Abstract
Objective
To describe trends in the prevalence of diabetes among hospitalized HIV-infected patients between 1997 and 2012 in Spain and compare them with those of age- and sex-matched non–HIV-infected patients.
Methods
The study was based on Spanish national hospital discharge data. We performed a retrospective study for the period 1997–2012. HIV infection (HIV-infected versus non–HIV-infected [control group])and calendar period in relation to widespread use of combination antiretroviral therapy (cART) (1997–1999; 2000–2003; 2004–2007 and 2008–2012), were the exposure variables The outcome variables were diagnosis of diabetes and in-hospital mortality (IHM).
Results
From 1997 to 2012, we identified 91,752 cases of diabetes: 15,398 in the HIV-infected group (403,277 hospital admissions) and 76,354 in the non–HIV-infected group (1,503,467 hospital admissions). Overall, HIV-infected patients had lower prevalence values for diabetes than non–HIV-infected patients throughout the follow-up (3.8% vs. 5.1%; p<0.001). The prevalence of diabetes increased 1.56-fold among non–HIV-infected patients and 4.2-fold among HIV-infected patients. The prevalence of diabetes in females was almost twice as high in HIV-infected patients as in non–HIV-infected patients during the last study period (4.72% vs. 2.88%; p<0.001). Diabetes showed a protective effect against IHM throughout the study period (aOR = 0.70; 95%CI, 0.65–0.75).
Conclusions
During the cART era, the prevalence of diabetes has increased sharply among HIV-infected hospitalized patients compared with matched non–HIV-infected subjects. The prevalence of diabetes is rising very fast among HIV-infected women. Diabetes has a protective effect on IHM among HIV-infected patients. Nevertheless, our study has several limitations. No information is available in the database used on important sociodemographic characteristics and relevant clinical variables including duration of the HIV infection, treatments used, drug resistance, treatment adherence or CD4 count, among others. Also, it is possible that increase of diabetes prevalence could reflect the improvement in recording habits.
Introduction
HIV treatment has improved substantially since the introduction of combination antiretroviral therapy (cART). However, the subsequent improvement in life expectancy has been characterized by an aging HIV-infected population who are increasingly affected by age-related non-communicable diseases [1,2]. Associated comorbidities frequently include metabolic complications that increase the risk of diabetes mellitus. The prevalence of diabetes among HIV-infected patients has been reported to be between 2% and 14% [3–5] and is expected to continue to increase in aging HIV-infected patients.
HIV-infected patients may be at increased risk of developing diabetes as a result of viral coinfection and adverse effects of treatment [6,7]. Previous studies have reported a wide spectrum of metabolic alterations associated with cART, including changes in glucose homeostasis and fat redistribution [8,9]. Protease inhibitors and nucleotide reverse transcriptase inhibitors (NRTIs) have been associated with diabetes [5,10]. Given that the insulin resistance and impaired glucose tolerance induced by cART might act as a precursor of diabetes, the risk of diabetes could have increased in the cART era.
Several studies have reported higher prevalence and/or incidence rates for diabetes in the HIV-infected population than in the general population [11–14], whereas others report similar [6] or even lower [15] rates. After a follow-up of 5.2 years, the DAD study revealed a crude incidence of new diabetes of 4.2 cases per 1000 person-years, which is similar to that described in the Swiss cohort (4.6 cases per 1000 person-years) [16,17]. In Spain, Araujo et al [18] reported that the rate of incident diabetes was 2.85 cases per 100 person-years in HIV-infected patients.
To our knowledge, no authors have investigated national trends in the prevalence of diabetes in hospitalized HIV-infected patients or the effect of diabetes on mortality in HIV-infected patients. In the present study, we used national hospital discharge data to describe trends in the prevalence of diabetes among hospitalized HIV-infected patients between 1997 and 2012 in Spain. We compared HIV-infected patients with age- and sex-matched non–HIV-infected patients. We analyzed in-hospital outcomes such as in-hospital mortality (IHM) in patients with and without diabetes and studied the effect of diabetes on mortality among these patients.
Methods
We performed a retrospective, observational study using the Spanish National Hospital Database (CMBD, Conjunto Minimo Básico de Datos, Ministry of Health, Social Services and Equality) which contains data on more than 95% of discharges from public and private hospitals [19], of all consecutive HIV-infected patients aged over 15 years from January 1, 1997 to December 31, 2012.
We subdivided the study period into four calendar periods according to the growing use of cART by HIV-infected patients in Spain [20], as follows: 1997–1999; 2000–2003; 2004–2007; 2008–2012.
A control group of non–HIV-infected patients (selective cohort) admitted to Spanish hospitals was selected in a ratio 4:1 with respect to HIV-infected patients. This approach is useful when the numbers of events is limited and increase the accuracy and power of the statistical test [21–23]. The control group was randomly selected from among HIV-negative patients aged ≥15 years. Patients were matched for gender and age, according to frequency [24], quartiles of age of HIV-infected patients, and assuming an approximate percentage of men, to avoid confounding factors. If a HIV patient was admitted more than once in the study period’s we would only use the data for the first hospital admission. To do this, even if data is anonymous, we could identify the same subject using an algorism including date of birth, sex and province of residence.
