1. Introduction
Human co-exposure to aflatoxins (AFs) and fumonisins (FBs) is an important public health concern in parts of the developing world where maize is a dietary staple (IARC, 2016). Populations at high risk for co-exposure to AFs and FBs include humans in Sub-saharan Africa, Southeast Asia, Mexico, Central America, and parts of South America (Kang et al., 2015; Marroquin-Cardona et al., 2014; Torres et al., 2014; Wild and Gong, 2010). AFs are known to cause human diseases, including aflatoxicoses resulting from acute exposure to high levels of AFs, and primary liver cancer as a result of chronic low dose exposure (Kensler et al., 2011; Khlangwiset et al., 2011). Both AF and FB exposure have been linked to growth retardation in children (Kimanya et al., 2010; Shirima et al., 2015; Wild and Gong, 2010) and AF exposure has been correlated with immune modulating effects (IARC, 2016; Wu et al., 2014). FBs have been linked to human esophageal cancer and neural tube defects in infants although the human data remains insufficient to draw a firm conclusion (Bulder, 2012; Wu et al., 2014). In Sub-Sahara African and Southeast Asian countries, dietary exposure to both mycotoxins is common and AFs exposure has been estimated to be associated with approximately 40% of the disease burden in the developing countries (Williams et al., 2004). Synergistic interaction between AFB1 exposure and hepatitis B virus infection in the development of human hepatocellular carcinoma has also been confirmed (IARC, 2002) and it is likely that when maize is a dietary staple there is also exposure to FB (Bulder, 2012; IARC, 2016). The human co-exposure to both AFB1 and FB1 is of particular interest with regards to the synergistic effects in enhancing early preneoplastic lesions in rat liver and the promotion of liver tumors in rainbow trout, respectively (Carlson et al., 2001; Gelderblom et al., 2002).
AFB1 is a potent mutagen and carcinogen that targets DNA while FB1 is an effective cancer promoter and a complete carcinogen without directly affecting DNA sequence (Bulder, 2012; Gelderblom et al., 1988; IARC, 2002). AFB1 induced liver cancer is characterized by a G to T mutation at codon 249 of the p53 gene (Aguilar et al., 1993) and possibly epigenetic effects involving DNA methylation (Wu et al., 2013). On the other hand, FB1 induces cancer in rodents and trout through the disruption of sphingolipid metabolism via inhibition of ceramide synthase leading to alterations of key signaling pathways, induction of oxidative damage, and alterations in growth regulation (Bulder, 2012; Gelderblom et al., 2001a; IARC, 2002; Merrill et al., 2001; Riedel et al., 2015; Riley et al., 2001). Based on relatively scarce evidence of co-exposure in animal models as compared to single mycotoxin research, AFB1 and FB1 co-treatment demonstrates additive or synergistic effects in enhancing liver toxicity (Carlson et al., 2001; Casado et al., 2001; Gelderblom et al., 2002; McKean et al., 2006; Orsi et al., 2007; Theumer et al., 2008). For example, a seven-day bioassay in F344 rats showed that co-exposure to both mycotoxins resulted in a strong additive effect for acute toxicity of AFB1; the LD50 of AFB1 alone was 2.71 mg/kg bw whereas the LD50 for AFB1 plus FB1 was 1.37 mg/kg bw (McKean et al., 2006). In a 3-month feeding assay in Wister rats co-exposed to 40 ppb AFB1 and 100 ppm FB1 it was concluded that the nephrotoxicity resulting from co-exposure using Fusarium verticilioides culture material was additive (Theumer et al., 2008). A similar 3-month feeding experiment in mice (co-exposure to 10 ppb AFB1 and 10 ppm FB1) also showed greater serum aspartate aminotransferase (AST) levels in the co-exposure group versus the single mycotoxin treatment group, however, a clear interaction was not apparent (Casado et al., 2001). When New Zealand rabbits were given AFB1 (30 µg/kg body weight), FB1 (1.5 mg/kg body weight), or the combination by gavage for 21 days, elevated serum AST and ALT and histopathological changes in liver and kidney were most pronounced in the co-exposure group, suggesting a synergistic effect (Orsi et al., 2007). In the only chronic dose-response feeding study exploring the ability of FB1 to promote AFB1 carcinogenicity, trout fry were initiated with a single dose of AFB1 (immersion in 100 ppb AFB1 for 30 min) and then fed diets containing pure FB1 at 3 mg/kg diet, 23 mg/kg diet, and 104 mg/kg diet for 42 weeks (Carlson et al., 2001). No liver tumors were found in any of the trout fed only FB1 but there was a dose-dependent increase in the incidence of liver tumors in trout initiated with AFB1 and fed the FB1 diets. The incidence was significantly (p<0.05) increased in the trout initiated with AFB1 and fed the 23 mg FB1/kg (61% incidence) and 104 mg FB1/kg (74% incidence) diets compared to the group fed the AFB1 only diet (35%).
The concept of multi-stage carcinogenesis derives from chemical-induced tumor models in mouse skin (Berenblum and Shubik, 1947a, b) and facilitates the evaluation of chemical carcinogens. While the two-year carcinogenesis model remains a “gold standard” to verify the carcinogenicity of certain environmental toxicants, short-term carcinogenesis models are both valuable for revealing potential carcinogens and less costly as an alternative approach. The Solt–Farber resistant hepatocyte model has been widely used as a short-term bioassay to detect the cancer initiating capacity of environmental agents (Espandiari et al., 2005; Gelderblom et al., 2002; Solt et al., 1977). Gelderblom et al. (2002) investigated the cancer initiating potential of AFB1 and FB1 using the resistant hepatocyte model and more importantly, explored the co-carcinogenic effects after sequential treatment with both mycotoxins (Gelderblom et al., 2002). They found that FB1 had a lower initiating capacity than AFB1, however, FB1 synergized with AFB1 in a sequential exposure liver carcinogenesis model to increase the number and mean size of rat liver GST-P+ foci. Their work provides solid evidence of the interaction between AFB1 and FB1 in rat liver. In view of the increasing concern for frequent human co-exposure to these two mycotoxins (IARC, 2016), we used a modified procedure to validate the co-carcinogenic effect of AFB1 and FB1 in a similar short-term rat model. The major modifications include administration of mycotoxins through feeding instead of oral gavage and without using partial hepatectomy (PH) to avoid potential harm to the animals (Ying et al., 1980). Outcome evaluations include an integrative approach monitoring serum biochemistry, histopathological alterations as well as liver GST-P+ foci formation. The aim of this study was to validate the co-carcinogenic rat model in a feeding study to provide further evidence for using this model for translational and dietary intervention studies.
