Abstract
Objective
Pathological ocular neovascularization is a major cause of blindness. Increased dietary intake of ω-3 long-chain polyunsaturated fatty acids (LCPUFA) reduces retinal and choroidal neovascularization, but ω-3 LCPUFA metabolites of a major metabolizing pathway, cytochrome P450 oxidase (CYP) 2C, promote ocular pathological angiogenesis. We hypothesized that inhibition of CYP2C activity will add to the protective effects of ω-3 LCPUFA on neovascular eye diseases.
Approach and Results
The mouse models of oxygen-induced retinopathy (OIR) and laser-induced choroidal neovascularization (CNV) were used to investigate pathological angiogenesis in the retina and choroid respectively. The plasma levels of ω-3 LCPUFA metabolites of CYP2C were determined by mass spectroscopy. Aortic ring and choroidal explant sprouting assays were used to investigate the effects of CYP2C inhibition and ω-3 LCPUFA derived CYP2C metabolic products on angiogenesis ex vivo. We found that inhibition of CYP2C activity by montelukast added to the protective effects of ω-3 LCPUFA on retinal and choroidal neovascularization by 30% and 20% respectively. In CYP2C8 over-expressing mice fed a ω-3 LCPUFA diet, montelukast suppressed retinal and choroidal neovascularization by 36% and 39% and reduced the plasma levels of CYP2C8 products. Soluble epoxide hydrolase inhibition, which blocks breakdown and inactivation of CYP2C ω-3 LCPUFA-derived active metabolites, increased OIR and CNV in vivo. Exposure to selected ω-3 LCPUFA metabolites of CYP2C significantly reversed the suppression of both angiogenesis ex vivo and endothelial cell functions in vitro by the CYP2C inhibitor montelukast.
Conclusion
Inhibition of CYP2C activity adds to the protective effects of ω-3 LCPUFA on pathological retinal and choroidal neovascularization.
Keywords: CYP2C inhibitor, ω-3 LCPUFA, pathological angiogenesis
Introduction
Pathological ocular angiogenesis comprising retinopathy and choroidal neovascularization is a leading cause of vision loss in all age groups including retinopathy of prematurity (ROP) in children, diabetic retinopathy (DR) and age-related macular degeneration (AMD) in adults1–3. Pathological retinal and choroidal neovascularization can be suppressed temporarily with anti-angiogenic agents. In particular, anti-vascular endothelial growth factor (VEGF) molecules have been used successfully for the treatment of neovascular AMD, DR and ROP, but with some adverse effects4, 5. Suppressing VEGF signaling does not address the underlying causes of neovascularization. Frequent intraocular injections of anti-VEGF drugs also carry a cumulative risk of complications, including the potential of long-term suppression of the beneficial neurotrophic effects of VEGF on neural retina6, 7. Identification of additional therapies with fewer adverse effects is highly desirable.
Beyond their roles as energy substrates and structural constituents of membranes, essential dietary lipids and their metabolites also regulate retinal and choroidal neovascularization8–10. In particular, long-chain polyunsaturated fatty acids (LCPUFA) influence eye diseases11. The ω-3 LCPUFA, docosahexaenoic acid (DHA) and ω-6 LCPUFA, arachidonic acid (AA), are present in retinal neural and vascular cell membrane phospholipids12. DHA is present in the retina at a higher concentration (20% of lipids) than in any other tissue, and adequate dietary intake is associated with a reduced risk of ROP, DR and AMD13–16. Proliferative retinopathy and neovascular AMD are respectively modeled in the mouse eye by oxygen-induced retinopathy (OIR) and laser-induced choroidal neovascularization (CNV)17, 18. Dietary intake of ω-3 versus ω-6 LCPUFA reduces pathological angiogenesis in retina and choroid in these models8, 18.
Both ω-3 and ω-6 LCPUFA are metabolized by at least three major pathways: cyclooxygenases (COXs), lipoxygenases (LOXs) and cytochrome P450 oxidases (CYPs). The metabolic products of ω-6 LCPUFA from these pathways are generally proangiogenic11 whereas ω-3 LCPUFA metabolites of the first 2 pathways, such as COX-2 derived prostaglandin E3 and 5-LOX derived 4-hydroxy-docosahexaenoic acid, show anti-angiogenic effects10, 19. Our previous studies found that CYP2C products derived from ω-3 and ω-6 LCPUFA, particularly 19,20-epoxydocosapentaenoic acid (EDP) and 14,15-epoxyeicosatrienoic acid (EET), are pro-angiogenic and involved in CYP2C regulation of retinal neovascularization20, partially counteracting the overall anti-angiogenic effects of ω-3 LCPUFA. These bioactive epoxides are further hydrolyzed by soluble epoxide hydrolase (sEH) into less active diols, such as 19,20-dihydroxy-docosapentaenoic acid (19,20-DiHDPA) and 14,15-dihydroxy-eicosatrienoic acid (14,15-DHET)20, 21. These findings suggest that inhibition of CYP2C activity might add to the protective effects of ω-3 LCPUFA on pathological retinal and choroidal neovascularization.
(R,E)-2-1-1-3-2-7-chloroquinolin-2-yl vinyl phenyl-3-2-2-hydroxypropan-2-yl phenyl propylthio methyl cyclopropyl acetic acid, also known as montelukast, was identified as a potent and selective CYP2C8 inhibitor (among human CYP2C enzymes) with a very high affinity (IC50 = 9.22 ± 0.88 nM) in vitro22, 23 suggesting its potential as an effective antagonist of CYP2C-catalyzed metabolism22, 23. Montelukast suppresses colon cancer development by inhibiting tumor angiogenesis and vascular permeability24, 25. Montelukast was originally developed as a cysteinyl leukotriene receptor 1 (CysLTR1) antagonist, and is widely used for the treatment of asthma and seasonal allergies26. Whereas CYP2C enzymes are expressed in endothelial cells20, 27, CysLTR1 is primarily expressed in leukocytes and macrophages, but not in endothelial cells28, 29, suggesting the effects of montelukast on angiogenesis and endothelial cells may be mediated by inhibition of CYP2C activity unrelated to its role as a CysLTR1 antagonist.