We classified diseases and procedures according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [25]. We selected all patients hospitalized with a diagnosis of HIV infection (codes 042 or V08). We classified discharges according to HIV status as randomly selected non-HIV-infected patients and HIV-infected patients.
The primary outcome was presence of diabetes, which was defined as a hospital discharge with a diagnosis of diabetes (any field) (ICD-9-CM codes: 250.XX). The secondary outcomes were length of hospital stay (LOHS) and IHM.
Comorbidity was assessed using the Charlson Comorbidity Index (CCI) excluding diabetes and HIV infection [26]. The prevalence of the diseases included in the CCI in the study groups was analyzed. Finally, information on tobacco use (ICD-9-CM codes 305.1 and V158.2), alcohol abuse (ICD-9-CM codes 305.0, 303.0, 303.9, 291.0, 291.1, 291.2, 291.3, 291.4, 291.5, 291.8, 291.9, 571.0, 571.1, 571.2, 571.3, 425.5, 535.3, 357.5, 265.2, V11.3, 790.3 and 980.0) and other disorders such as obesity (ICD-9-CM 278.00, 278.01, 278.02, 278.03, 278.0, 278.1 and 278.8) and high blood pressure (HBP) (ICD-9-CM 401.XX) were analyzed. According to the CMBD methodology the physician who is discharging the patient fulfills the discharge report only including those diagnosis that, in his opinion, have affected the hospital admission, the duration and outcome.
Continuous variables are presented as means and 95% confidence intervals and for categorical variables as frequencies and percentages. We matched HIV-infected patients and non–HIV-infected patients by frequency, thus enabling statistical tests to be used for independent groups.
We used chi-square or Fisher exact test to analyze categorical data and proportions. We used t test or Mann-Whitney test to compare continuous variables. Prevalence was compared using a Poisson distribution. Temporal trends in the prevalence of diabetes were evaluated using a Poisson distribution. We also calculated the odds for prevalence and IHM in patients diagnosed with diabetes according to HIV status using logistic regression models, which were adjusted for age, sex, CCI, obesity and HBP.
Finally, to assess the effect of diabetes on IHM among HIV-infected patients, we used a logistic regression model adjusted for these same variables for each calendar period and for the entire study period. Statistical analyses were performed using package R (version 3.1.2) [27]. Statistical significance was set at p<0.05 (2-tailed).
Data confidentiality was maintained at all times in accordance with Spanish legislation. Patient identifiers were deleted by the Spanish Ministry of Health before the database was provided to the authors in order to maintain patient anonymity. It is not possible to identify patients individually, either in this article or in the database. Since the dataset was anonymous and mandatory, informed consent was unnecessary. The Spanish Ministry of Health considered that our study protocol fulfilled all ethical requirements according to Spanish legislation and provided us with the database. Given the nature of the investigation and according to the Spanish Legislation it is NOT needed the approval of an Ethics Committee S1 Document. Anonymized data was used and authors were NOT involved with the patients' medical treatment nor had any interaction with the participants. None of the authors were affiliated with the hospitals/clinics where patients were treated.
Results
During the study period, there were 403,277 hospital admissions of HIV-infected patients and 1,503,467 hospital admissions of non–HIV-infected patients. We identified a total of 15,398 hospital admissions of patients with a diagnosis of diabetes in the HIV-infected group from 1997 to 2012 and 76,354 hospital admissions with a diagnosis of diabetes in the non–HIV-infected group.
Table 1 shows the clinical characteristics, risk factors, and outcomes for all hospital admissions due to diabetes according to HIV status during the study period.
Table 1. Clinical characteristics, risk factors and outcomes of patients admitted with diabetes stratified by HIV status in Spain, 1997–2012.
| HIV-uninfected patients N = 1,503,467 | HIV-infected patients N = 403,277 | p-value* | |
|---|---|---|---|
| No. of patients with diabetes, N(%) | 76,354 (5.07) | 15,398 (3.81) | <0.001 |
| Males, N (%) | 65,321 (85.6) | 12,467 (81) | <0.001 |
| Age (years), Mean (95%CI) | 49.05 (48.97–49.14) | 50.45 (50.26–50.64) | <0.001 |
| Charlson Comorbidity Index, Mean (95%CI) | 1.09 (1.07–1.1) | 1.59 (1.57–1.62) | <0.001 |
| Obesity N (%) | 9,771 (12.8) | 668 (4.3) | <0.001 |
| High Blood Pressure, N (%) | 25,262 (33.1) | 3,294 (21.4) | <0.001 |
| Alcohol, N (%) | 4,233 (5.5) | 878 (5.7) | 0.447 |
| Tobacco, N (%) | 17,173 (22.5) | 3,452 (22.4) | 0.852 |
| Length of stay (days), Mean (95%CI) | 9.1 (9.01–9.19) | 11.18 (10.94–11.42) | <0.001 |
| In-hospital mortality, N (%) | 2,019 (2.6) | 946 (6.1) | <0.001 |
* Chi-square or Fisher exact test were used to compare categorical data and proportions and t test or Mann-Whitney test to compare continuous variables between HIV-uninfected and infected patients.
