±11.44ab | 63.00±5.16b | 54.57±20.12b |
| Lactate (mg/dL) | 8.60±0.91a | 7.59±0.95a | 5.97±0.73b | 4.59±0.45c |
Notes: Data is presented as mean ± standard deviation. Superscripts with different alphabets differ significantly at P<0.05.
Abbreviations: C, control; CW, curcumin, Ex, exercise; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDL-C, LDL cholesterol; total-C, total cholesterol.
Muscle MDA and antioxidant enzymes
Muscle MDA concentration was decreased by 15.5% (P<0.0001; Table 5) in the Ex group. CW treatment reduced the serum MDA concentration by 43.5% (P<0.001). Exercised rats had higher muscle SOD (0.35 vs 0.26; P<0.0001), GPx (161 vs 148; P< 0.003) activities (U/mg protein), and muscle GSH (11.8 μg/mg vs 8.8 μg/mg protein; P<0.0001) levels than controls. A decrease in MDA and an increase in muscle superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GHS-Px) concentrations in response to CW treatment were more notable than in the other groups (P<0.001 for all; Table 5).
Table 5.
Effect of CW supplementation in the Ex group on muscle oxidative stress metabolites and antioxidant enzymes
| Variable | C | CW | Ex | Ex + CW |
|---|---|---|---|---|
| MDA (nmol/mg protein) | 74.29±7.48a | 56.43±6.90b | 62.71±2.69b | 42.00±2.65c |
| SOD (U/mg protein) | 0.26±0.05c | 0.36±0.03b | 0.35±0.05b | 0.45±0.06a |
| GPx (U/mg protein) | 148.00±21.63b | 163.71±17.76ab | 161.57±19.45b | 192.43±21.51a |
| GSH (µg/mg protein) | 8.80± 1.95c | 12.40±1.87b | 11.89± 1.80b | 15.47±2.45a |
Notes: Data is presented as mean ± standard deviation. Superscripts differ by alphabets represent significance at P<0.05.
Abbreviations: C, control; CW, curcumin, Ex, exercise; GPx, glutathione peroxidase; GSH, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase.
Muscle protein levels
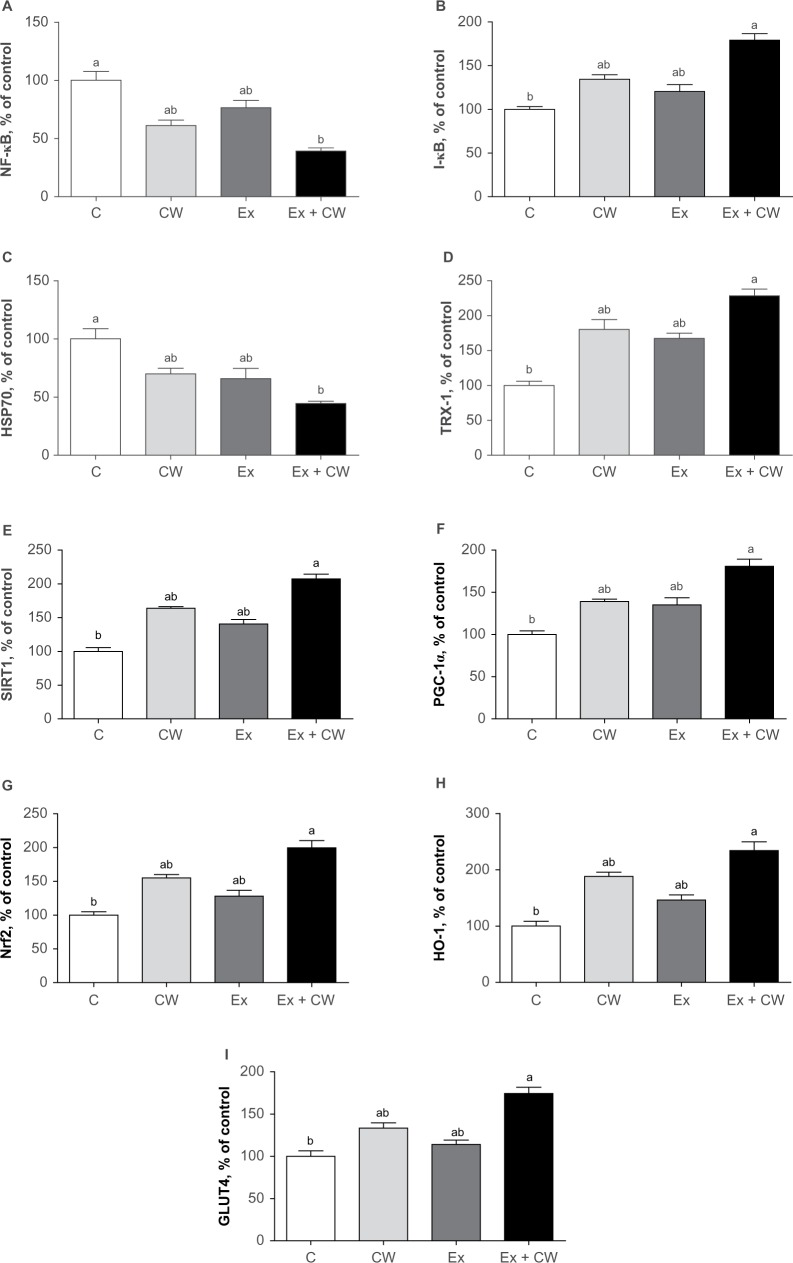