This study showed that inhibition of CYP2C activity by montelukast decreased the plasma levels of CYP2C metabolites and added to the protective effects of ω-3 LCPUFA on pathological ocular neovascularization. In contrast to inhibition of CYP2C, increasing plasma levels of CYP2C products with a sEH inhibitor, that blocks the breakdown of CYP2C ω-3 LCPUFA bioactive epoxides products into less active diols20, 30, 31 promoted ocular neovascularization in vivo. We also found that selected ω-3 LCPUFA metabolites of CYP2C reversed the inhibition of montelukast on angiogenesis ex vivo and endothelial cell functions in vitro.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
CYP2C inhibition added to the protective effects of ω-3 LCPUFA on retinal and choroidal neovascularization
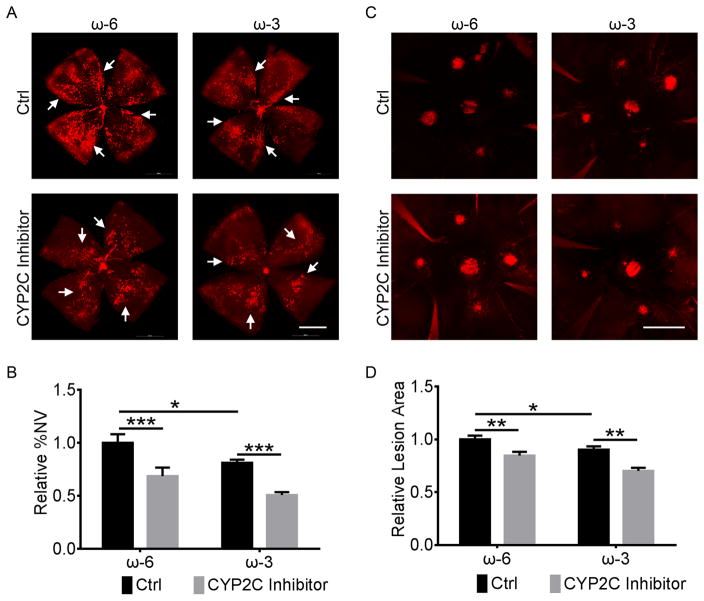
To test the hypothesis that inhibiting CYP2C activity adds to ω-3 LCPUFA inhibition of pathological retinal and choroidal neovascularization, we subjected C57BL/6 mice fed with either ω-6 or ω-3 LCPUFA enriched diets (as 2% of total dietary fatty acids (2% AA without any ω-3 LCPUFA or 1% DHA and 1% EPA with any ω-6 LCPUFA) to either OIR or laser-induced CNV, and treated them with either the CYP2C inhibitor montelukast or vehicle control. At postnatal day (P) 17, OIR pups fed a ω-3 (versus ω-6) LCPUFA enriched diet had 19% (P = 0.046) less retinal neovascularization, which was further reduced by the inhibition of CYP2C by 30% (P = 4.4×10−7) (Figure 1A&B). Moreover, adult mice fed a ω-3 (versus ω-6) LCPUFA enriched diet had 10% (P = 0.020) reduction of CNV lesion area at 7 days after laser photocoagulation, and inhibition of CYP2C further reduced the CNV lesion area by 20% (P = 6.6×10−6) (Figure 1C&D). Although the effects of CYP2C inhibition are not specific for, or require ω-3 LCPUFA, these results suggested that the CYP2C inhibitor montelukast enhances the overall protective effects of ω-3 LCPUFA on both retinal and choroidal neovascularization.
Figure 1. CYP2C inhibitor added to the protective effects of ω-3 LCPUFA on retinal and choroidal neovascularization.
Representative images of retinal (A) and choroidal (C) flat-mounts from OIR mice fed with a ω-6 or ω-3 LCPUFA enriched diet and intraperitoneally injected with the CYP2C inhibitor montelukast (1 mg/kg) or 10% DMSO control daily from postnatal day (P) 12 to P16 for OIR or from day 0 to day 6 after laser photocoagulation for CNV. Scale bar, 1 mm (A), 500 μm (C). CYP2C inhibitor augmented the suppression of retinal (B) and choroidal (D) neovascularization (NV) by ω-3 LCPUFA. n = 11 mice/group. * P < 0.05; ** P < 0.01; *** P < 0.001.