2. Materials and Methods
2.1. Chemicals
AFB1, diethylnitrosamine (DEN) and 2-Acetylaminofluorene (2-AAF) were purchased as powder at purity higher than 98% from Sigma-Aldrich Inc. (St. Louis, MO). FB1 was provided from PROMEC Unit, Medical Research Council, South Africa and the USDA-ARS Toxicology and Mycotoxin Research Unit. All other chemicals were purchased commercially at the highest degree of purity available.
2.2. Animals
Male F344 rats (120–140g) were purchased from Harlan Laboratories Inc. (Indianapolis, IN). Animals were housed in a 12h/12h light/dark cycle with controlled temperature (20–22°C) and humidity (50–70%), and fed with AIN-76A diet (Harland Tekland, Madison, WI). Animals were acclimated for one week before treatment.
2.3. Preparation of test feed
The procedure for preparing test feed with FB1 was similar to a previous study (Gelderblom et al., 2002). In the study by Gelderblom et al. (2002) the AFB1 and 2-AAF were dosed by oral gavage but in this study the 2-AAF and AFB1 were dosed in the diet. Briefly, the test chemicals in powder form were weighed out using a designated analytical balance and dissolved in methanol. The solution with each test compound was thoroughly mixed with 100 g AIN-76A diet under a chemical hood and dried. This material served as “stock feed” and was stored under nitrogen at −20°C. The “stock feeds” were mixed with additional AIN-76A feed to the final dosing concentrations and aliquoted in 500 g lots and stored at −20°C until administration to animals within one week. The final concentrations for 2-AAF, AFB1 and FB1 were 150 µg/kg, 150 µg/kg and 250 mg/kg feed, respectively.
2.4. Experimental design
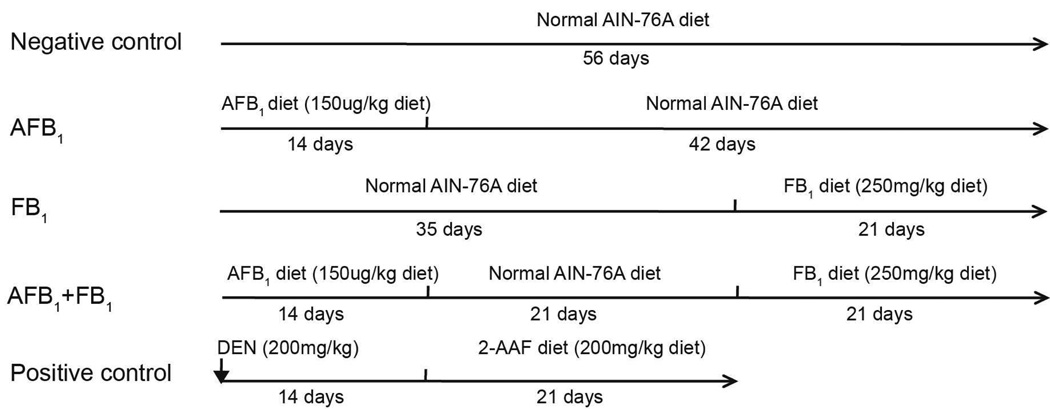
The experimental design is shown in Fig. 1. A total of 62 male F344 rats (6-weeks old) were randomly divided into 5 groups. Animals in the negative control group (n=10) were fed with normal AIN-76A diet throughout the 56 day experimental period. The AFB1-only treatment group: animals (n=13) were fed with AFB1-containing feed (150 µg/kg diet) for the initial 14 days, followed by normal AIN-76A diet for an additional 42 days; the FB1-only treatment group: animals (n=13) were fed with normal AIN-76A diet for 35 days, and then were fed with FB1-containing feed (250 mg/kg diet) for an additional 21 days; the AFB1 and FB1 co-treatment group: animals (n=13) were fed with AFB1-only containing feed (150 µg/kg diet) for the initial 14 days, followed by normal AIN-76A diet for 21 days, and then switched to FB1-only containing feed (250 mg/kg diet) for an additional 21 days. The dose selection was derived from previous studies (Gelderblom et al., 1994; Gelderblom et al., 2002; Qian et al., 2013). The positive control-group animals (n=13) were given a single i.p injection of DEN (dissolved in DMSO) at 200 mg/kg body weight (50µL/100g body weight) and then maintained on the normal AIN-76A diet for 14 days followed by feeding with the 2-AAF containing diet (150 µg/kg diet) for 21 days. The purpose of the positive control-group was to ensure that the initiation/promotion protocol, as used in this study, did in fact induce preneoplastic lesions. Animal body weight was recorded three times a week and feed intake was recorded daily. Animals were fasted 12 h before sacrifice. The study protocol was reviewed and approved by the UGA Animal Use and Care Committee.
Fig. 1.
Experimental design. The negative control animals (n=10), AFB1-only group (n=13), FB1-only group (n=13), and the AFB1+FB1 group (n=13) were fed AIN-76 diet for a total of 56 days. The negative control group received un-amended diet for the entire 56 days of feeding. The AFB1-only group received diet amended with pure AFB1 for the first 14 days followed by un-amended diet for 42 days. The FB1-only group received un-amended diet for the first 35 days and then FB1 amended diet for the final 21 days. The AFB1+FB1-group received the AFB1-only amended diet for the first 14 days followed by 21 days on un-amended diet followed by 21 days on the FB1-only amended diet. The positive control group was injected with the known cancer initiator DEN on day 1 and then fed un-amended diet for 14 days followed by 21 days consuming the AIN 76A diet amended with 2-AAF for a total of 35 days.
2.5. Serum biochemistry
Serum was separated from whole blood collected by cardiac puncture and stored at −20°C before biochemical analysis. The serum biochemical analyses were done with a Roche Hitachi 912 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN).The clinical parameters measured included total serum protein (g/dL), albumin (g/dL), globulin (g/dL), albumin:globulin ratio (A/G ratio), serum calcium (mg/dL), phosphorus (mg/dL), glucose (mg/dL), BUN (mg/dL), creatinine (mg/dL), total bilirubin (mg/dL), alkaline phosphatase (ALP, U/L), creatine kinase (CK, U/L), alanine transaminase (ALT, U/L), aspartate transaminase (AST, U/L), g-glutamyl transpeptidase (GGT, U/L), amylase (U/L), cholesterol (mg/dL), serum sodium (mmol/L), potassium (mmol/L), Na/K ratio, and chloride (mmol/L).
2.6. Liver histopathology
Immediately after sacrifice, rat liver tissues were dissected, briefly washed in cold saline, and then fixed in 4% neutral buffered formalin. The routine procedure for paraffin embedding and Harris hematoxylin and eosin (H&E) staining were followed and 5 µm sections were cut. Approximately 7–8 serial sections were cut from each of the three rat liver lobes, left lateral, median, and right lateral lobes for examination of H&E staining and immunohistochemical staining. Light microscopic examination of H&E stained slides were performed by two independent pathologists. Sections were coded and the group information was kept blind to pathologists. Photographs were taken on an Olympus XC30 microscope (Germany) with a digital camera linked to an image analysis system (Olympus UC30 camera, Cellsense image analysis software with a measure and count package).