Clear bands for different muscle proteins were observed in the CW-treated groups with or without Ex compared to the control group (Figure 1). Oxidative stress protein levels of muscle NF-κB (Figure 2A) and HSP70 (Figure 2C) were decreased in the CW-treated groups. Antioxidant muscle protein levels of I-κB (Figure 2B), TRX-1 (Figure 2D), SIRT1 (Figure 2E), PGC-1α (Figure 2F), Nrf2 (Figure 2G), HO-1 (Figure 2H), and GLUT4 (Figure 2I) were increased in the CW group compared to those in the other groups (P<0.05; Figure 2).
Figure 1.
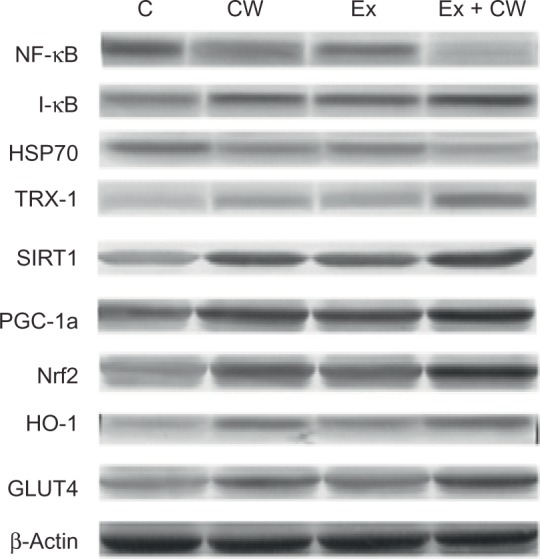
Effect of different treatments on protein expression levels (Western blot strips) of muscle tissues.
Notes: The intensity of the bands was quantified by densitometric analysis. Data are expressed as the ratio of control (sedentary untreated rats) value (set to 100%). The bar represents standard deviation of mean. Blots were repeated at least three times (n=3) and a representative blot is shown. Protein loading was controlled using β-actin.
Abbreviations: GLUT4; glucose transporter 4; HO-1, hemeoxygenase-1; HSP70, heat shock protein 70; I-κB, inhibitors of kappa B; NF-kB; nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor (erythroid-derived 2)-like 2; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SIRT1, sirtuin 1; TRX-1; thioredoxin-1; C, control; CW, curcumin, Ex, exercise.
Figure 2.
Effect of different treatments on NF-kB (A), I-kB (B), HSP70 (C), TRX-1 (D), SIRT1 (E), PGC-1α (F), Nrf2 (G), HO-1 (H), and GLUT4 (I) protein expression levels of muscle.
Note: Superscripts differ by alphabets represent significance at P<0.05.
Abbreviations: GLUT4; glucose transporter 4; HO-1, hemeoxygenase-1; HSP70, heat shock protein 70; I-κB, inhibitors of kappa B; NF-kB; nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor (erythroid-derived 2)-like 2; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SIRT1, sirtuin 1; TRX-1; thioredoxin-1; C, control; CW, curcumin, Ex, exercise.