Inhibition of CYP2C8 activity suppressed retinal and choroidal neovascularization and CYP2C8 products
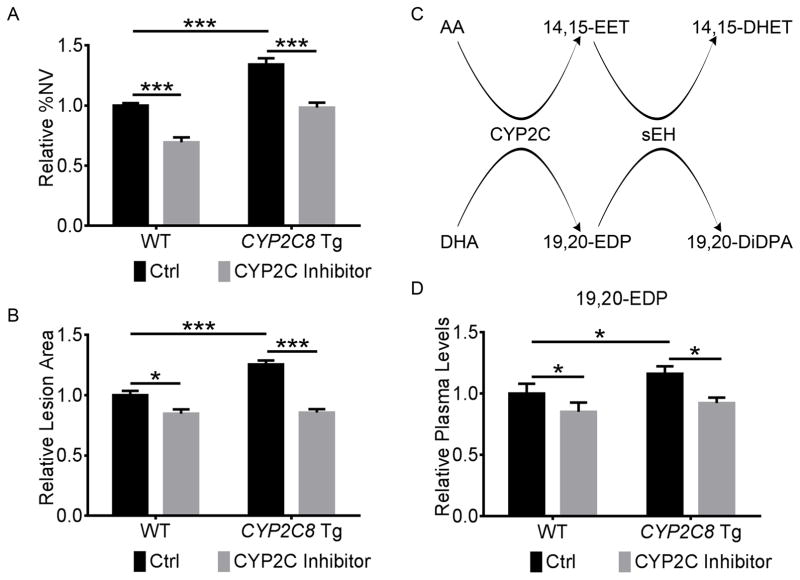
To further examine the potential for CYP2C inhibition to augment the protective effect of ω-3 LCPUFA on ocular neovascularization, we treated Tie2-driven human CYP2C8 overexpressing mice and their wild-type littermates on a ω-3 LCPUFA enriched diet with montelukast or vehicle control daily in the OIR and laser-induced CNV models. CYP2C8 overexpression increased retinal neovascularization in OIR by 34% (P = 3.0×10−5), which was suppressed by 36% (P = 1.7×10−5) at P17 with montelukast treatment (Figure 2A & Supplemental Figure IA). CYP2C8 transgenic mice fed a ω-3 LCPUFA enriched diet had 25% (P = 3.4×10−7) more choroidal neovascularization than wild-type littermates, which was suppressed by 39% (P = 3.4×10−16) with montelukast treatment (Figure 2B & Supplemental Figure IB). We chose to examine the plasma levels of AA and DHA metabolites of CYP2C, 14,15-EET and 19,20-EDP, and their metabolic products of sEH (Figure 2C) because our previous studies revealed their pro-angiogenic effects on pathological retinal and choroidal neovascularization. The decreased pathological angiogenesis with CYP2C inhibition was accompanied by 24% (P = 0.011) lower plasma levels of the bioactive CYP2C8 product derived from ω-3 LCPUFA, 19,20-EDP (Figure 2D). The production of other LCPUFA metabolites of CYP2C8 was also suppressed by montelukast (Supplemental Table I). Also, montelukast reversed the induction of retinal (OIR) and choroidal neovascularization (CNV) in the Tie2-driven CYP2C8 transgenic mice on a ω-6 LCPUFA enriched diet by 51% (P = 8.6×10−4) and 47% (P = 6.5×10−7) respectively (Supplemental Figure I&II), which was accompanied by 23% (P = 0.031) lower plasma levels of CYP2C8 products derived from ω-6 LCPUFA, such as 14,15-EET, without changing CYP2C8 transcriptional levels (Supplemental Figure II & Table II). These results suggested that inhibition of CYP2C activity by montelukast is associated with suppression of pathological angiogenesis in OIR and laser-induced CNV.
Figure 2. CYP2C inhibitor reduced retinal and choroidal neovascularization and CYP2C8 products.
CYP2C inhibitor reversed the induction of retinal (A) and choroidal (B) neovascularization (NV) by CYP2C8 overexpression in the mouse OIR and laser-induced CNV models. Tie2-driven CYP2C8 transgenic (Tg) mice and wild-type (WT) littermate controls fed with a ω-3 LCPUFA enriched diet were intraperitoneally injected with the CYP2C inhibitor montelukast (1 mg/kg) or 10% DMSO control daily from P12 to P16 for OIR or from day 0 to day 6 after laser photocoagulation for CNV. C, Schematic diagram of CYP2C8 and sEH metabolism of DHA and AA. D, CYP2C inhibitor reversed the induction of plasma levels of DHA metabolites downstream of CYP2C8, 19,20-EDP, by CYP2C8 overexpression in mice. n = 10–16 mice/group. *P < 0.05; *** P < 0.001.
sEH inhibition increased retinal and choroidal neovascularization in vivo and angiogenesis ex vivo
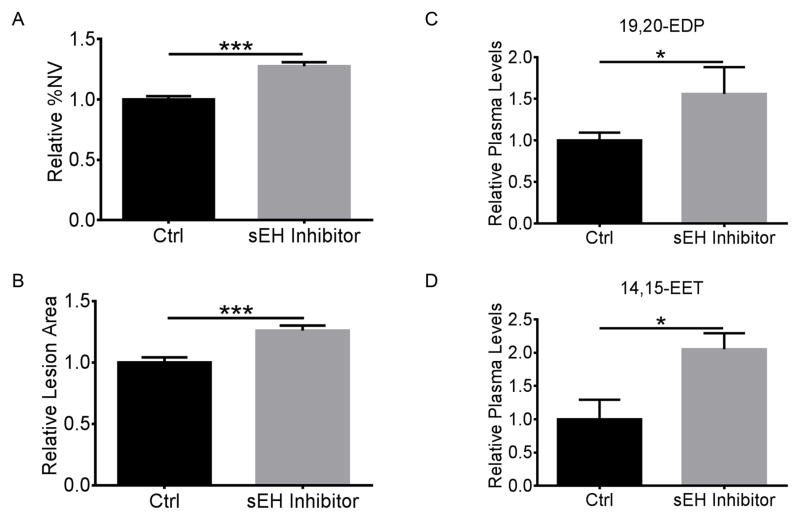
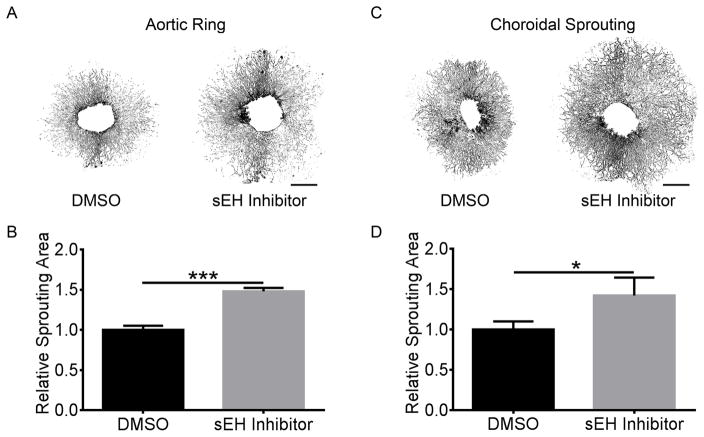
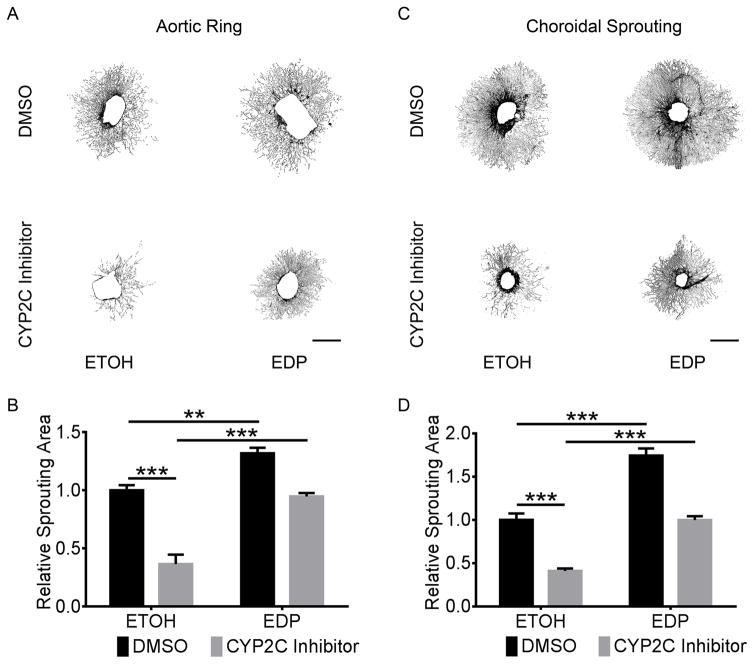