2.7. Liver GST-P staining
Paraffin embedded liver sections were stained for liver GST-P using the ABC method following manufacture’s protocol (Vector Labs, Burlingame, CA). Briefly, liver sections were routinely hydrated and then placed into 3% hydrogen peroxide in methanol to quench endogenous peroxidase activity, followed by washing with deionized (DI) water once and 1 × PBS three times. These liver sections were boiled in 30 mM sodium citrate buffer (pH 6.0) for 10 min for retrieval of antigen. Cooled sections were blocked using diluted normal goat serum for 30 min at 37°C. Primary rabbit anti-rat GST-P antibody (1:800) was directly applied onto these sections which were incubated overnight at 4°C. After three washes with 1 × PBS, secondary goat anti-rabbit antibody (1:200) was applied onto these sections for an additional 30 min incubation at 37°C. After three washes with 1 × PBS, ABC complex was applied into sections which were further incubated for 45 min at 37°C. 3,3’-Diaminobenzidine (DAB) staining was then done for approximately 5 min at room temperature, followed by washing with running tap water. Finally, these sections were counterstained with hematoxylin and mounted for light microscopic examination. GST-P+ foci were defined as a focus that had more than 5 hepatocytes positively stained with brown color. Photographs were taken on an Olympus XC30 microscope (Germany) with a digital camera linked to an image analysis system. The areas of live GST-P+ foci and the whole tissue section were calculated using the count & measure package of the CellSense digital imaging software (Olympus, Center Valley, PA).
2.8. Statistical analysis
All the data were presented as mean ± standard deviation (SD) or standard error (SE). Body weights, serum biochemical parameters, and histological alterations were compared using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison procedure. The number of liver GST-P+ foci, foci area ratio, and mean focus area were compared using the Kruskal-Wallis test of one-way ANOVA followed by a Mann-Whitney test with Bonferroni correction. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Comparison of effects observed in the positive control and negative control groups
3.1.1. Changes of body weight and feed intake
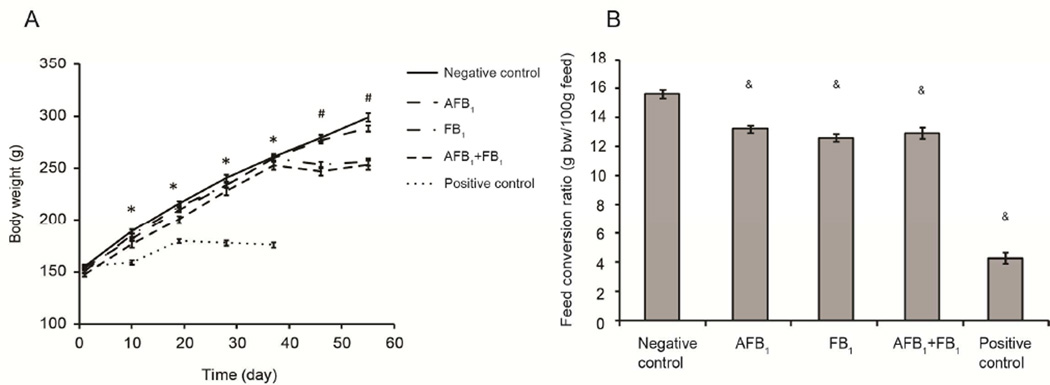
Treatment of F344 rats with a single injection of the known liver cancer initiator (DEN) followed by consumption of diets containing a tumor promoter (2-AAF) resulted in significant reduction in weight gain at all sampling times compared to the negative control group (Fig. 2A). Feed conversion was also significantly reduced in the positive control group compared to the negative control group (Fig. 2B).
Fig. 2.
(A) Effects of different treatment protocols with AFB1 and FB1 on body weight compared to the negative and positive groups. An asterisk (*) indicates that the mean weight of the positive control group was significantly less (p<0.05) than any of the other groups; the pound sign (#) indicates that the mean weights of the FB1-only group and the AFB1+FB1 group were significantly less (p < 0.05) than the time-matched negative control and the AFB1-only group. Values are shown as means ± SE. (B) Effects of different treatment protocols on feed conversion ratio. Feed conversion ratio is described as the body weight gain in g per 100g feed consumed during the 56 days experimental period. An ampersand (&) indicates that the mean ratio is significantly (p < 0.001) different compared to the negative control group. Values are shown as means ± SE.
3.1.2. Alterations of serum biochemical parameters
Alterations in serum biochemical parameters are shown in Table 1 and Supplemental Table S1. As compared to the negative control group, the positive control group animals showed significantly decreased concentration of serum proteins (total protein, globulin, and albumin), glucose, and cholesterol (p < 0.001). Levels of AST, ALP, and total bilirubin were significantly elevated (p < 0.01).
Table 1.
Alterations of serum biochemistry induced by single or sequential AFB1 and FB1 treatments.
| Parameters | Negative control | AFB1 | FB1 | AFB1 + FB1 | Positive control |
|---|---|---|---|---|---|
| Total Protein (g/dl) | 6.78±0.28 | 7.12±0.12# | 7.23±0.25*,# | 6.75±0.18 | 3.97±0.15* |
| AST (U/l) | 103.33±9.63 | 109.33±16.94# | 221.50±23.16*,# | 353.33±52.81* | 151.50±14.68*,# |
| ALT (U/l) | 41.50±1.22 | 37.83±3.71# | 109.17±14.44*,# | 182.17±28.02* | 29.00±6.00 |
| BUN (mg/dl) | 13.32±1.41 | 17.20±1.11* | 18.27±1.30* | 17.48±1.42* | 13.52±.0.61# |
| Creatinine (mg/dl) | 0.20±0.01 | 0.22±0.01# | 0.43±0.04* | 0.37±0.03* | 0.20±0.01# |
| Total bilirubin (mg/dl) | 0.05±0.00 (<0.1) | 0.12±0.02* | 0.20±0.06* | 0.18±0.03* | 0.66±0.33* |
| ALP (U/l) | 139.17±7.41 | 125.33±7.79# | 161.50±7.92*,# | 231.67±12.23* | 157.33±14.61* |
| CK (U/l) | 809.00±46.87 | 738.80±205.76 | 698.00±138.45 | 1284.80±349.25* | 631.40±94.24 |
| Glucose (mg/dl) | 202.17±16.07 | 220.83±18.30# | 157.00±14.04*,# | 113.50±12.47* | 90.17±15.55* |
| Cholesterol (mg/dl) | 112.07±8.61 | 112.97±11.61# | 189.30±21.01* | 195.73±13.15* | 34.92±3.78*,# |
p < 0.05, compared to negative control;
p < 0.05, compared to the AFB1+FB1 group.