Mean age was slightly but significantly higher among HIV-infected than non–HIV-infected patients with diabetes (50.5 years vs. 49.1 years). Males accounted for 85.6% of the non–HIV-infected patients and 81% of the HIV-infected patients (p<0.01).
In comparison with the non–HIV-infected group, the HIV-infected patients had higher IHM, longer LOHS and higher CCI values, whereas non-infected patients had a higher prevalence of HBP and obesity.
Table 2 shows the clinical and epidemiological trends of diabetes in Spain by time period and according to HIV status. Overall, the prevalence of diabetes was lower in the HIV-infected group than in the non–HIV-infected group (3.8% vs. 5.1%; p<0.001). However, the prevalence of diabetes increased sharply among HIV-infected patients, from 1.5% in 1997–1999 to 6.3% in 2008–2012 (p<0.001), and was slightly higher in the HIV-infected group than in the non–HIV-infected group during 2008–2012 (6.3% vs. 6.0%; p<0.001). The prevalence of diabetes rose 1.56 times among non–HIV-infected patients and 4.2 times among HIV-infected patients.
Table 2. Clinical and epidemiological trends of hospitalization with diabetes according to HIV status and calendar period in Spain, 1997–2012.
| Calendar Periods | Time trend | |||||
|---|---|---|---|---|---|---|
| 1997–1999 | 2000–2003 | 2004–2007 | 2008–2012 | p-value | ||
| Total number of admissions | HIV-uninfected | 254,919 | 368,063 | 389,572 | 490,913 | |
| HIV-infected | 75,513 | 104,559 | 105,016 | 118,189 | ||
| Diabetes diagnosis N (Prevalence) | HIV-uninfected | 9789 (3.84) | 16600 (4.51) | 20475 (5.26) | 29490 (6.01) | <0.001 |
| HIV-infected | 1134 (1.5)* | 2671 (2.55)* | 4121 (3.92)* | 7472 (6.32)* | <0.001 | |
| Age Mean (95%CI) | HIV-uninfected | 48.2 (47.9–48.4) | 48.7 (48.5–48.9) | 49 (48.9–49.2) | 49.6 (49.4–49.7) | <0.001 |
| HIV-infected | 45.1 (44.4–45.8)* | 47.5 (47.1–48)* | 49.4 (49.1–49.8) | 52.9 (52.6–53.1)* | <0.001 | |
| Diabetic male N (Prevalence) | HIV-uninfected | 8282 (4.51) | 14109 (5.36) | 17625 (6.43) | 25305 (7.32) | <0.001 |
| HIV-infected | 933 (1.66)* | 2238 (2.92)* | 3396 (4.45)* | 5900 (6.95)* | <0.001 | |
| Diabetic female N (Prevalence) | HIV-uninfected | 1507 (2.12) | 2491 (2.37) | 2850 (2.47) | 4185 (2.88) | <0.001 |
| HIV-infected | 201 (1.04)* | 433 (1.56)* | 725 (2.53) | 1572 (4.72)* | <0.001 | |
| Obesity N (Prevalence) | HIV-uninfected | 668 (16.36) | 1650 (18.78) | 2637 (22.75) | 4816 (23.01) | <0.001 |
| HIV-infected | 21 (17.8) | 62 (18.67) | 161 (22.93) | 424 (26.43)* | <0.001 | |
| High Blood Pressure N (Prevalence) | HIV-uninfected | 2151 (15.9) | 4727 (18.87) | 7035 (21.93) | 11349 (23.98) | <0.001 |
| HIV-infected | 84 (15.11) | 386 (20.72) | 832 (21.85) | 1992 (25.03)* | <0.001 | |
| Alcohol N (Prevalence) | HIV-uninfected | 519 (8.03) | 917 (10.75) | 1197 (13.28) | 1600 (14.98) | <0.001 |
| HIV-infected | 38 (2.65)* | 122 (3.96)* | 251 (5.79)* | 467 (7.94)* | <0.001 | |
| Tobacco N (Prevalence) | HIV-uninfected | 1428 (6.2) | 3255 (7.12) | 5010 (8.96) | 7480 (9.8) | <0.001 |
| HIV-infected | 115 (1.3)* | 476 (2.34)* | 915 (3.06)* | 1946 (4.94)* | <0.001 | |
| In-hospital mortality N (%) | HIV-uninfected | 269 (2.75) | 449 (2.7) | 563 (2.75) | 738 (2.5) | 0.122 |
| HIV-infected | 77 (6.79)* | 179 (6.7)* | 253 (6.14)* | 437 (5.85)* | 0.072 | |
| Length of stay Mean (95%CI) | HIV-uninfected | 10.2 (10; 10.5) | 9.6 (9.4; 9.8) | 9.1 (9; 9.3) | 8.4 (8.3; 8.6) | <0.001 |
| HIV-infected | 13 (12.2–13.9)* | 11.8 (11.3–12.3)* | 12.2 (11.6–12.8)* | 10.1 (9.8–10.4)* | <0.001 | |
| Charlson Comorbidity Index Mean (95%CI) | HIV-uninfected | 0.9 (0.9–0.9) | 1 (1–1.1) | 1.1 (1.1–1.1) | 1.2 (1.2–1.2) | <0.001 |
| HIV-infected | 0.9 (0.8–0.9) | 1.3 (1.2–1.3)* | 1.6 (1.6–1.7)* | 1.9 (1.9–1.9)* | <0.001 | |
* Chi-square or Fisher exact test were used to compare categorical data and proportions and t test or Mann-Whitney test to compare continuous variables between HIV-uninfected and infected patients within each time period and in the total period.
