Abstract
Background
Terrestrial Trunked Radio (TETRA) is a telecommunications system widely used by police and emergency services around the world. The Stewart Report on mobile telephony and health raised questions about possible health effects associated with TETRA signals. This study investigates possible effects of TETRA signals on the electroencephalogram and electrocardiogram in human volunteers.
Methods
Blinded randomized provocation study with a standardized TETRA signal or sham exposure. In the first of two experiments, police officers had a TETRA set placed first against the left temple and then the upper-left quadrant of the chest and the electroencephalogram was recorded during rest and active cognitive processing. In the second experiment, volunteers were subject to chest exposure of TETRA whilst their electroencephalogram and heart rate variability derived from the electrocardiogram were recorded.
Results
In the first experiment, we found that exposure to TETRA had consistent neurophysiological effects on the electroencephalogram, but only during chest exposure, in a pattern suggestive of vagal nerve stimulation. In the second experiment, we observed changes in heart rate variability during exposure to TETRA but the electroencephalogram effects were not replicated.
Conclusions
Observed effects of exposure to TETRA signals on the electroencephalogram (first experiment) and electrocardiogram are consistent with vagal nerve stimulation in the chest by TETRA. However given the small effect on heart rate variability and the lack of consistency on the electroencephalogram, it seems unlikely that this will have a significant impact on health. Long-term monitoring of the health of the police force in relation to TETRA use is on-going.
Abbreviations: TETRA, Terrestrial Trunked Radio; ELF, extremely low frequency; EMF, electromagnetic fields; EEG, electroencephalogram; EKG, electrocardiogram; ERPs, event-related potentials; HRV, heart rate variability; SAR, specific absorption rate; ABT, Attentional Blink Task; SART, Sustained Attention to Response Task; VNS, vagal nerve stimulation
Keywords: Electromagnetic fields, Exposure, Neurophysiological effects, Occupational cohort, Radio frequency
Highlights
-
•
Two provocation experiments of Terrestrial Trunked Radio (TETRA) in healthy volunteers.
-
•
In one experiment, chest exposure to TETRA had significant effect on the electroencephalogram (EEG).
-
•
Chest exposure to TETRA also induced changes in heart rate variability (HRV).
-
•
Pattern of effects on the EEG and HRV suggestive of vagal nerve stimulation.
-
•
Physiological effects of short-term TETRA exposure warrants further investigation
1. Introduction
Terrestrial Trunked Radio (TETRA) is an open standard telecommunications system for private mobile radios used by the emergency services, utility companies and the military in more than 100 countries. TETRA employs time division multiplexing such that the radio frequency (RF) signal (380–395 MHz) is transmitted in a series of bursts (timeslots) with a pulse rate of 17.6 Hz (Challis, 2007, MTHR, 2007). This pulsing may induce an extremely low frequency (ELF) modulation of the magnetic field at 17.6 Hz in addition to, and synchronized with, the pulse-modulated RF electromagnetic fields (EMF).
The UK’s Independent Expert Group on Mobile Phones (Stewart Report) concluded in 2000 that ‘…as a precautionary measure, amplitude modulation around 16 Hz should be avoided, if possible, in future developments in signal coding’ (IEGMP, 2000). This recommendation was based largely on the results of a study which claimed that RF signals pulsed at around 16 Hz had an effect on calcium efflux from cells (Bawin et al. 1975), though later and better designed studies using live tissue failed to confirm this finding (MTHR, 2007, Green et al., 2006, NRPB, 2001). From 2001, TETRA was introduced in police forces across the UK. To address the concerns raised by the Stewart Report, in 2003 the UK Home Office commissioned i) the Airwave Health Monitoring Study (Airwave is the commercial name of the TETRA-based digital telecommunications system adopted by the police forces in the UK), an epidemiological (cohort) study into the possible long-term health effects of TETRA (Elliott et al., 2014), and ii) investigation of possible acute effects by comparing effects of TETRA emissions and sham exposure in a blinded randomized provocation study.