N=6 for each group. Additional serum biochemical data are shown in the Supplemental Table S1.
3.1.3. Histopathological alterations and formation of liver GST-P+ foci
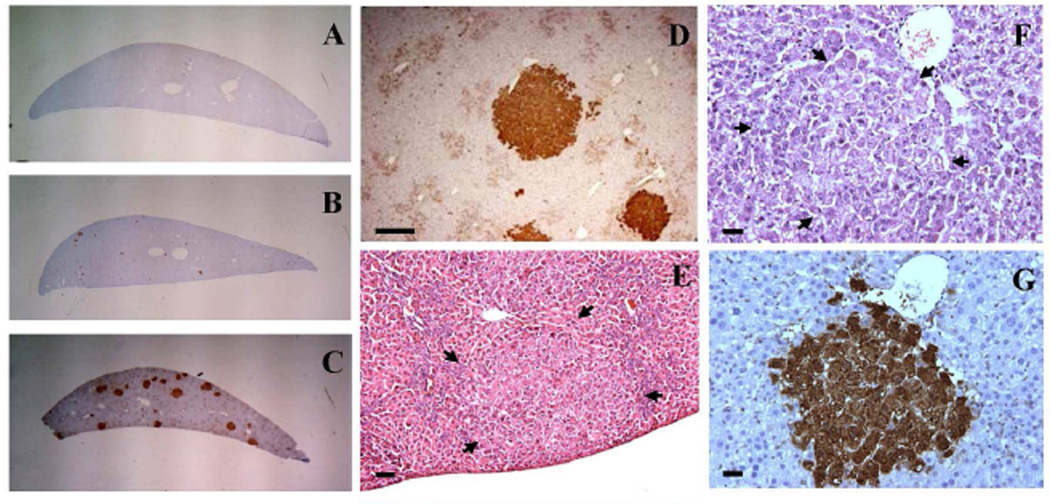
Changes indicative of induction of hyperplastic nodules and an increase in markers of preneoplastic liver lesions in the positive control group compared to the negative control group were observed as expected (Fig. 3A, 3C-3E). In the positive control group treated with DEN and 2-AAF the major histopathological alterations found were formation of dysplasia, foci of altered hepatocytes, mitoses, apoptosis, and necrosis as summarized in Table 2. Proliferative nodules were observed in the livers of all positive control rats (Table 2, Fig. 3E). In comparison to the negative control and other treatment groups, the positive control group displayed a significantly higher number of liver GST-P+ foci as shown in Table 3.
Fig. 3.
Representative images of liver gross view, liver GST-P+ foci, hepatocellular proliferative nodule and foci of altered hepatocytes. A–C: Gross view of liver sections from the negative control group, AFB1+FB1 group, and the positive control (DEN+ 2-AAF) group, respectively. D: IHC staining of liver GST-P+ foci from the positive control group at lower magnification, bar represents 200µm. E: Basophilic nodule of hepatocellular proliferation from the positive control group as indicated by arrowhead, bar represents 50µm. F and G are the same area of the sections in AFB1+FB1 group, bar represents 20µm. F: Basophilic focus by H&E staining, arrowhead indicates focus area. G: GST-P+ focus by IHC staining.
Table 2.
Semi-quantitative evaluation of histological alterations induced by single or sequential AFB1 and FB1 treatments.
| Groups | N | No. of dysplasia | Mitoses | Apoptosis | No. of foci of altered hepatocytes |
No. of proliferative nodule |
Necrosis |
|---|---|---|---|---|---|---|---|
| Negative control | 10 | ND | ND | ND | ND | ND | ND |
| AFB1 | 13 | 194.7±41.4* | ND | 2.5±2.1* | 2.8±1.1* | ND | 1.6±0.4* |
| FB1 | 13 | 97.5±23.6* | 2.3±2.0 | 3.4±1.6* | ND | ND | 1.9±0.4 |
| AFB1+ FB1 | 13 | 258.5±40.2 | 3.5±1.9 | 26.5±7.8 | 4.7±2.2 | 0.1±0.3 (1/13) | 2.1±0.5 |
| Positive control | 13 | 249.1±26.6 | 0.28±0.6* | 3.7±3.8* | 51.3±15.6* | 14.8±9.8 (15/15) | 1.8±0.4 |
ND, not detectable. The positive control group was not included in the statistical analysis of differences among the AFB1-only, FB1-only, and AFB1+FB1 treatment groups.
p < 0.05, compared to the AFB1+FB1 group.
No. of dysplasia, mitoses, and apoptoses were mean values from 3 slides per rat liver, 5 random views per slides at 400 × magnification.
No. of foci of altered hepatocytes and proliferative nodules were mean values from 3 whole liver sections per rat at 200 × magnification.
Necrosis: Scoring was from 1 to 3 where a score of “1” indicated < 10 individual hepatocyte necroses or a small necrotic focus with ≤ 5 hepatocytes; “2” indicated ≥ 10 individual necrototic hepatocytes or a small necrotic focus with > 5 hepatocytes but ≤ 20 hepatocytes; “3” indicated a large necrotic focus with > 20 hepatocytes.
Table 3.
Effects of single or sequential AFB1 and FB1 treatments on liver GST-P+ foci development.
| Groups | N | No. of foci (No./cm2) |
Foci area ratio (mm2)/(cm2)×1000 |
Mean foci area (mm 2)×1000 |
Detection rate of foci |
|---|---|---|---|---|---|
| Negative control | 10 | 0.2±0.3* | 1±3* | 1±2* | 1/10 |
| AFB1 | 13 | 1.6±0.6* | 7±6* | 5±5* | 13/13 |
| FB1 | 13 | 0.9±1.0* | 6±10* | 4±4* | 9/13 |
| AFB1 + FB1 | 13 | 11.6±4.7 | 350±210 | 30±1 | 13/13 |
| Positive control | 13 | 50.4±10.0 | 5600±1200 | 105±20 | 13/13 |
The positive control group (DEN/2-AAF initiation promotion model) was not included in the statistical analysis of differences among the AFB1-only, FB1-only, and AFB1+FB1 treatment groups.
p < 0.05, compared to the AFB1+FB1 group.
3.2. Comparison of effects observed in AFB1-only, FB1-only, and AFB1+FB1-groups compared to the negative control group
3.2.1. Changes of body weight and feed intake
Animals treated with AFB1-only (150 µg/kg diet) for 2 weeks did not show any significant change in body weight gain compared to the negative control throughout the experiment. Conversely, FB1-only treatment (250 mg/kg diet) and AFB1 plus FB1 co-treatment significantly inhibited body weight gain on day 37 and until the end of the 56 day experiment (Fig. 2A). The feed conversion ratio was significantly lower in the single and the combination treatment groups, with approximately 15% to 19% decrease as compared to the negative control group (p < 0.001, Fig. 2C).