Time trend p-values for the prevalence of diabetes were estimated using a Poisson regression model.
Mean age rose significantly from 48.2 to 49.6 years in HIV-infected patients and from 45.1 to 52.9 years among non–HIV-infected patients.
In the HIV-infected group, the prevalence of diabetes increased in both sexes from 1.7% and 1.0% (1997–1999) to 6.9% and 4.7% (2008–2012) in males and females, respectively (p<0.001). The prevalence of diabetes in HIV-infected males was lower than in non–HIV-infected males throughout the study period (p<0.001). However, the prevalence of diabetes in females was almost two times higher in HIV-infected than in non–HIV-infected patients during the last study period (4.7% vs. 2.9%; p<0.001) (Table 2). The prevalence of diabetes was higher among men in all the periods studied, regardless of HIV status.
The prevalence of diabetes was higher in patients with obesity or HBP. In both groups, and for both conditions, the prevalence of diabetes increased over time. Furthermore, in the last period analyzed, the prevalence of diabetes was significantly higher in HIV-infected patients with obesity or HBP than in non–HIV-infected patients (26.4% vs. 23.0% for obesity and 25.0% vs 24% for HBP).
We found the prevalence of diabetes to be higher among non–HIV-infected patients who smoked or drank in all the years analyzed (Table 2).
In-hospital mortality was higher in the HIV-infected group for all four calendar periods: 6.8% vs. 2.8% in 1997–1999 (p<0.001), 6.7% vs. 2.7% in 2000–2003 (p<0.001), 6.1% vs. 2.8% in 2004–2007 (p<0.001) and 5.9% vs. 2.5% in 2008–2012 (p<0.001) (Table 2).
LOHS was also higher in the HIV-infected group in 1997–1999 and 2008–2012; LOHS decreased in both groups from 1997 to 2012 (p<0.001) (Table 2).
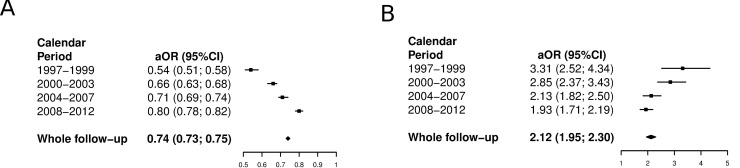
Throughout the study period, the adjusted prevalence for diabetes was 0.74 times lower in the HIV-infected group (Fig 1A). By time period, the adjusted OR (aOR) for prevalence among the HIV-infected group increased when compared with the non–HIV-infected group (aOR = 0.54 in 1997–1999, aOR = 0.66 in 2000–2003, aOR = 0.71 in 2004–2007 and aOR = 0.80 in 2008–2012), suggesting that the adjusted prevalence of diabetes among HIV-infected patients is becoming similar to that found in non–HIV-infected patients (Fig 1A).
Fig 1.
A. Adjusted likelihood of prevalence among HIV-infected subjects diagnosed with diabetes in Spain (1997–2012) stratified by HIV status. Fig 1B. Adjusted likelihood of death among HIV-infected subjects diagnosed with diabetes in Spain (1997–2012) stratified by HIV status. (A) Reference category HIV uninfected subjects. Model adjusted by age, sex, CCI, obesity and HBP. (B) Reference category HIV uninfected subjects. Model adjusted by age, sex, CCI, obesity and HBP.
The adjusted likelihood of IHM among patients with diabetes was 2.12 times higher in the HIV-infected group throughout the study period (Fig 1B). By time period, the likelihood of death was greater among HIV-infected patients in 1997–1999 (aOR = 3.31), 2000–2003 (aOR = 2.85), 2004–2007 (aOR = 2.13) and in 2008–2012 (aOR = 1.93) (Fig 1B). As with the prevalence of diabetes, the risk of death during hospitalization seems to be increasingly similar over time for both HIV-infected and non–HIV-infected patients.
Table 3 shows the factors associated with IHM among hospitalized HIV-infected patients in Spain from 1997 to 2012 for the total period and each of the calendar periods. Diabetes showed a protective effect for in-hospital mortality throughout the study period (aOR = 0.70; 95%CI, 0.65–0.75) and in each calendar period analyzed.