We report here the results of the provocation study. This was designed initially (Experiment 1) to investigate whether emissions from TETRA handsets may produce detectable effects on the electroencephalogram (EEG). The EEG measures the brain’s naturally occurring, spontaneous, electrical oscillations, which occur at frequencies that overlap the pulsing rate of TETRA. It was therefore hypothesized that the EEG would provide a particularly sensitive test of any neurophysiological effects of TETRA. Based on the results of Experiment 1, we hypothesized that TETRA-RF might have an effect on the vagal nerve and for this reason the study was later extended to include possible effects on heart rate variability (HRV) measured by the electrocardiogram (EKG).
2. Materials and methods
We conducted two experiments. The first studied the effects of TETRA signals on the EEG with placement of the radio against the head and left-side of the chest (Experiment 1). We then included simultaneous recording of the EEG and the EKG, focusing only on the chest placement (Experiment 2).
2.1. Participants
The first sample (Experiment 1) comprised 164 police officers who had joined the Airwave Health Monitoring Study (Elliott et al., 2014), including 107 officers who reported health symptoms they attributed to TETRA. Paid leave was provided by the police force and participants had their expenses paid. The second sample (Experiment 2) comprised 60 volunteers recruited by advertisement and paid £25 for their time. The study was conducted in accordance with the Declaration of Helsinki. All participants gave individual informed consent.
2.2. TETRA exposure system
2.2.1. Experiment 1
TETRA was generated by a radio system that had been especially commissioned by the UK’s Mobile Telecommunications Health Research (MTHR) programme (Barker et al., 2007, Butler, 2005, Nieto-Hernandez et al., 2011, Smith, 2007). The radios were designed to transmit in the TETRA range (390–400 MHz) and were calibrated to give a peak specific absorption rate (SAR) of 1.3 Wkg−1±30% averaged over 10 g, in TETRA mode for head exposure (MTHR, 2001). SAR for chest exposure was not available. We previously showed that TETRA could interfere with the EEG recording system by inducing an electronic artefact at 17.6 Hz and higher harmonics, seen in both human and phantom head recordings (Fouquet et al., 2013). Despite extensive shielding, TETRA interference continued to be found in ~2% of the channels.
2.2.2. Experiment 2
The MTHR radios were no longer available so TETRA was generated using a specially commissioned radio developed by the National Physical Laboratory that transmitted at 381 MHz (just outside the TETRA range to reduce interference with other TETRA users) (MTHR, 2001) and was calibrated to give a peak head SAR of 1.35 Wkg−1±22% averaged over 10 g and a peak body SAR of 1.0 Wkg−1±22% averaged over 10 g in TETRA mode (Loader, 2013).
In both experiments, maximum SAR was generated close to the antenna and the distribution of SAR in the head from commercially available TETRA handsets is given in Dimbylow et al. (2003) and for the MTHR handsets in National Physics Laboratory (2013).
2.3. Electroencephalogram
2.3.1. Experiment 1
EEG was recorded from 28 scalp electrodes (see Supplementary Fig. 1a) using a Neuroscan Synamps-II amplifier (Compumedics Neuroscan, USA). EEG was recorded in the resting state (Eyes Closed) and during performance of the Attentional Blink Task (ABT) and Sustained Attention to Response Task (SART) (see Supplementary Material). Channels showing TETRA interference were identified using an automatic algorithm (Fouquet et al., 2013), affecting approximately 2% of channels for head exposure (none for chest exposure); for these channels, data were imputed by interpolation using a weighted average of the nearest four channels. This affected just under one third of the sample (53/164). Participant data were excluded if insufficient artefact-free EEG recordings were available or because of aberrant performance on the tasks leaving 156 (95%) participants for the analyses of the EEG amplitude spectra, 151 (92%) for the SART and 146 (89%) for the ABT.
2.3.2. Experiment 2
EEG was recorded from 21 scalp electrodes (see Supplementary Fig. 1b, which shows the electrode positions for Experiment 2), using a Galileo NT system (EBNeuro, Florence, Italy), during two rest conditions (Eyes Closed and Eyes Open) and during performance of the SART. Data from three people were corrupted due to a failure in the storage medium leaving a total of 57 people for the EEG and HRV analyses. TETRA interference was visible on the EEG recordings for 8 participants and it became apparent following spectral analysis for a further 6, thus un-blinding the experimenters in 14/57 (25%) of the sample. In these cases, data imputation was used for all conditions whether or not they showed evidence of artefact. As both un-blinding and data imputation might bias results, all EEG analyses were performed on the full sample and on the subset of 43 individuals for whom no data were imputed.