To investigate the regulatory effects of the CYP2C/sEH pathway and the metabolites on pathological retinal and choroidal neovascularization, we treated C57BL/6J mice with the sEH inhibitor in the piperidine series 1770 (1-trifluoromethoxyphenyl1-3-(1-propionylpiperidin-4-yl)urea)32, 33 or vehicle control in both OIR and laser-induced CNV models. We found sEH inhibition increased ocular neovascularization by 27% (P = 3.3×10−8) and 26% (P = 9.7×10−6) respectively in OIR and laser-induced CNV (Figure 3A&B & Supplemental Figure IIIA&B), which was also accompanied by 56±17% (P = 0.016) and 105% (P = 0.032) higher plasma levels of the bioactive epoxides 19,20-EDP and 14,15-EET (Figure 3C&D). However, neither sEH nor CYP2C inhibitor affected vaso-obliteration in OIR (Supplemental Figure IIIC&D). The sEH inhibitor 1770 significantly decreased the plasma levels of diols but had no effect on LCPUFA metabolites through other pathways (Supplemental Table III). The ratios of both 19,20-EDP:DiHDPA and 14,15-EET:DHET were increased by 153% (P = 0.035) and 44% (P = 0.020) respectively by addition of the sEH inhibitor 1770 (Supplemental Figure IVA&B). The transcriptional levels of neither CYP2C nor sEH were significantly altered by sEH inhibition (Supplemental Figure IVC&D). Moreover, sEH inhibition yielded 48% (P = 5.0×10−5) and 42% (P = 0.046) increases in sprouting of both aortic rings and choroidal explants (Figure 4). These results indicated the association of ω-3 LCPUFA metabolites of CYP2C with neovascularization.
Figure 3. sEH inhibitor aggravated retinal and choroidal neovascularization.
sEH inhibitor increased P17 retinal (A) and choroidal (B) neovascularization (NV) in OIR and laser-induced CNV mice. C57BL/6J mice were intraperitoneally injected with the sEH inhibitor 1770 (0.3 mg/kg) and 10% DMSO control daily from P12 to P16 for OIR (n = 26–27 mice/group) or from day 0 to day 6 after laser photocoagulation for CNV (n = 11 mice/group). sEH inhibitor increased plasma levels of 19,20-EDP (C) and 14,15-EET (D) in mice. n = 4 mice/group. * P < 0.05; *** P < 0.001.
Figure 4. sEH inhibitor promoted angiogenesis ex vivo.
Representative images of aortic rings (A) and choroidal (C) sprouting treated with the sEH inhibitor 1770 (20 μg/ml) or 0.2% DMSO as control for 6 days after tissue planting. Scale bar, 1 mm. sEH inhibitor promoted aortic ring (B) and choroidal (D) sprouting. n = 5. * P < 0.05; *** P < 0.001.
19,20-EDP reversed the inhibition of angiogenesis ex vivo by CYP2C inhibition
To further investigate if inhibition of CYP2C and lower levels of CYP2C products derived from ω-3 LCPUFA suppressed angiogenesis, we examined the effects of the CYP2C inhibitor montelukast in the presence of DHA, which would provide metabolites from many pathways including beneficial products or one of its CYP2C metabolites, 19,20-EDP, on tissue explants. In the aortic ring sprouting assay, there was 19% (P = 0.030) less sprouting area with DHA compared with control at day 6 (Supplemental Figure VA&B). Addition of montelukast further suppressed sprouting by 46% (P = 0.0037), which was consistent with results from the animal models in vivo suggesting that CYP2C products were pro-angiogenic. In the choroid sprouting assay, montelukast not only decreased the sprouting area by 29% (P = 0.0087), but also further increased the inhibitory effects of DHA by 28% (P = 0.013) (Supplemental Figure VC&D). In contrast, one of the DHA metabolites of CYP2C, 19,20-EDP, reversed the suppression of sprouting by montelukast in aortic rings and choroidal explants by 58% (P = 4.3×10−4) and 59% (P = 4.2×10−4) respectively (Figure 5). These data suggested that inhibition of CYP2C by montelukast suppressed angiogenesis ex vivo.
Figure 5. 19,20-EDP reversed the inhibition of angiogenesis ex vivo by CYP2C inhibition.
Representative images of aortic rings (A) and choroidal (C) treated with the CYP2C inhibitor montelukast (20 μg/ml) or 0.2% DMSO control, and 19,20-EDP (1 μM) or ethanol (ETOH) vehicle control for 6 days after tissue planting. Scale bar, 1 mm. 19,20-EDP rescued the inhibition of aortic ring (B) and choroidal (D) sprouting by CYP2C inhibition. n = 5. ** P < 0.01; *** P < 0.001.