Table 3. Multivariable analysis of the factors associated with mortality among HIV-infected patients in Spain, 1997–2012.
| 1997–1999 N = 330,432 | 2000–2003 N = 472,622 | 2004–2007 N = 494,588 | 2008–2012 N = 609,102 | Whole follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| aOR (95%CI) | P-value | aOR (95%CI) | P-value | aOR (95%CI) | P-value | aOR (95%CI) | P-value | aOR (95%CI) | P-value | |
| Diabetes | 0.69 (0.54–0.87) | <0.001 | 0.66 (0.56–0.78) | <0.001 | 0.65 (0.55–0.75) | <0.001 | 0.76 (0.68–0.85) | <0.001 | 0.70 (0.65–0.75) | <0.001 |
| Age | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.02–1.03) | <0.001 |
| Charlson Comorbidity Index | 1.40 (1.36–1.44) | <0.001 | 1.49 (1.46–1.52) | <0.001 | 1.41 (1.39–1.44) | <0.001 | 1.32 (1.30–1.35) | <0.001 | 1.40 (1.39–1.41) | <0.001 |
| Sex (male) | 1.44 (1.39–1.55) | <0.001 | 1.24 (1.16–1.33) | <0.001 | 1.39 (1.30–1.48) | <0.001 | 1.24 (1.17–1.32) | <0.001 | 1.31 (1.27–1.36) | <0.001 |
| Length of stay | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Calendar Period | N/A | N/A | N/A | N/A | ||||||
| 1997–1999 | 1 | |||||||||
| 2000–2003 | 0.77 (0.74–0.81) | <0.001 | ||||||||
| 2004–2007 | 0.64 (0.61–0.66) | <0.001 | ||||||||
| 2008–2012 | 0.51 (0.49–0.54) | <0.001 | ||||||||
Older age, higher CCI, male sex, and longer hospital stay were significantly associated with a higher risk of IHM.
Finally, when the calendar period was entered into the model, we observed that the probability of dying during hospitalization decreased significantly by half (aOR = 0.51; 95%CI, 0.49–0.54) from 1997–1999 (reference) to 2008–2012.
Discussion
Based on the Spanish National Hospital Database, our results reveal that almost 4% of hospitalized HIV-infected patients have an associated diagnosis of diabetes and that the prevalence of diabetes increased significantly from 1997 to 2012. In Italy, Galli et al [28] reported that prevalence of diabetes in HIV-infected patients to be 4%, compared with 2.5% in non–HIV-infected patients.
We found that the prevalence of diabetes was lower for HIV-infected patients than for non–HIV-infected patients. In the Veterans Cohort Study in the US, Butt et al [29] concluded that the risk of diabetes at baseline was lower in the HIV-infected group than in the non–HIV-infected group (OR, 0.84; 95%CI, 0.72–0.97). The authors also indicated that the net risk of diabetes is determined by a complex interplay of individual factors, with the traditional risk factors (increasing age, minority race and obesity) dominating the profile and leading to an overall lower risk in HIV-infected persons. However our results reveal that, in the following years, the prevalence of diabetes increased sharply among HIV-infected patients, resulting in a higher prevalence of diabetes among HIV-infected patients in the fourth period (2008–2012). One possible explanation is that the use of cART was associated with a significantly higher risk of insulin resistance and diabetes in HIV-infected patients [6,7, 29]. In the Multicenter AIDS Cohort Study (MACS) performed in the US, the prevalence of diabetes adjusted for age and BMI among seropositive patients taking antiretroviral therapy was 4.6 times greater than among seronegative patients (14% in HIV-infected patients vs. 5% in non–HIV-infected patients) [13]. However, in their cohort of 1046 HIV-infected patients, Capeu et al [5] found a decreasing trend for the incidence of diabetes mellitus over time, from 23.2 cases/1000 person-years in 1999–2000 to 2.7 cases/1000 person-years in 2005–2006, and concluded that the decline in incidence over time may reflect changes in cART practices and the development of less toxic cART alternatives.
Our investigation reinforces the well-known association between obesity and HBP and diabetes in HIV-infected patients [17,28]. We found that the prevalence of diabetes was higher in patients with obesity or HBP and HIV infection. In a population-based cohort study in Denmark, Rasmussen et al. [30] found an increased risk of diabetes in HIV-infected individuals with increasing age (IRR, 4.88 [95%CI, 1.17–20.37] in patients aged 50–59 years; IRR, 10.21 [95%CI, 2.43–42.95] in patients aged >60 years) and BMI (IRR, 9.25 [95%CI 5.37–15.94] in obese patients) and indicated that traditional risk factors seem to be similar in both HIV-infected individuals and the background population. The authors suggested that HIV-infected patients be screened for diabetes in accordance with local general guidelines.