Discussion
This study was carried out to explain the efficacy and potential mechanism of action of a water-soluble CW formulation (20% curcuminoids) on muscle proteins, oxidative stress, andconcentration associated with modulation of the antioxidant Nrf2 and decreased oxidative stress expression of NF-κB. CW supplementation enhances the antioxidant activity by increasing serum SOD, GPx, and GSH (Table 5) compared to the other groups. The molecular basis of the antioxidant and anti-inflammatory properties of CW is linked to transcription factors, growth regulators, and cellular signaling molecules.4,16,18,19 Several studies have reported that CW inhibits the scavenging of superoxide radicals, hydrogen peroxide, and nitric oxide from activated macrophages, reducing the iron complex and inhibiting the lipid peroxidation.20–22 Results of our study suggest that Ex may enhance lipid peroxidation and reduce the oxidative damage of proteins and DNA.23 Reduced oxidative stress results from an enhanced antioxidant defense system.24,25 Chronic Ex reduces oxidative stress by upregulating the activity of antioxidant enzymes.26 Belviranli et al25 have reported that plasma MDA levels were lowered in chronically exercised groups compared to controls, as well as by an antioxidant (grape seed extract) supplementation. They also reported the plasma activities of SOD, an antioxidant defense for superoxide radicals that catalyzes the dismutation of superoxide and the formation of H2O2 and GPx. The levels of these enzymes improved after chronic Ex and antioxidant supplementation (grape seed extract). Takahashi et al27 reported that the serum biological antioxidant potential concentrations after Ex were much higher in single and double CW supplementation trials compared to those before Ex. CW supplementation can attenuate Ex-induced oxidative stress and increase blood’s antioxidant capacity. CW directly influences the activity of inflammatory regulators.18,19,28,29 Total cholesterol and LDL-C were reduced in the Ex + CW groups. Ramírez-Tortosa et al30 reported that the administration of turmeric extract inhibits the oxidation of LDL and potential hypocholesterolemic effect. A study by Arafa31 in experimental animals fed with high-cholesterol diet indicated that CW had a hypocholesterolemic effect by reducing the serum total cholesterol and LDL-C and increasing the HDL cholesterol. In order to understand the mechanism of lowering cholesterol in a CW diet, the activity of hepatic cholesterol-7a-hydroxylase and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG Co A) reductase was measured. Hepatic cholesterol-7a-hydroxylase level and cholesterol catabolism rate were significantly higher in diabetic rats fed with CW32 Yiu et al33 demonstrated an increase in the expression of cholesterol 7 α-hydroxylase, HO-1, and LDL receptors but a decrease in 3-hydroxy-3-methyl-glutaryl-CoA reductase level in high-cholesterol diet. These results suggest that turmeric prevents hypercholesterolemia.
Our study demonstrates different regulatory pathways to prevent muscle damage and soreness and inflammation, as shown in Figure 2A–I. CW bioavailability is very important to realize the efficacy of molecular physiological effects. CW uptake in cells is significant.34 Ex induces transient changes in metabolic genes in human skeletal muscle and enhances redox regulation of NF-κB and expression of antioxidant enzymes.35 NF-κB is a transcription factor that controls gene expression and many inflammatory proteins, cellular growth, and apoptosis.36 Increased NF-κB signaling decreases insulin action and promotes muscle wasting. NF-κB is activated in a redox-sensitive manner during muscular contraction due to an increased oxidant production. These data suggest that the novel CW form downregulates NF-κB and upregulates Nrf2.
In the present study, HSP70 expression decreased after CW treatment compared to the other groups (Figure 2C). The upregulation of HSP70 potentially contributes to muscle fiber integrity, muscle regeneration, and recovery. HSP70 is an indicator of cellular stress and a molecular chaperone, maintains cellular homeostasis and apoptosis, influences energy metabolism, facilitates cellular processes of muscular adaptation, and interacts with signaling pathways.37 Evidence supports that the loss of HSP70 drives muscle atrophy, contractile dysfunction, and reduced regenerative capacity38 CW-treated groups showed upregulation of the stress protein HSP70. TRX system, an antioxidant system, controls the cellular redox status.39 TRX-1 has antioxidative and anti-apoptosis properties.40 CW-treated groups showed upregulation of TRX-1, which when combined with Ex was highly significant over control (Figure 2D). Due to the limitation of the literature on the effect of CW on TRX-1, TRX-1 data are not comparable. However, TRX-1 ameliorates the depletion of GSH and restores the GSH/GSSG ratio.41
SIRT1 promotes mitochondrial biogenesis via deacetylation of PGC-1α and mitochondrial biogenesis.42 SIRT1 levels increase in skeletal muscles in response to chronic Ex, in parallel to the upregulation of mitochondrial content.42 CW activates SIRT1 and potentially enhances mitochondrial biogenesis and fatty acid oxidation in adipocytes and myotubes.43,44 CW regulates mitochondrial biogenesis (SIRT1), including PGC-1α. Consistent with our results, Ray Hamidie et al44 have shown that CW and Ex increased cytosol and the NAD+/NADH ratio and SIRT1 protein in muscle. In addition, some polyphenols, including CW, activate SIRT1 directly or indirectly, as shown in a variety of research models.45 Our study demonstrates an increase in SIRT1 (Figure 2E) and PGC-1α (Figure 2F) levels in skeletal muscle in response to CW treatment compared to the other groups.