3.2.2. Alterations of serum biochemical parameters
Treatment with AFB1 (150 µg/kg diet) for 2 weeks did not cause significant alterations in biochemical parameters, except for a significant increase in levels of blood urea nitrogen (BUN) and potassium (p < 0.01, Table 1 and Supplemental Table S1) as compared to the negative control group. FB1 treatment significantly increased serum levels of AST and ALT by 1.14- and 1.63-fold, respectively, as compared to the negative control group (Table 1, p < 0.05). However, sequential treatment with AFB1 and FB1 led to a 2.42- and 3.39-fold increase in the serum levels of AST and ALT, respectively. Similarly, treatment with AFB1 alone did not change the serum level of ALP, and FB1 treatment only increased the serum level of ALP by 16% (p < 0.05). However, sequential treatment with both mycotoxins led to a significant increase in ALP level by 66%, as compared to the negative control group (p < 0.05). AFB1 treatment alone did not alter serum glucose concentration, FB1 treatment decreased glucose concentration by 22% (p > 0.05), whereas the sequential treatment with both mycotoxins significantly decreased serum glucose concentration by 44% (p < 0.05) as compared to the negative control group. AFB1 treatment alone did not alter serum cholesterol level, but treatment with FB1 increased cholesterol level by 69% (p < 0.05), and sequential treatment with both mycotoxins increased cholesterol level by 75% (p < 0.05) as compared to the negative control group.
3.2.3. Histopathological alterations and formation of liver GST-P+ foci
Foci of altered hepatocytes were observed in animals treated with AFB1 only and AFB1+FB1 (Fig. 3B and 3F). Although treatment with FB1 only did not induce observable foci of altered hepatocytes, sequential AFB1+FB1 treatment induced almost twice as many foci as that induced by AFB1-only treatment. These foci were mainly consisted of basophilic (Fig. 3F). For comparison purpose, all different foci were generalized as foci of altered hepatocytes (Thoolen et al., 2010). A synergistic induction of apoptosis was found in the AFB1+FB1 group compared to the single mycotoxin treatment. Similarly, the amount of dysplasia was much higher in the sequential co-exposure group compared to the single toxin treatment groups (p < 0.05, Table 2). Proliferative nodules were only found in one rat of the AFB1+FB1 group. Mitoses were observed in both the FB1-only and AFB1+FB1 groups but not in the AFB1-only group, possibly due to recovery after withdrawal of the AFB1-only treatment. Necrosis was significantly lower in the AFB1-only treatment group (p < 0.05). Gross examination of rat liver did not show any other observable gross alterations in either the single mycotoxin or sequential co-exposure group.
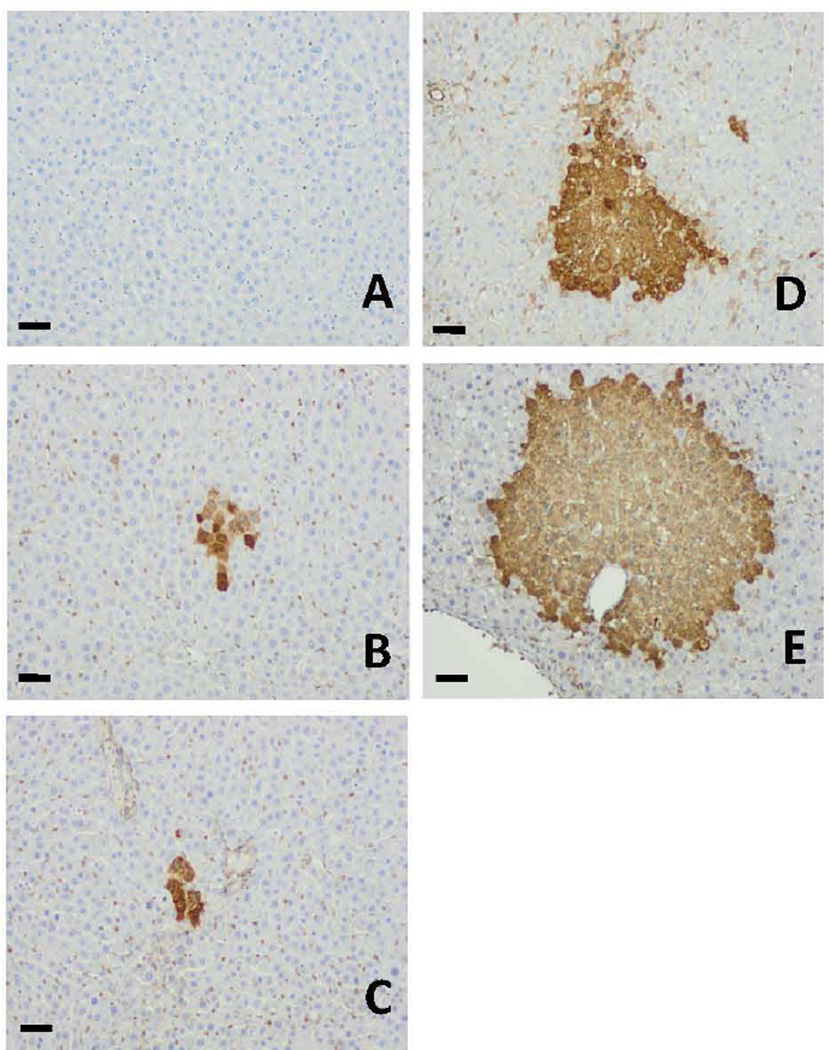
The gross appearance of liver GST-P+ foci induction by different treatment is shown in Fig. 3A-3C and the representative liver GST-P+ foci from different treatment groups are shown in Fig. 4. We also observed a correlation between foci of altered hepatocytes by H&E staining and liver GST-P+ foci as shown in Fig. 3F and 3G (AFB1+FB1 group) and supplementary Fig. S1 (Positive control group). Treatment with AFB1-only or FB1-only slightly induced formation of liver GST-P+ foci, however, the sequential co-treatment with AFB1+FB1 significantly increased the numbers of liver GTP-P+ foci by approximately 7.3- and 12.9-fold and the mean sizes of GST-P+ foci by 6- and 7.5-fold, respectively, as compared to AFB1-only and FB1-only treatment alone groups (p < 0.05). The ratio of foci/section was significantly increased by approximately 50-fold by the sequential treatment as compared to either single mycotoxin treatment alone (p < 0.05). These quantitative data clearly demonstrated the synergistic effects of sequential exposure to both AFB1 and FB1 in inducing liver GST-P+ foci formation (Table 3).
Fig. 4.
Representative photomicrograph images of liver GST-P+ foci induced by different treatment protocols. (A) the negative control, (B) AFB1-only, (C) FB1-only, (D) AFB1+FB1, and (E) the positive control groups. Bar represents 50 µm.