19,20-EDP reversed the inhibition of endothelial cell tubule formation in vitro by CYP2C inhibition
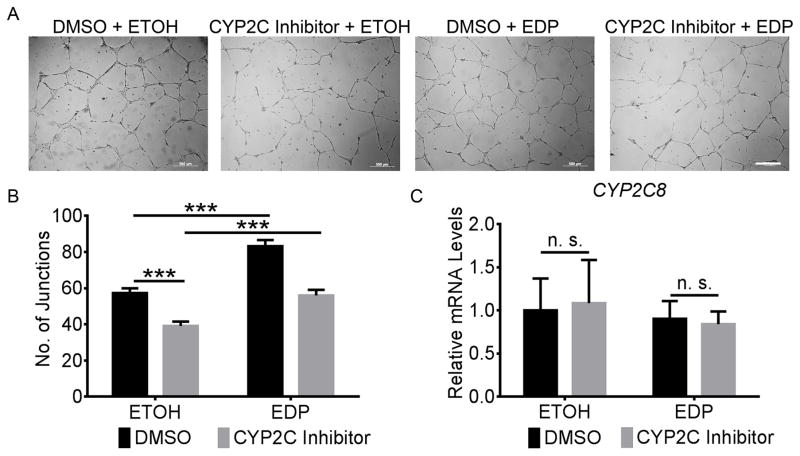
We examined the anti-angiogenic effects of the CYP2C inhibitor montelukast on endothelial cell tubule formation in vitro using human retinal microvascular endothelial cells (HRMECs). Cells treated with montelukast showed a 32% reduction (P = 3.5×10−4) in tubule formation compared with cells treated with control (Figure 6A&B). Addition of DHA further inhibited HRMEC tubule formation by 93% (P = 0.0025) without any effect on CYP2C8 expression (Supplemental Figure VI). However, 19,20-EDP reversed the suppression of HRMEC tubule formation by montelukast by 43% (P = 7.4×10−4) (Figure 6A&B). Neither montelukast nor 19,20-EDP had an effect on the transcription of CYP2C8 in HRMECs (Figure 6C). To further distinguish between CYP2C8 and CysLTR1 inhibitory effects of montelukast, we treated HRMECs with specific CYP2C8 or CysLTR1 siRNAs. CYP2C8, but not CysLTR1, knockdown inhibited HRMEC tubule formation by 64% (Supplemental Figure VIIA&B). Moreover, CysLTR1 expression levels were undetectable in HRMECs (Supplemental Figure VIIC). These data suggested that the lower levels of bioactive CYP2C products derived from ω-3 LCPUFA are involved in the impairment of endothelial cell functions by CYP2C inhibition.
Figure 6. 19,20-EDP reversed the inhibition of human retinal endothelial cell (HRMEC) tubule formation by CYP2C inhibition.
A, Representative photos of HRMECs grown on Matrigel for 6 hour under the conditions of the CYP2C inhibitor montelukast (20 μg/ml) or 0.2% DMSO control, and 19,20-EDP (1 μM) or ETOH vehicle control. Scale bar, 500 μm. B, 19,20-EDP rescued the inhibition of endothelial cell tubule formation by CYP2C inhibition. C, Neither CYP2C inhibitor nor 19,20-EDP had effects on CYP2C8 transcriptional levels in HRMECs. n = 8. *** P < 0.001; n. s., not significant.
Discussion
Increased dietary intake of ω-3 LCPUFA reduces pathological retinal and choroidal neovascularization8. While many ω-3 LCPUFA metabolites biosynthesized through COX and LOX pathways have anti-angiogenic effects, ω-3 LCPUFA metabolites of CYP2C promote both retinal neovascularization (OIR) and choroidal neovascularization (laser-induced CNV)10, 19, 20. In this study, we showed that inhibition of CYP2C activity by montelukast added to the protective effects of ω-3 LCPUFA on ocular neovascularization in the animal models of both OIR and laser-induced CNV. The increased neovascularization observed in transgenic mice overexpressing human CYP2C8 on a ω-3 LCPUFA enriched diet was substantially reversed with CYP2C inhibition, associated with lower plasma levels of CYP2C8 products derived from ω-3 LCPUFA without affecting CYP2C8 transcriptional levels. Montelukast treatment reduced both CNV and OIR in CYP2C8 transgenic mice to the level of or below that of untreated wild-type littermates. In addition, inhibition of sEH significantly increased ocular neovascularization in vivo in association with increased plasma levels of the bioactive ω-3 LCPUFA metabolites of CYP2C. Addition of ω-3 LCPUFA metabolites of CYP2C reversed the suppression of angiogenesis ex vivo and endothelial cell functions in vitro by CYP2C inhibition.
CYP2C8 is present at high levels in human tissues and is involved in endothelial cell functions27, 34. In an in vitro screening of 209 frequently prescribed drugs to examine their potential to inhibit CYP2C8, montelukast was identified as a potent suppressor22 and a selective inhibitor of CYP2C8 activity among all tested human CYP2C isoforms23. Mouse Cyp2C55 shares a high homology with human CYP2C835. In our studies, montelukast reduced retinal and choroidal neovascularization not only in Tie2-driven CYP2C8 (human) transgenic but also in wild-type mice, which suggests that montelukast inhibits not only exogenous human CYP2C8 but also endogenous mouse Cyp2C activity, such as Cyp2C55.