Nrf2 is a transcription factor that binds to antioxidant response element, thereby increasing a variety of cytoprotective genes.46,47 The levels of Nrf2 and HO-1 are increased in CW and Ex groups over the control group (Figure 2G and H). Consistent with our results, Ex stimulates transcription factors, decreases oxidative stress, and increases antioxidantdefenses.35
GLUT4 isoform of insulin-regulated glucose transporter increases during Ex. GLUT4 may enhance mitochondrial biogenesis.43 GLUT4 is found in heart tissue, skeletal muscles, and adipose tissues.48 The data suggest the immunomodulatory properties of CW and its potential for altering the expression of inflammatory genes. In summary, the combination of Ex and CW with a unique water-soluble formulation9 may accelerate mitochondrial biogenesis in the skeletal muscle and regulate the NF-kB, Nrf2, SIRT1, and PGC-1α pathways. CW’s protective effects are significant for cholesterol metabolism, improved antioxidant status, and reduction of oxidative stress metabolites.
Acknowledgments
The authors thank OmniActive Health Technologies Inc. (Morristown, NJ, USA) for financial support. This work was also supported in part by the Turkish Academy of Sciences (KS). This article was presented at the Experimental Biology Meeting, Boston, 2015.
Footnotes
Disclosure
VJ is an employee of OmniActive Health Technologies Inc. The authors report no other conflicts of interest in this work.
References
- 1.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem. 2013;253(1–2):307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 2.Newham DJ, Jones DA, Clarkson PM. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol (1985) 1987;63(4):1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- 3.Michailidis Y, Karagounis LG, Terzis G, et al. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am J Clin Nutr. 2013;98(1):233–245. doi: 10.3945/ajcn.112.049163. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39(3):283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62(7):919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 9.Jäger R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative absorption of curcumin formulations. Nutr J. 2014;13:11. doi: 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heunks LM, Viña J, van Herwaarden CL, Folgering HT, Gimeno A, Dekhuijzen PN. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277(6 Pt 2):R1697–R1704. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- 11.Mastaloudis A, Morrow JD, Hopkins DW, Devaraj S, Traber MG. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med. 2004;36(10):1329–1341. doi: 10.1016/j.freeradbiomed.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 12.Sciberras JN, Galloway SD, Fenech A, et al. The effect of turmeric (curcumin) supplementation on cytokine and inflammatory marker responses following 2 hours of endurance cycling. J Int Soc Sports Nutr. 2015;12(1):5. doi: 10.1186/s12970-014-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drobnic F, Riera J, Appendino G, et al. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): a randomised, placebo-controlled trial. J Int Soc Sports Nutr. 2014;11:31. doi: 10.1186/1550-2783-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Liu J, Yu H, Wang Q, Chen Y, Xiang L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci Lett. 2013;547:26–31. doi: 10.1016/j.neulet.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 15.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Yeo HC, Overvik-Douki E, et al. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol (1985) 2000;89(1):21–28. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- 17.Sahin K, Tuzcu M, Orhan C, et al. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br J Nutr. 2013;110(2):197–205. doi: 10.1017/S0007114512004850. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 19.Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Cai W, Zhang B, Duan D, Wu J, Fang J. Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol Appl Pharmacol. 2012;262(3):341–348. doi: 10.1016/j.taap.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Bayomi SM, El-Kashef HA, El-Ashmawy MB, et al. Synthesis and biological evaluation of new curcumin derivatives as antioxidant and antitumor agents. Med Chem Res. 2013;22(3):1147–1162. [Google Scholar]
- 22.Sankar P, Telang AG, Manimaran A. Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp Toxicol Pathol. 2012;64(5):487–493. doi: 10.1016/j.etp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Leeuwenburgh C, Ji LL. Glutathione and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exercise. J Nutr. 1998;128(12):2420–2426. doi: 10.1093/jn/128.12.2420. [DOI] [PubMed] [Google Scholar]