EEG data preparation was performed in BESA ver. 5.1.8 (BESA, 2014) and the spectral analysis in MatLab ver. 2014a (MatLab, 2014a). Two bipolar polygraphic channels were used to record vertical and horizontal eye movements. Electro-oculographic artefact correction was performed using Schlogl’s algorithm (Schlogl et al., 2007) in Experiment 1 and principal component analysis in BESA ver. 5.3 (Ille et al., 2002) in Experiment 2. Impedances (maintained below 5 kΩ) were measured at the beginning of each recording period using an impedance meter. Data were divided into short epochs; values outside the range ±100 μV were treated as artefact and excluded from further analysis. EEG from the Eyes Closed baseline recordings was segmented into epochs of 2.048 ms, de-trended and subjected to multi-taper fast Fourier transform (Percival and Walden, 1993) with time-half bandwidth product=4 (MatLab, 2014a, MatLab, 2014b) and average amplitudes in the 0.5–45 Hz range estimated in 0.5 Hz intervals. For event-related potentials (ERPs), EEG data for the ABT were segmented into epochs from −50 ms to +1550 ms around the 1st target stimulus. For the SART, segmentation was −50 ms to +800 ms around the stimulus presentation. In both cases, data were baseline corrected (−50 ms to 0 ms), filtered using a 5th order 20 Hz low-pass Butterworth filter, and averaged.
2.4. Electrocardiogram (Experiment 2)
EKG was recorded during rest (Eyes Closed and Eyes Open) with a single polygraphic channel using the Galileo NT system at the V1 position. Participants were seated in the upright position and instructed to maintain a respiratory rate of 12 cycles/minute. To measure HRV, we calculated the timing of each R wave of the QRS complex on the EKG using the matching template algorithm available in the NPXLab software (Bianchi et al., 2007, Niskanen et al., 2004) and analysed the time intervals between consecutive R waves using Kubios HRV (Tarvainen et al., 2014). We estimated autonomic indices in both the time and frequency domains (Saul et al., 1990). In the time domain these were the N-N (normal to normal) interval, an index of cardio-vagal function, SDNN (the standard deviation of the N-N interval), and the pNN50 (the number of interval differences of successive N-N intervals greater than 50 ms divided by the total number of N-N intervals) which is a measure of parasympathetic activity. In the frequency domain, we estimated spectral power in the High Frequency (HF) range (0.15–0.4 Hz), a marker of parasympathetic activity, spectral power in the Low Frequency (LF) range (0.04–0.15 Hz), and the ratio of the two (LF:HF ratio). Conventionally, LF is considered to be an index of sympathetic modulation and LF:HF an index of sympathovagal balance, but recent evidence suggests that neither measure provides a valid marker of sympathetic enervation of the heart (Reyes del Paso et al., 2013, Shaffer et al., 2014).
2.5. Experimental procedures
Participants were exposed to TETRA and sham exposure under blinded randomized conditions in counterbalanced order. At the end of each experiment participants were asked if they could identify the TETRA or sham condition. In experiment 2, participants also completed a symptom checklist at the end of each condition that included symptoms associated with vagal nerve stimulation (Supplementary Material - Appendix).
2.5.1. Experiment 1
EEG was recorded with the radio in two positions: against the head on the left-side (Fig. 1) and on the upper-left quadrant of the chest with the tip of the antenna at the level of the collarbone. The recording sequence for each session is shown in Supplementary Table 1, which demonstrates the sequence of events and approximate durations for each participant in Experiment 1. As the ABT was self-paced, total exposure time varied; mean duration was 51 (range 36–81) minutes with the handset positioned against the head and 14 (range 12–16) minutes against the chest. There was a 10 min break between the two exposure conditions (one TETRA, one sham exposure) for both head and chest, and 20 min between the head and chest exposure conditions.
Fig. 1.