Montelukast was originally formulated as a specific antagonist for CysLTR1 and is broadly used to treat chronic asthma26. CysLTR1 is expressed in lung, spleen, leukocytes, macrophages and smooth muscle cells, and its activation leads to contraction and proliferation of smooth muscle, edema, and eosinophic migration29, 36. Studies of the effect of montelukast on angiogenesis are limited. Montelukast suppresses colon cancer growth through the inhibition of angiogenesis and inhibits angiogenesis ex vivo in rat thoracic aortic rings24. Our data show that montelukast inhibited angiogenesis ex vivo in mouse aortic rings and choroid explants. However, there is some controversy about the effects of montelukast on endothelial cells. Montelukast was reported to reduce vascular permeability by reducing VEGF expression25, whereas others found that montelukast increases inter-cellular adhesion molecule 1 expression in human primary endothelial cells37. CysLTR1 is generally reported as absent in primary endothelial cells28, 29, although one group reported its expression in a human endothelial cell line and that montelukast inhibited endothelial cell migration by inhibiting the extracellular signal-regulated kinase pathway38. We also observed the inhibition of human retinal endothelial cell migration and VEGF-induced extracellular signal-regulated kinase activation by montelukast (Supplemental Figure VIII), but failed to detect the expression of CysLTR1 in human primary retinal endothelial cells (Supplemental VIIC). Our results suggested that montelukast impairs endothelial cell functions by inhibiting CYP2C activity. The CYP2C inhibitor montelukast is approved by the United States Food and Drug Administration and has been used to treat asthma for decades so could be repurposed to treat neovascular eye diseases. Our findings enhance our knowledge of tissue-specific effects of montelukast and its mechanism of angiogenesis regulation.
Compared with ω-6 LCPUFA, ω-3 LCPUFA reduce pathological retinal and choroidal neovascularization8, 18. The ω-3 LCPUFA DHA inhibits angiogenesis ex vivo and endothelial functions in vitro, which is consistent with previous reports and likely related to its metabolites produced through COX and LOX, rather than CYP pathways9, 10, 20. DHA does not appreciably alter the effects of CYP2C inhibition, and further increases the inhibition of angiogenesis, suggesting that DHA has parallel effects in the co-treatment with a CYP2C inhibitor. In contrast to the modest effects of DHA on the inhibition of montelukast, addition of CYP2C products derived from DHA, such as 19,20-EDP, reversed the inhibitory effects of this CYP2C inhibitor on angiogenesis ex vivo and endothelial cell functions in vitro, suggesting that montelukast functions upstream of CYP2C products. Moreover, increased plasma levels of CYP2C products derived from ω-3 LCPUFA with inhibition of sEH activity also promoted neovascularization, which was accompanied by increased levels of ω-3 LCPUFA metabolites of CYP2C in plasma. Our results suggested that inhibition of CYP2C activity suppresses endothelial cell growth during angiogenesis by lowering the levels of CYP2C products. More clinical research on biological effects of CYP2C inhibition on neovascularization will expand our understanding about the mechanism of CYP2C/sEH pathway regulation of angiogenesis.
CYP2C inhibition reversed the induction of retinal and choroidal neovascularization in CYP2C8 overexpressing mice fed with either a ω-3 or ω-6 LCPUFA enriched diet. These results suggest that CYP2C products derived from both ω-3 and ω-6 LCPUFA are pro-angiogenic in the retina and choroid. A previous study showed that 19,20-EDP inhibited tumor growth and human umbilical vein endothelial cell functions in vitro by suppressing VEGF-C, but not VEGF-A39. In our studies, no change in VEGF-C expression was observed in either Tie2-driven CYP2C8 transgenic or CYP2C inhibitor-treated retina (Supplemental Figure IX). The different expression pattern of VEGF-A and VEGF-C in the retina and in tumor might contribute to the different effects of the CYP2C metabolite observed, indicating a tissue-specific role of 19,20-EDP. Despite much research on the potent effects of other CYP2C metabolites, such as EDPs and EETs, in many physiological and pathological processes, knowledge about the molecular mechanism or regulation of angiogenesis and endothelial cell behaviors is still limited. A recent study indicated that 11,12-EET promotes hematopoietic stem and progenitor cell specification by increasing activator protein 1 and runx1 transcription through phosphatidylinositol-3-OH kinase pathway40, which has also been implicated in advanced AMD by our previous work41. More research on the direct target and downstream pathways of CYP2C metabolites is needed.
Our inferences were strengthened on the basis of 5 additional lines of evidence: 1) intravitreal injections of trimethoprim, an antibiotic known to inhibit CYP2C8, is effective in treatment of human toxoplasma retinochorioditis, a disease affecting the retinal vasculature42, 43; 2) injections of cationic liposome-encapsulated paclitaxel, a substrate of CYP2C8, reduces the volume of choroidal neovascularization in an animal model of pathologic choroidal angiogenesis44; 3) exposure to thiazolidinedione, an anti-diabetic agent known to inhibit CYP2C8, significantly reduces the extent of retinal neovascular pathology in OIR mice45 – effects in this report were attributed to a pioglitazone-induced increase in adiponectin, a ω-3 LCPUFA-modulated protective factor for pathological retinal and choroidal neovascularization46; 4) CYP2C8-inhibiting thiazolidinediones also act as trans-activators of the peroxisome proliferator activated receptor gamma transcription response element, which is part of an ω-3 LCPUFA-sensing signaling system implicated in ROP, DR and AMD9, 47, 48; and 5) pull-down and co-immunopreciptation studies confirmed the binding of CYP2C8 with alpha 2-macroglobulin, a major protease inhibitor that acts on and is cleaved by matrix metallopeptidase 9 an AMD-associated collagenase essential for clearing space for sprouting vessels within the angiogenic cascade49, 50.
In summary, our study found that inhibition of CYP2C inhibited pathological retinal and choroidal neovascularization by lowering the levels of CYP2C products from both ω-6 and ω-3 LCPUFA. ω-3 LCPUFA may help prevent retinal and choroidal neovascularization. Our findings suggest enhanced protective effects of ω-3 LCPUFA against pathological angiogenesis with CYP2C inhibition. Montelukast is a potential therapeutic to treat neovascular eye diseases. Dietary ω-3 LCPUFA DHA supplementation with CYP2C inhibition is likely to benefit retinal and choroidal neovascularization.
Supplementary Material
Highlights.
Cytochrome P450 oxidase 2C inhibitor reduces retinal and choroidal neovascularization.
Soluble epoxide hydrolase inhibitor induces retinal and choroidal neovascularization.
Cytochrome P450 oxidase 2C inhibitor adds to the protective effects of ω-3 long-chain polyunsaturated fatty acids on pathological retinal and choroidal neovascularization.
cytochrome P450 oxidase Inhibitor presents a new therapeutic approach for prevention of neovascular eye diseases.