In our study, the prevalence of diabetes in women was almost two times higher in HIV-infected patients than in non–HIV-infected patients in the last time period (2008–2012). In the Women’s Interagency HIV Study, Tien et al. [12] reported that among HIV-infected women, longer cumulative exposure to NRTIs was associated with a greater risk of diabetes than no exposure to NRTIs (relative hazard, 2.64 [95%CI, 1.11–6.32] for >3 years’ exposure). In the US, Brar et al. [31] found female sex to be significantly associated with diabetes in HIV-infected patients (OR, 2.30; 95%CI, 1.47–3.60) but not in the non–HIV-infected control group. These results suggest that traditional risk factors for diabetes (including loss of ovarian function earlier in life in HIV-infected women than in non–HIV-infected women) appeared to be the most important predictors [32].
The adjusted prevalence of diabetes and the risk of dying from diabetes seem to be similar over time for HIV-infected and non–HIV-infected patients. Our results point to the need for good care of HIV-infected patients based on the assessment of factors such as HIV-related and non–HIV-related comorbidities, patients’ readiness to start cART, and prevention and management of non-infectious comorbidities such as HBP and diabetes [33].
Several factors may explain the protective effect of diabetes on IHM among HIV-infected patients. One explanation is that the presence of diabetes might be an additional factor to be taken into account when selecting the best clinical strategy for these patients. It has been shown that management of HIV-infected patients in accordance with continually updated national and international guidelines and recommendations decreased the risk of death (less than 5% of HIV-infected patients identified during 2007–2012 in Sweden) [33].
Another factor could be the “obesity paradox”—elevated BMI in patients with diabetes could be associated with decreased HIV-related mortality—which has recently been reported in coronary heart disease and other diseases, including incident diabetes [34].
It has been found that HIV infection without satisfactory treatment (as it was in 1997 or before) is associated with lower insulin resistance, that the impact of first HAART was associated with an increased likelihood of survival but also with developing lipodystrophy, and that lipodystrophy was associated with a higher risk of diabetes mellitus [35]. Therefore, diabetes could be a proxy for the bad effects of antiretroviral therapy (lipodystrophy) but also for the good ones (better HIV control and longer survival).
Finally, a possible explanation for this result would be that diabetes may be under-recorded in patients deceased due to severe HIV-related conditions. However we don’t think this should happen more frequently among patients deceased due to severe HIV-related conditions than among those non-HIV patients who died. In our opinion this misclassification bias, is exists, would be non-differential and affect equally both groups therefore, reducing the significance of the association. The main strengths of our study are its large sample size, 15-year follow-up period, and standardized methodology. Nevertheless, our study is subject to a series of limitations.
No information is available in the Spanish Hospital Discharge Database on important sociodemographic characteristics such as race and educational or economical level. Furthermore, relevant clinical variables including duration of the HIV infection, treatments used, drug resistance, treatment adherence or CD4 count, among others, are not included in the database. However we consider that given that we conducted an epidemiological, not clinical investigation, our conclusions are valid and relevant and that most of the residual confounding bias that could be introduced by this clinical variables is controlled by including the CCI as a proxy for the overall health condition of the patients. Also, it is possible that increase of diabetes prevalence could reflect the improvement in recording habits. Ribera et al indicated that as the quality of the coding is apparently gradually improving in Spain [36]. However this improvement would affect equally subjects with and without HIV infection therefore not affecting the conclusions of the investigation. Furthermore, the increase in the age of the study population and the results of other studies conducted in Spain on diabetes prevalence makes us think that the increase in diabetes in our investigation, as a consequence of changes in recording practices, is possibly of a very small magnitude [37–39].
However, beside the limitations of administrative databases for clinical investigation on HIV many studies have used this data sources for relevant epidemiological studies on different aspects of HIV [40–44].
Nevertheless, the CMBD, are periodically audited and the validity of the “diabetes diagnosis” in hospital discharge reports has been demonstrated in the past [36, 45–47].
In conclusion, we found that in the cART era, the prevalence of diabetes has increased sharply among HIV-infected patients compared with age- and sex-matched non–HIV-infected patients. The prevalence of diabetes is rising very fast among HIV-infected women. Diabetes has a protective effect against IHM among HIV-infected patients.
Our findings suggest that diabetes is a health problem that affects HIV-infected and non–HIV-infected patients in much the same way.
Supporting Information
(PDF)
Acknowledgments
We wish to thank to Spanish Ministry of Health and Social Policy for providing the records of Minimum Basic Data Set (CMBD).
Abbreviations
- cART
combination antiretroviral therapy
- CCI
Charlson Comorbidity Index
- HBP
high blood pressure
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IHM
in-hospital mortality
- LOHS
length of hospital stay
- MBDS
Minimum Basic Data Set
- NRTIs
nucleotide reverse transcriptase inhibitors
Data Availability
All relevant data are within the paper.