4. Discussion
Human data documenting co-exposure to AFB1 and FB1 are increasing (IARC, 2016). Development of animal models that can be used to evaluate the potential adverse consequences from co-exposure will be useful for understanding the potential of these two carcinogenic mycotoxins as contributing factors in human disease and for designing and conducting translational research for evaluating the efficacy of intervention strategies to reduce risk.
To reveal the interaction of AFB1 and FB1, the sequential treatment protocol was adopted in this study. Both AFB1-only and FB1-only caused a significant reduction in feed conversion but only FB1 and AFB1+FB1 treatment resulted in reduced weight gain (Fig. 2), which is consistent with a 90-day feeding assay in male F344 rats (Theumer et al., 2008). The data suggest that the mechanism of FB1-induced growth inhibition is likely to be different from the mechanism by which AFB1 causes reduced feed conversion, due to different serum biochemistry profiles observed among the positive control group (DEN+2-AAF treated) and single mycotoxin or sequential mycotoxin co-treatment groups (Table 1 and supplementary Table 1). Damage to hepatocytes was evident in the positive control group as the levels of total protein, glucose, and cholesterol were significantly decreased. The AFB1-only treatment did not significantly alter serum concentrations of ALT, AST, and ALP on day 56. However, when FB1 was involved in the sequential protocol, an overall 2- to 4-fold change in these enzymes was found as compared to the FB1 -only treatment, suggesting that AFB1 has sensitized the liver to FB1-induced cytotoxic effects.
Mild histopathological alterations were induced by AFB1- or FB1-only treatment, including increased dysplasia, apoptosis, and mitosis. In contrast, the sequential treatment showed a more than 7-fold increase of apoptosis compared to AFB1- or FB1-only treatment and additive effects in inducing dysplasia, mitosis, and foci of altered hepatocytes. These findings were consistent with previous reports (Gelderblom et al., 2001c; Gelderblom et al., 2002).
Under the current experimental condition only the sequential treatment produced proliferative nodules in one out of 13 animals (Table 2), which is likely due to low dose treatment, a short period of exposure, and a lack of stimuli for regenerative proliferation. The cancer initiation potential of FB1 in rat liver was thought to be related to the hepatotoxic effect and an increase in lipid peroxidation. The initiation potential could also be enhanced by regenerative proliferation in response to hepatotoxicity (Gelderblom et al., 2001b; Gelderblom et al., 2008). However, the hepatoxic effect in the male F344 is confounded by the fact that the primary carcinogenic target of FB1 is the kidney, whereas, in female B63F1 mice (IARC, 2002; NTP, 2002) and male BD9 rats the liver is the primary target (IARC, 2002). In rat kidney and mouse liver the mechanism of action clearly involves the elevation in free sphingoid bases, sphingoid base 1-phosphates, decreased ceramide biosynthesis (Riley and Voss, 2006) and the resulting global disruption of lipid metabolism (Bolger, 2001; Bulder, 2012; Gelderblom and Marasas, 2012; IARC, 2002). The balance between the levels of the sphingolipid molecules elevated and/or depleted in liver, kidney, blood, and other tissues will determine which sphingolipid-dependent signaling pathways are affected: increased cell death or survival (Torres et al., 2015). The potential mechanisms proposed for FB1 cancer induction include “the possible role of oxidative damage during initiation and the disruption of lipid metabolism, integrity of cellular membranes, and altered growth-regulatory responses as important events during promotion” (Gelderblom et al., 2001a; Gelderblom and Marasas, 2012; Riedel et al., 2015).
GST-P+ preneoplastic hepatic foci have been recommended as a useful alternative endpoint in place of tumors for studying hepatocellular carcinogenesis (Tsuda et al., 2003). In our study the number and mean size of GST-P+ foci were higher in the AFB1-only group than that of FB1-only treated group (Table 3). The discrepancy of foci of altered hepatocytes observed between H&E and GST-P+ staining for FB1-only treatment group could be explained by the fact that a focus determined by H&E apparently requires more altered cells than that of a GST-P+ focus. In this study the sequential treatment markedly and significantly increased the number and size of GST-P+ foci by approximately 7-fold and 12-fold as compared to the AFB1- or FB1- only treatment groups, respectively (Table 3 and Fig 4), which clearly indicates a synergistic effect on preneoplastic foci induction. Early studies showed that regenerative hepatocyte proliferation is possibly required for “fixing” the mutation and driving the clonal expansion of already initiated cells to a focal/nodular stage (Columbano et al., 1981), which can be induced by PH or carbon tetrachloride (CCl4) at a necrogenic dose (Columbano et al., 1987; Columbano et al., 1991). AFB1 and FB1 showed inhibitory effects on cell proliferation and enhancing effects on apoptotic cell death, and these effect are thought to account for the slow/weak cancer initiating potential of these two mycotoxins (Gelderblom et al., 1996; Neal and Cabral, 1980). It is also noticeable that the number and size of GST-P+ foci were smaller in the current study compared to those reported by Gelderblom et al. (2002), possibly due to different route of exposure employed
The mechanisms underlying the interaction between AFB1 and FB1 are complex and not well understood. AFB1 induced toxicity to liver may predispose a portion of hepatocytes to DNA damage and make them more susceptible to FB1-induced promotion, which is thought to be associated with several factors including the disruption of lipid-dependent signaling pathways controlling the balance between cell survival and death (Bolger, 2001; Bulder, 2012; IARC, 2002), hepatotoxicity, and an increase in lipid peroxidation (Gelderblom et al., 2001b). These effects may act together to disturb the genomic stability and increase the error of DNA replication, which could favor the clonal expansion of AFB1 initiated hepatocytes. Another possible explanation for the synergy relates to cell proliferation. Cell proliferation may act at each stage of the carcinogenesis process and is potentially important in converting DNA adducts to mutation and enhancing the mutation frequency by increasing the number of DNA replication errors (Tomatis, 1993). FB1-induced DNA and histone modifications are also possible (Gardner, 2016). Several lines of evidence support the hypothesis that a cytotoxic/proliferative threshold exists for cancer initiation by FB1 in rat liver and levels that fail to induce a toxic effect lack cancer initiating activity (Gelderblom et al., 1994; Gelderblom et al., 2001c). In this case, the regenerative cell proliferation) has been suggested to be responsible for the cancer induction by FB1 (Abel and Gelderblom, 1998). Taken together, FB1 induced regenerative cell proliferation may promote the initiating events driven by AFB1.
In summary, we have shown in this study the synergistic preneoplastic effects of sequential dietary exposure to AFB1 and FB1 in an 8-week assay. The sequential feeding of rats with diets amended with AFB1 and FB1, in the absence of the additional stress of partial hepatectomy, demonstrated conclusively an interactive effect on liver GST-P+ foci induction. This model provides a useful assay for assessment of the efficacy of potential intervention strategies that aim to reduce the possible adverse effects from dietary co-exposure to AFB1 and FB1 in animals and humans.