Montelukast as an approved pharmaceutical on the market could be repurposed for treating neovascular eye diseases.
Acknowledgments
We thank Drs. Jing Chen and Ye Sun for helpful discussions and critical reading of the manuscript, Drs. Dipak Panigrahy and Raffael Liegl for helpful discussions, and Dr. Jie Li, Christian G. Hurst, Ricky Z. Cui, Lucy P. Evans, Katherine T. Tian and James D. Loewke for excellent technical support.
Sources of Funding
This work was supported by the National Institutes of Health/National Eye Institute (R01 EY022275, EY024864, EY017017, and P01 HD18655), Lowy Medical Foundation, European Commission FP7 PREVENT-ROP project (305485 LEHS), Knights Templar Eye Foundation and Bernadotte Foundation (ZF), Republic of China Ministry of Science and Technology Postdoctoral Research Abroad Program (104-2917-I-564-026 CHL), and in part by National Institutes of Health/National Institute of Environmental Health Sciences (1K99ES024806-01 KSSL, Z01 025034 DCZ, R01 ES002710 and P42 ES04699 BDH).
Abbreviations
- AA
arachidonic acid
- AMD
age-related macular degeneration
- CNV
choroidal neovascularization
- COX
cyclooxygenase
- CYP
cytochrome P450 oxidase
- CysLTR
cysteinyl leukotriene receptor
- DHA
docosahexaenoic acid
- DHET
dihydroxy-eicosatrienoic acid
- DiHDPA
dihydroxy-docosapentaenoic acid
- DR
diabetic retinopathy
- EDP
epoxydocosapentaenoic acid
- EET
epoxyeicosatrienoic acid
- HRMEC
human retinal microvascular endothelial cell
- LCPUFA
long-chain polyunsaturated fatty acids
- LOX
lipoxygenase
- OIR
oxygen-induced retinopathy
- ROP
retinopathy of prematurity
- sEH
soluble epoxide hydrolase
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None
References
- 1.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. The New England journal of medicine. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson DM. Diabetic retinopathy and age-related macular degeneration in the u.S. American journal of preventive medicine. 2012;43:48–54. doi: 10.1016/j.amepre.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Cheung N, Lam DS, Wong TY. Anti-vascular endothelial growth factor treatment for eye diseases. Bmj. 2012;344:e2970. doi: 10.1136/bmj.e2970. [DOI] [PubMed] [Google Scholar]
- 5.Liu CH, Sun Y, Li J, Gong Y, Tian KT, Evans LP, Morss PC, Fredrick TW, Saba NJ, Chen J. Endothelial microrna-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:12163–12168. doi: 10.1073/pnas.1508426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki M, Ozawa Y, Kubota S, Hirasawa M, Miyake S, Noda K, Tsubota K, Kadonosono K, Ishida S. Neuroprotective response after photodynamic therapy: Role of vascular endothelial growth factor. Journal of neuroinflammation. 2011;8:176. doi: 10.1186/1742-2094-8-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, Kusaka S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. American journal of ophthalmology. 2012;153:327–333. e321. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, Guerin KI, Hua J, Smith LE. Short communication: Ppar gamma mediates a direct antiangiogenic effect of omega 3-pufas in proliferative retinopathy. Circulation research. 2010;107:495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapieha P, Stahl A, Chen J, et al. 5-lipoxygenase metabolite 4-hdha is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Science translational medicine. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahl A, Krohne TU, Sapieha P, Chen J, Hellstrom A, Chew E, Holz FG, Smith LE. Lipid metabolites in the pathogenesis and treatment of neovascular eye disease. The British journal of ophthalmology. 2011;95:1496–1501. doi: 10.1136/bjo.2010.194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY. {omega}-3 long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: Areds report 30, a prospective cohort study from the age-related eye disease study. The American journal of clinical nutrition. 2009;90:1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Progress in retinal and eye research. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: A prospective, randomized study. JPEN. Journal of parenteral and enteral nutrition. 2014;38:711–716. doi: 10.1177/0148607113499373. [DOI] [PubMed] [Google Scholar]
- 15.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: The blue mountains eye study. Archives of ophthalmology. 2009;127:656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 16.Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ, Tang J, Kern TS, Akula JD, Smith LE. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutrition & diabetes. 2012;2:e36. doi: 10.1038/nutd.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Investigative ophthalmology & visual science. 1994;35:101–111. [PubMed] [Google Scholar]
- 18.Gong Y, Li J, Sun Y, Fu Z, Liu CH, Evans L, Tian K, Saba N, Fredrick T, Morss P, Chen J, Smith LE. Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PloS one. 2015;10:e0132643. doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymczak M, Murray M, Petrovic N. Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood. 2008;111:3514–3521. doi: 10.1182/blood-2007-08-109934. [DOI] [PubMed] [Google Scholar]
- 20.Shao Z, Fu Z, Stahl A, et al. Cytochrome p450 2c8 omega3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:581–586. doi: 10.1161/ATVBAHA.113.302927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome p450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacological reports: PR. 2010;62:536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 22.Walsky RL, Gaman EA, Obach RS. Examination of 209 drugs for inhibition of cytochrome p450 2c8. J Clin Pharmacol. 2005;45:68–78. doi: 10.1177/0091270004270642. [DOI] [PubMed] [Google Scholar]
- 23.Walsky RL, Obach RS, Gaman EA, Gleeson JP, Proctor WR. Selective inhibition of human cytochrome p4502c8 by montelukast. Drug metabolism and disposition: the biological fate of chemicals. 2005;33:413–418. doi: 10.1124/dmd.104.002766. [DOI] [PubMed] [Google Scholar]