Funding Statement
This study forms part of research funded by the FIS (Fondo de Investigaciones Sanitarias—Health Research Fund, Instituto de Salud Carlos III grants no. PI13/00118, PI11/00245 & PI14CIII/00011 and PI12/00019) co-financed by the European Union through the Fondo Europeo de Desarrollo Regional (FEDER, “Una manera de hacer Europa”) and by the Grupo de Excelencia Investigadora URJC-Banco Santander Nº30VCPIGI03: Investigación traslacional en el proceso de salud—enfermedad (ITPSE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord, Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 2012;41:433–445. 10.1093/ije/dyr164 [DOI] [PubMed] [Google Scholar]
- 2.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem AV, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015;15:810–818. 10.1016/S1473-3099(15)00056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visnegarwala F, Chen L, Raghavan S, Tedaldi E. Prevalence of diabetes mellitus and dyslipidemia among antiretroviral naive patients co-infected with hepatitis C virus (HCV) and HIV-1 compared to patients without co-infection. J Infect 2005;50:331–337. [DOI] [PubMed] [Google Scholar]
- 4.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2009;50: 499–505. 10.1097/QAI.0b013e31819c291b [DOI] [PubMed] [Google Scholar]
- 5.Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Salmon-Ceron D, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012;26:303–314. 10.1097/QAD.0b013e32834e8776 [DOI] [PubMed] [Google Scholar]
- 6.Howard AA, Floris-Moore M, Arnsten JH, Santoro N, Fleischer N, Lo Y, et al. Disorders of glucose metabolism among HIV-infected women. Clin Infect Dis 2005;40:1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young F, Critchley JA, Johnstone LK, Unwin NC. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Global Health 2009;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calza L, Manfredi R, Chiodo F. Insulin Resistance and Diabetes Mellitus in HIV-Infected Patients Receiving Antiretroviral Therapy. Metab Syndr Relat Disord 2004;2:241–250. 10.1089/met.2004.2.241 [DOI] [PubMed] [Google Scholar]
- 9.Grinspoon S. Mechanisms and strategies for insulin resistance in acquired immune deficiency syndrome. Clin Infect Dis 2003;37 (Suppl 2):S85–S90. [DOI] [PubMed] [Google Scholar]
- 10.Justman JE, Benning L, Danoff A, Minkoff H, Levine A, Greenblatt RM, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. J Acquir Immune Defic Syndro 2003;32:298–302. [DOI] [PubMed] [Google Scholar]
- 11.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med 2010; 11:462–468. 10.1111/j.1468-1293.2009.00815.x [DOI] [PubMed] [Google Scholar]
- 12.Tien PC, Schneider MF, Cole SR, Levine AM, Cohen M, DeHovitz J, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. AIDS 2007;21:1739–1745. [DOI] [PubMed] [Google Scholar]
- 13.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–1184. [DOI] [PubMed] [Google Scholar]
- 14.Silva EF, Bassichetto KC, Lewi DS. Lipid profile, cardiovascular risk factors and metabolic syndrome in a group of AIDS patients. Arq Bras Cariol 2009;93:113–118. [DOI] [PubMed] [Google Scholar]
- 15.Cahn P, Leite O, Rosales A, Cabello R, Alvarez CA, Seas C, et al. Metabolic profile and cardiovascular risk factors among Latin American HIV-infected patients receiving HAART. Braz J Infect Dis 2010;14:158–166. [DOI] [PubMed] [Google Scholar]
- 16.Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007;45:111–119. [DOI] [PubMed] [Google Scholar]
- 17.Petoumenos K, Worm SW, Fontas E, Weber R, De Wit S, Bruyand M, et al. Predicting the short-term risk of diabetes in HIV-positive patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. J Int AIDS Soc 2012;15:17426 10.7448/IAS.15.2.17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araujo S, Bañón S, Machuca I, Moreno A, Pérez-Elías MJ, Casado JL. Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur J Endocrinol 2014;171:545–554. 10.1530/EJE-14-0337 [DOI] [PubMed] [Google Scholar]
- 19.Subdireccion General de Desarrollo, Instituto Nacional de Salud, Ministerio de Sanidad y Consumo. Conjunto Mínimo Basico de Datos de Datos Hospitales de Insalud. In Spanish; 2001. Available: http://www.ingesa.msc.es/estadEstudios/documPublica/CMBD-2001.htm.
- 20.Nosyk B, Min J, Lima VD, Yip B, Hogg RS, Montaner J, et al. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. J Acquir Immune Defic Syndr 2013;63:653–659. 10.1097/QAI.0b013e3182976891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce N, Pope D, Stanistreet D. Quantitative methods for health research: a practical interactive guide to epidemiology and statistics John Wiley & Sons;2008. [Google Scholar]
- 22.Ury HK. Eciency of case-control studies with multiple controls per case: continuous or dichotomous data. Biometric 1975;643–649. [PubMed] [Google Scholar]
- 23.Taylor JM. Choosing the number of controls in a matched case-control study, some sample size, power and eciency considerations. Statistics in medicine 1986;5:29–36. [DOI] [PubMed] [Google Scholar]
- 24.Gail MH. In: Frequency Matching. John Wiley & Sons, Ltd; 2005. 10.1002/0470011815.b2a03067. [DOI] [Google Scholar]
- 25.2007 ICD-9-CM Diagnosis codes. ICD9Data.com. Available: http://www.icd9data.com/2007/Volume1/140-239/default.htm.