Supplementary Material
Highlights.
A modified and less invasive treatment procedure was used to validate the co-carcinogenic effects of AFB1 and FB1.
Serum biochemistry, histopathological alterations, and liver GST-P+ foci formation were monitored.
Sequential exposure increased dysplasia, apoptosis and foci of altered hepatocytes as compared to either toxin alone.
Sequential exposure increased liver GTP-P+ foci number by 7.3- and 12.9-fold as compared to AFB1 or FB1 only treatment.
Synergistic effect of AFB1 and FB1 in the rat model holds promise for future dietary intervention studies.
The co-carcinogenic rat model holds promise for future dietary intervention studies.
Acknowledgments
This study was supported by the research grant (1R01MD005819-01) from the National Institute on Minority Health and Health Disparity (NIMHD) and the research contract (ECG-A00-0700001-00) from the United States Agency for International Development (USAID) via Peanut Collaborative Research Support Program (Peanut CRSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- Abel S, Gelderblom WC. Oxidative damage and fumonisin B1-induced toxicity in primary rat hepatocytes and rat liver in vivo. Toxicology. 1998;131:121–131. doi: 10.1016/s0300-483x(98)00123-1. [DOI] [PubMed] [Google Scholar]
- Aguilar F, Hussain SP, Cerutti P. Aflatoxin B1 induces the transversion of G-->T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc Natl Acad Sci U S A. 1993;90:8586–8590. doi: 10.1073/pnas.90.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I, Shubik P. A new, quantitative, approach to the study of the stages of chemical cartinogenesis in the mouse’s skin. Br J Cancer. 1947a;1:383–391. doi: 10.1038/bjc.1947.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I, Shubik P. The role of croton oil applications, associated with a single painting of a carcinogen, in tumour induction of the mouse’s skin. Br J Cancer. 1947b;1:379–382. doi: 10.1038/bjc.1947.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger MCRD, Dinovi M, Gaylor D, Gelderblom MO, Paster N, Riley RT, Shephard G, Speijers JA. Fumonisins. Safety evaluation of certain mycotoxins in food. Food and Agriculture Organization of the United Nations, paper 74. World Health Organization Food Additives, Series 47. 2001:103–279. [Google Scholar]
- Bulder AS, Arcella D, Bolger M, Carrington C, Kpodo K, Resnik S, Riley RT, Wolterink G, Wu F. Fumonisins. Safety evaluation of certain food additives and contaminants. Seventy-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECTA). WHO Food Additives Series No. 65. 2012:325–527. [Google Scholar]
- Carlson DB, Williams DE, Spitsbergen JM, Ross PF, Bacon CW, Meredith FI, Riley RT. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N’-nitro-nitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol Appl Pharmacol. 2001;172:29–36. doi: 10.1006/taap.2001.9129. [DOI] [PubMed] [Google Scholar]
- Casado JM, Theumer M, Masih DT, Chulze S, Rubinstein HR. Experimental subchronic mycotoxicoses in mice: individual and combined effects of dietary exposure to fumonisins and aflatoxin B1. Food Chem Toxicol. 2001;39:579–586. doi: 10.1016/s0278-6915(00)00174-5. [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Coni P, Pani P. Failure of mitogen-induced cell proliferation to achieve initiation of rat liver carcinogenesis. Carcinogenesis. 1987;8:345–347. doi: 10.1093/carcin/8.2.345. [DOI] [PubMed] [Google Scholar]
- Columbano A, Ledda-Columbano GM, Coni P, Pichiri-Coni G, Curto M, Pani P. Chemically induced cell proliferation and carcinogenesis: differential effect of compensatory cell proliferation and mitogen-induced direct hyperplasia on hepatocarcinogenesis in the rat. Prog Clin Biol Res. 1991;369:217–225. [PubMed] [Google Scholar]
- Columbano A, Rajalakshmi S, Sarma DS. Requirement of cell proliferation for the initiation of liver carcinogenesis as assayed by three different procedures. Cancer Res. 1981;41:2079–2083. [PubMed] [Google Scholar]
- Espandiari P, Robertson LW, Srinivasan C, Glauert HP. Comparison of different initiation protocols in the resistant hepatocyte model. Toxicology. 2005;206:373–381. doi: 10.1016/j.tox.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Gardner NM, Riley RT, Showker JL, Voss KA, Sachs AJ, Maddox JR, Gelineau-van Waes JB. Elevated sphingoid base-1-phosphates and decreased histone deacetylase activity after fumonisin B1 treatment in mouse embryonic fibroblasts. Toxicol Appl Pharmacol. 2016 doi: 10.1016/j.taap.2016.02.018. In press. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Abel S, Smuts CM, Marnewick J, Marasas WF, Lemmer ER, Ramljak D. Fumonisin-induced hepatocarcinogenesis: mechanisms related to cancer initiation and promotion. Environ Health Perspect. 2001a;109(Suppl 2):291–300. doi: 10.1289/ehp.01109s2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom WC, Cawood ME, Snyman SD, Marasas WF. Fumonisin B1 dosimetry in relation to cancer initiation in rat liver. Carcinogenesis. 1994;15:209–214. doi: 10.1093/carcin/15.2.209. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Galendo D, Abel S, Swanevelder S, Marasas WF, Wild CP. Cancer initiation by fumonisin B(1) in rat liver--role of cell proliferation. Cancer Lett. 2001b;169:127–137. doi: 10.1016/s0304-3835(01)00542-0. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Jaskiewicz K, Marasas WF, Thiel PG, Horak RM, Vleggaar R, Kriek NP. Fumonisins--novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol. 1988;54:1806–1811. doi: 10.1128/aem.54.7.1806-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom WC, Lebepe-Mazur S, Snijman PW, Abel S, Swanevelder S, Kriek NP, Marasas WF. Toxicological effects in rats chronically fed low dietary levels of fumonisin B(1) Toxicology. 2001c;161:39–51. doi: 10.1016/s0300-483x(00)00459-5. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Marasas WF. Controversies in fumonisin mycotoxicology and risk assessment. Hum Exp Toxicol. 2012;31:215–235. doi: 10.1177/0960327110395338. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Marasas WF, Lebepe-Mazur S, Swanevelder S, Abel S. Cancer initiating properties of fumonisin B1 in a short-term rat liver carcinogenesis assay. Toxicology. 2008;250:89–95. doi: 10.1016/j.tox.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Marasas WF, Lebepe-Mazur S, Swanevelder S, Vessey CJ, Hall Pde L. Interaction of fumonisin B(1) and aflatoxin B(1) in a short-term carcinogenesis model in rat liver. Toxicology. 2002;171:161–173. doi: 10.1016/s0300-483x(01)00573-x. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Snyman SD, Abel S, Lebepe-Mazur S, Smuts CM, Van der Westhuizen L, Marasas WF, Victor TC, Knasmuller S, Huber W. Hepatotoxicity and -carcinogenicity of the fumonisins in rats. A review regarding mechanistic implications for establishing risk in humans. Adv Exp Med Biol. 1996;392:279–296. [PubMed] [Google Scholar]