- 24.Savari S, Liu M, Zhang Y, Sime W, Sjolander A. Cyslt(1)r antagonists inhibit tumor growth in a xenograft model of colon cancer. PloS one. 2013;8:e73466. doi: 10.1371/journal.pone.0073466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KS, Kim SR, Park HS, Jin GY, Lee YC. Cysteinyl leukotriene receptor antagonist regulates vascular permeability by reducing vascular endothelial growth factor expression. The Journal of allergy and clinical immunology. 2004;114:1093–1099. doi: 10.1016/j.jaci.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Jones TR, Labelle M, Belley M, Champion E, Charette L, Evans J, Ford-Hutchinson AW, Gauthier JY, Lord A, Masson P, et al. Pharmacology of montelukast sodium (singulair), a potent and selective leukotriene d4 receptor antagonist. Canadian journal of physiology and pharmacology. 1995;73:191–201. doi: 10.1139/y95-028. [DOI] [PubMed] [Google Scholar]
- 27.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, Foley JF, Torphy R, Ronnekleiv OK, Tomer KB, Lee CR, Zeldin DC. Endothelial expression of human cytochrome p450 epoxygenase cyp2c8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virchow JC, Jr, Faehndrich S, Nassenstein C, Bock S, Matthys H, Luttmann W. Effect of a specific cysteinyl leukotriene-receptor 1-antagonist (montelukast) on the transmigration of eosinophils across human umbilical vein endothelial cells. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2001;31:836–844. doi: 10.1046/j.1365-2222.2001.01051.x. [DOI] [PubMed] [Google Scholar]
- 29.Lotzer K, Spanbroek R, Hildner M, Urbach A, Heller R, Bretschneider E, Galczenski H, Evans JF, Habenicht AJ. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:e32–36. doi: 10.1161/01.ATV.0000082690.23131.CB. [DOI] [PubMed] [Google Scholar]
- 30.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (eets) Prostaglandins & other lipid mediators. 2007;82:42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nature reviews Drug discovery. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones PD, Tsai HJ, Do ZN, Morisseau C, Hammock BD. Synthesis and sar of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorganic & medicinal chemistry letters. 2006;16:5212–5216. doi: 10.1016/j.bmcl.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. Journal of medicinal chemistry. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsao CC, Coulter SJ, Chien A, Luo G, Clayton NP, Maronpot R, Goldstein JA, Zeldin DC. Identification and localization of five cyp2cs in murine extrahepatic tissues and their metabolism of arachidonic acid to regio- and stereoselective products. The Journal of pharmacology and experimental therapeutics. 2001;299:39–47. [PubMed] [Google Scholar]
- 35.Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome p450 (cyp) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Lynch KR, O’Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene cyslt1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 37.Andersson K, Shebani EB, Makeeva N, Roomans GM, Servetnyk Z. Corticosteroids and montelukast: Effects on airway epithelial and human umbilical vein endothelial cells. Lung. 2010;188:209–216. doi: 10.1007/s00408-010-9227-6. [DOI] [PubMed] [Google Scholar]
- 38.Yuan YM, Fang SH, Qian XD, Liu LY, Xu LH, Shi WZ, Zhang LH, Lu YB, Zhang WP, Wei EQ. Leukotriene d4 stimulates the migration but not proliferation of endothelial cells mediated by the cysteinyl leukotriene cyslt(1) receptor via the extracellular signal-regulated kinase pathway. Journal of pharmacological sciences. 2009;109:285–292. doi: 10.1254/jphs.08321fp. [DOI] [PubMed] [Google Scholar]
- 39.Zhang G, Panigrahy D, Mahakian LM, et al. Epoxy metabolites of docosahexaenoic acid (dha) inhibit angiogenesis, tumor growth, and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Lahvic JL, Binder V, et al. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature. 2015;523:468–471. doi: 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SanGiovanni JP, Mehta S, Mehta S. Variation in lipid-associated genes as they relate to risk of advanced age-related macular degeneration. World review of nutrition and dietetics. 2009;99:105–158. doi: 10.1159/000193002. [DOI] [PubMed] [Google Scholar]
- 42.Matsuo T, Yamaoka A, Shiraga F, Takasu I, Okanouchi T, Nagayama M, Baba T, Hayashi M, Sarada K. Clinical and angiographic characteristics of retinal manifestations in cat scratch disease. Japanese journal of ophthalmology. 2000;44:182–186. doi: 10.1016/s0021-5155(99)00195-1. [DOI] [PubMed] [Google Scholar]
- 43.Choudhury H, Jindal A, Pathengay A, Bawdekar A, Albini T, Flynn HW., Jr The role of intravitreal trimethoprim/sulfamethoxazole in the treatment of toxoplasma retinochoroiditis. Ophthalmic surgery, lasers & imaging retina. 2015;46:137–140. doi: 10.3928/23258160-20150101-27. [DOI] [PubMed] [Google Scholar]
- 44.Gross N, Ranjbar M, Evers C, Hua J, Martin G, Schulze B, Michaelis U, Hansen LL, Agostini HT. Choroidal neovascularization reduced by targeted drug delivery with cationic liposome-encapsulated paclitaxel or targeted photodynamic therapy with verteporfin encapsulated in cationic liposomes. Molecular vision. 2013;19:54–61. [PMC free article] [PubMed] [Google Scholar]
- 45.Higuchi A, Ohashi K, Shibata R, Sono-Romanelli S, Walsh K, Ouchi N. Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:46–53. doi: 10.1161/ATVBAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu Z, Lofqvist CA, Shao Z, et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. The American journal of clinical nutrition. 2015;101:879–888. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (field study): A randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 48.SanGiovanni JP, Chen J, Sapieha P, Aderman CM, Stahl A, Clemons TE, Chew EY, Smith LE. DNA sequence variants in ppargc1a, a gene encoding a coactivator of the omega-3 lcpufa sensing ppar-rxr transcription complex, are associated with nv amd and amd-associated loci in genes of complement and vegf signaling pathways. PloS one. 2013;8:e53155. doi: 10.1371/journal.pone.0053155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sottrup-Jensen L, Birkedal-Hansen H. Human fibroblast collagenase-alpha-macroglobulin interactions. Localization of cleavage sites in the bait regions of five mammalian alpha-macroglobulins. The Journal of biological chemistry. 1989;264:393–401. [PubMed] [Google Scholar]
- 50.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annual review of cell and developmental biology. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.