- 24.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Can J Appl Physiol. 2004;29(3):245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 25.Belviranlı M, Gökbel H, Okudan N, Başaralı K. Effects of grape seed extract supplementation on exercise-induced oxidative stress in rats. Br J Nutr. 2012;108(2):249–256. doi: 10.1017/S0007114511005496. [DOI] [PubMed] [Google Scholar]
- 26.Greathouse KL, Samuels M, DiMarco NM, Criswell DS. Effects of increased dietary fat and exercise on skeletal muscle lipid peroxidation and antioxidant capacity in male rats. Eur J Nutr. 2005;44(7):429–435. doi: 10.1007/s00394-005-0548-9. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Suzuki K, Kim HK, et al. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int J Sports Med. 2014;35(6):469–475. doi: 10.1055/s-0033-1357185. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169(8):1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra LS, Mertia PN, Nandedkar T, et al. CurcuWIN™ cellular uptake in human glioblastoma cells further proves bioavailability; 4th World Ayurveda Congress & AROGYA EXPO; Bangalore. 2010. [Google Scholar]
- 30.Ramírez-Tortosa MC, Mesa MD, Aguilera MC, et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147(2):371–378. doi: 10.1016/s0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 31.Arafa HM. Curcumin attenuate diet-induced hypercholesterolemia in rats. Med Sci Monit. 2005;11(7):BR228–BR234. [PubMed] [Google Scholar]
- 32.Babu PS, Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol Cell Biochem. 1997;166(1–2):169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- 33.Yiu WF, Kwan PL, Wong CY, et al. Attenuation of fatty liver and prevention of hypercholesterolemia by extract of Curcuma longa through regulating the expression of CYP7A1, LDL-receptor, HO-1, and HMG-CoA reductase. J Food Sci. 2011;76(3):H80–H89. doi: 10.1111/j.1750-3841.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 34.Angelo LS, Wu JY, Meng F, et al. Combining curcumin (diferuloylmethane) and heat shock protein inhibition for neurofibromatosis 2 treatment: analysis of response and resistance pathways. Mol Cancer Ther. 2011;10(11):2094–2103. doi: 10.1158/1535-7163.MCT-11-0243. [DOI] [PubMed] [Google Scholar]
- 35.George L, Asghar M, Lokhandwala MF. Exercise stimulates transcription factors (Nrf2 & NFκB), increases antioxidant defenses, decreases oxidative stress, and restores renal dopamine D1 receptor function in aging. FASEB J. 2008;22:1159.6. [Google Scholar]
- 36.Ali S, Mann DA. Signal transduction via the NFκB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22(2):67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- 37.Senf SM. Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front Physiol. 2013;4:330. doi: 10.3389/fphys.2013.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandal MN, Patlolla JM, Zheng L, et al. Curcumin protects retinal cells from light- and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46(5):672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu J, Holmgren A. Thioredoxin system in cell death progression. Antioxid Redox Signal. 2012;17(12):1738–1747. doi: 10.1089/ars.2012.4650. [DOI] [PubMed] [Google Scholar]
- 40.Kuo JJ, Chang HH, Tsai TH, Lee TY. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int J Mol Med. 2012;30(3):643–649. doi: 10.3892/ijmm.2012.1020. [DOI] [PubMed] [Google Scholar]
- 41.Iwata S, Hori T, Sato N, et al. Adult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiolmediated redox regulation in apoptosis caused by pro-oxidant state. J Immunol. 1997;158(7):3108–3117. [PubMed] [Google Scholar]
- 42.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism. 2008;57(7):986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Holloszy JO. Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol. 2011;1(2):921–940. doi: 10.1002/cphy.c100052. [DOI] [PubMed] [Google Scholar]
- 44.Ray Hamidie RD, Yamada T, Ishizawa R, Saito Y, Masuda K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism. 2015;64(10):1334–1347. doi: 10.1016/j.metabol.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Queen BL, Tollefsbol TO. Polyphenols and aging. Curr Aging Sci. 2010;3(1):34–42. doi: 10.2174/1874609811003010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair S, Li W, Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007;28(4):459–472. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 47.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46(4):1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Stenbit AE, Tsao TS, Li J, et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med. 1997;3(10):1096–1101. doi: 10.1038/nm1097-1096. [DOI] [PubMed] [Google Scholar]