- IARC. Working Group on the Evaluation of Carcinogenic Risk to Humans. Vol. 82. Lyon, France: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene IARC; 2002. pp. 301–366. [PMC free article] [PubMed] [Google Scholar]
- IARC. Working Group Report. In: Wild CP, Miller JD, Groopman JD, editors. Mycrotoxin control measuresin low and middle income countries. IARC Working Group Report No. 9. 2016. [Google Scholar]
- Kang MS, Nkurunziza P, Muwanika R, Qian G, Tang L, Song X, Xue K, Nkwata A, Ssempebwa J, Lutalo T, Asiki G, Serwadda D, Seeley J, Kaleebu P, Nalugoda F, Newton R, William JH, Wang JS. Longitudinal evaluation of aflatoxin exposure in two cohorts in south-western Uganda. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32:1322–1330. doi: 10.1080/19440049.2015.1048749. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci. 2011;120(Suppl 1):S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41:740–755. doi: 10.3109/10408444.2011.575766. [DOI] [PubMed] [Google Scholar]
- Kimanya ME, De Meulenaer B, Roberfroid D, Lachat C, Kolsteren P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol Nutr Food Res. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- Marroquin-Cardona AG, Johnson NM, Phillips TD, Hayes AW. Mycotoxins in a changing global environment-A review. Food Chem Toxicol. 2014;69:220–230. doi: 10.1016/j.fct.2014.04.025. [DOI] [PubMed] [Google Scholar]
- McKean C, Tang L, Tang M, Billam M, Wang Z, Theodorakis CW, Kendall RJ, Wang JS. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem Toxicol. 2006;44:868–876. doi: 10.1016/j.fct.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Sullards MC, Wang E, Voss KA, Riley RT. Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environ Health Perspect. 2001;109(Suppl 2):283–289. doi: 10.1289/ehp.01109s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal GE, Cabral JR. Effect of partial hepatectomy on the response of rat liver to aflatoxin B1. Cancer Res. 1980;40:4739–4743. [PubMed] [Google Scholar]
- NTP. Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 82. Lyon, France: International Agency for Research on Cancer; 2002. Aflatoxins; pp. 171–136. [PMC free article] [PubMed] [Google Scholar]
- Orsi RB, Oliveira CA, Dilkin P, Xavier JG, Direito GM, Correa B. Effects of oral administration of aflatoxin B1 and fumonisin B1 in rabbits (Oryctolagus cuniculus) Chem Biol Interact. 2007;170:201–208. doi: 10.1016/j.cbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Qian G, Wang F, Tang L, Massey ME, Mitchell NJ, Su J, Williams JH, Phillips TD, Wang JS. Integrative toxicopathological evaluation of aflatoxin B(1) exposure in F344 rats. Toxicol Pathol. 2013;41:1093–1105. doi: 10.1177/0192623313477256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel S, Abel S, Swanevelder S, Gelderblom WC. Induction of an altered lipid phenotype by two cancer promoting treatments in rat liver. Food Chem Toxicol. 2015;78:96–104. doi: 10.1016/j.fct.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Riley RT, Enongene E, Voss KA, Norred WP, Meredith FI, Sharma RP, Spitsbergen J, Williams DE, Carlson DB, Merrill AH., Jr Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ Health Perspect. 2001;109(Suppl 2):301–308. doi: 10.1289/ehp.01109s2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RT, Voss KA. Differential sensitivity of rat kidney and liver to fumonisin toxicity: organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicol Sci. 2006;92:335–345. doi: 10.1093/toxsci/kfj198. [DOI] [PubMed] [Google Scholar]
- Shirima CP, Kimanya ME, Routledge MN, Srey C, Kinabo JL, Humpf HU, Wild CP, Tu YK, Gong YY. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ Health Perspect. 2015;123:173–178. doi: 10.1289/ehp.1408097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt DB, Medline A, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977;88:595–618. [PMC free article] [PubMed] [Google Scholar]
- Theumer MG, Lopez AG, Aoki MP, Canepa MC, Rubinstein HR. Subchronic mycotoxicoses in rats. Histopathological changes and modulation of the sphinganine to sphingosine (Sa/So) ratio imbalance induced by Fusarium verticillioides culture material, due to the coexistence of aflatoxin B1 in the diet. Food Chem Toxicol. 2008;46:967–977. doi: 10.1016/j.fct.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Kuttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38:5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- Tomatis L. Cell proliferation and carcinogenesis: a brief history and current view based on an IARC workshop report. International Agency for Research on Cancer. Environ Health Perspect. 1993;101(Suppl 5):149–151. doi: 10.1289/ehp.93101s5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres O, Matute J, Gelineau-van Waes J, Maddox JR, Gregory SG, Ashley-Koch AE, Showker JL, Voss KA, Riley RT. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin Journal. 2015;8:143–159. [Google Scholar]
- Torres O, Matute J, Gelineau-van Waes J, Maddox JR, Gregory SG, Ashley-Koch AE, Showker JL, Zitomer NC, Voss KA, Riley RT. Urinary fumonisin B1 and estimated fumonisin intake in women from high- and low-exposure communities in Guatemala. Mol Nutr Food Res. 2014;58:973–983. doi: 10.1002/mnfr.201300481. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Fukushima S, Wanibuchi H, Morimura K, Nakae D, Imaida K, Tatematsu M, Hirose M, Wakabayashi K, Moore MA. Value of GST-P positive preneoplastic hepatic foci in dose-response studies of hepatocarcinogenesis: evidence for practical thresholds with both genotoxic and nongenotoxic carcinogens. A review of recent work. Toxicol Pathol. 2003;31:80–86. doi: 10.1080/01926230390173879. [DOI] [PubMed] [Google Scholar]
- Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- Wu F, Groopman JD, Pestka JJ. Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol. 2014;5:351–372. doi: 10.1146/annurev-food-030713-092431. [DOI] [PubMed] [Google Scholar]
- Wu HC, Wang Q, Yang HI, Tsai WY, Chen CJ, Santella RM. Global DNA methylation in a population with aflatoxin B1 exposure. Epigenetics. 2013;8:962–969. doi: 10.4161/epi.25696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying TS, Sarma DS, Farber E. The sequential analysis of liver cell necrosis: inhibition of diethylnitrosamine- and dimethylnitrosamine-induced acute liver cell death by posttreatment with diethyldithiocarbamate. Am J Pathol. 1980;99:159–174. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.