- 26.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. R. A Language and Environment for Statistical Computing. Vienna, Austria; 2014. ISBN 3-900051-07-0. Available: http://www.R-project.org/.
- 28.Galli L, Salpietro S, Pellicciotta G, Galliani A, Piatti P, Hasson H, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur J Epidemiol 2012;27:657–65. 10.1007/s10654-012-9707-5 [DOI] [PubMed] [Google Scholar]
- 29.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–1234. 10.1097/QAD.0b013e32832bd7af [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen LD, Mathiesen ER, Kronborg G, Pedersen C, Gerstoft J, Obel N. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PLoS One 2012;7:e44575 10.1371/journal.pone.0044575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brar I, Shuter J, Thomas A, Daniels E, Absalon J; Minorities and Women's Task Force of Terry Beirn Community Programs for Clinical Research on AIDS. A comparison of factors associated with prevalent diabetes mellitus among HIV-Infected antiretroviral-naive individuals versus individuals in the National Health and Nutritional Examination Survey cohort. J Acquir Immune Defic Syndr 2007;45:66–71. [DOI] [PubMed] [Google Scholar]
- 32.Santoro N, Fan M, Maslow B, Schoenbaum E. Women and HIV infection: the makings of a midlife crisis. Maturitas 2009;64:160–164. 10.1016/j.maturitas.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jallow A, Ljunggren G, Wändell P, Carlsson AC. Prevalence, incidence, mortality and co-morbidities amongst human immunodeficiency virus (HIV) patients in Stockholm County, Sweden—the Greater Stockholm HIV Cohort Study. AIDS Care 2015;27:142–149. 10.1080/09540121.2014.963012 [DOI] [PubMed] [Google Scholar]
- 34.Carnethon MR, Rasmussen-Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep 2014;16:446 10.1007/s11886-013-0446-3 [DOI] [PubMed] [Google Scholar]
- 35.Bindlish S, Presswala LS, Schwartz F. Lipodystrophy: Syndrome of severe insulin resistance. Postgrad Med. 2015;127:511–6. 35. 10.1080/00325481.2015.1015927 [DOI] [PubMed] [Google Scholar]
- 36.Ribera A, Marsal JR, Ferreira-González I, Cascant P, Pons JM, Mitjavila F, et al. Predicting in-hospital mortality with coronary bypass surgery using hospital discharge data: comparison with a prospective observational study. Rev Esp Cardiol 2008;61:843–852. [PubMed] [Google Scholar]
- 37.de Burgos-Lunar C, Jiménez-García R, Salinero-Fort MA, Gómez-Campelo P, Gil A, Abánades-Herranz JC, et al. Trends in hypertension prevalence, awareness, treatment and control in an adult type 2 diabetes Spanish population between 2003 and 2009. PLoS One.2014;9:e86713 10.1371/journal.pone.0086713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiménez Mejías E, Olvera Porcel MC, Amezcua Prieto C, Olmedo-Requena R, Martínez Ruiz V, Jiménez Moleón JJ. [Effect of age on the prevalence of diabetes mellitus in Spain between 2001 and 2012]. Nutr Hosp. 2014;29:1335–8. 10.3305/nh.2014.29.6.7327 [DOI] [PubMed] [Google Scholar]
- 39.Basterra-Gortari FJ, Bes-Rastrollo M, Seguí-Gómez M, Forga L, Martínez JA,Martínez-González MA. [Trends in obesity, diabetes mellitus, hypertension and hypercholesterolemia in Spain (1997–2003)]. Med Clin (Barc). 2007;129:405–8. 36. [DOI] [PubMed] [Google Scholar]
- 40.George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. PLoS One. 2014;9:e104169 10.1371/journal.pone.0104169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82:443–51. 10.1212/WNL.0000000000000086 [DOI] [PubMed] [Google Scholar]
- 42.Klein D, Hurley LB, Quesenberry CP Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002; 30: 471–477. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20:561–5. [DOI] [PubMed] [Google Scholar]
- 44.Okeke NL, Hicks CB, McKellar MS, Fowler VG Jr, Federspiel JJ. History of AIDS in HIV-Infected Patients Is Associated With Higher In-Hospital Mortality Following Admission for Acute Myocardial Infarction and Stroke. J Infect Dis. 2016;213:1955–61. 10.1093/infdis/jiw082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leong A, Dasgupta K, Bernatsky S, Lacaille D, Avina-Zubieta A, Rahme E. Systematic review and meta-analysis of validation studies on a diabetes case definition from health administrative records. PLoS One. 2013;8:e75256 10.1371/journal.pone.0075256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zellweger U, Bopp M, Holzer BM, Djalali S, Kaplan V. Prevalence of chronic medical conditions in Switzerland: exploring estimates validity by comparing complementary data sources. BMC Public Health. 2014;14:1157 10.1186/1471-2458-14-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen G, Khan N, Walker R, Quan H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res Clin Pract. 2010;89:189–95. 10.1016/j.diabres.2010.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.