The TETRA radio was attached to the left side of the head using an elasticated net and a velcro band. The relative position of the radio and the EEG recording channels (indicated by white circles) is shown. SAR was maximal close to the antenna (contained within the yellow cylinder at the top left of the radio) which lay midway between T7 and P7 to midway between T7 and CP5. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5.2. Experiment 2
The handset was held over the left upper quadrant of the chest with the tip of the antenna at the level of the collarbone. Exposure time was increased compared with Experiment 1 to a minimum of 40 min. The recording sequence for each session is shown in Supplementary Table 2, which demonstrates the sequence of events and approximate durations for each participant in Experiment 2. This sequence was carried out twice (one TETRA, one sham exposure) with a 10-minute break between the two. EEG and EKG were recorded throughout the two sessions.
2.6. Statistical analysis
We checked participant blindness using Fisher’s exact test to compare actual and participant reported exposure conditions. We analysed effects of the provocation test on i) performance (accuracy and reaction times) on the SART and ABT using one-way repeated measures ANOVA with TETRA vs. sham exposure as the independent variable; ii) EEG and ERPs by Partial Least Squares (PLS; Lobaugh et al., 2001; see Supplementary Material). PLS is a multivariate method of analysis and, for the power spectrum analysis, the data were the mean EEG spectra for each frequency (0.5–45 Hz), for each channel and for each participant compared across the two conditions (TETRA vs. sham). For the event-related potential analysis, the data were the mean amplitude for each frequency time point (0–800 ms), for each channel and for each participant compared across the two conditions (TETRA vs. sham). Overall statistical difference between the conditions was determined using permutation testing (1000 permutations) and, if a difference was found, the reliability of differences (P<0.05) for each frequency at each electrode (for the spectral analysis) or at each time point at each electrode (for ERP analysis) was evaluated, by bootstrapping with 1000 re-samplings. We used Hotelling’s T-test to assess the effect of the provocation test on EEG at all electrode sites at the pulsing rate of TETRA (17.6 Hz).
We analysed data on HRV using two-way multivariate repeated measures analysis of variance with TETRA vs. sham exposure and Eyes Open vs. Eyes Closed as the independent variables. The LF:HF ratios showed a strong negative skew and so were log-transformed. The pNN50 was transformed by the arcsine of the square root of the raw data. All statistical analysis was on the transformed data but mean and standard deviations, where reported, refer to the untransformed data.
3. Results
The first sample (Experiment 1) comprised 140 male and 24 female police officers; mean age was 39 years (sd=7.3; range=22–62). The second sample (Experiment 2) comprised 20 male and 37 female volunteers with useable data on EEG and EKG; mean age was 34 years (sd=13.5; range=18–64). We found no statistically significant difference between TETRA and sham conditions on the SART and ABT (Supplementary Table 3). Participants were unable to determine the order of TETRA or sham condition (P>0.05 for all comparisons), but because TETRA-interference was visible to the experimenters on the EEG traces in a minority of cases, the experiments were not fully double blind for all conditions (see Supplementary Material – Double-blind Experimental Design). In Experiment 2, there was no difference in symptom reporting between the TETRA and sham conditions (p>0.05) and the symptoms that were reported were non-specific items such as fatigue and other minor discomforts and did not include symptoms associated with vagal stimulation.
3.1. Electroencephalogram
In Experiment 1, there was a significant effect of TETRA on the resting EEG (Eyes Closed, amplitude spectra 0.5–45 Hz) with the radio positioned against the chest (P=0.017) (Fig. 2), but not when the radio was positioned against the head. At low frequencies (delta,<4 Hz), EEG amplitude was significantly enhanced during TETRA exposure across much of the scalp surface, particularly on the right side and most notably at frontal sites (Fp2 and F7). At frequencies above 10 Hz, particularly in the high beta and gamma frequency ranges, this effect was reversed and EEG amplitude in the TETRA condition was enhanced most notably on the left and at posterior sites (Oz and O2) (Fig. 2). Head exposure to TETRA had no significant effect on the ERPs obtained from either the SART (P=0.179) or ABT (P=0.350). However, chest exposure to TETRA did have a significant effect on the SART ERPs (P=0.037) (Fig. 3) which was maintained when individuals with TETRA interference were excluded (P=0.034). The TETRA effect was strongest at 300–600 ms and was most prominent at right frontal sites (Fp2, Fz, F4, FC6) when the ERP was more negative to TETRA, and at posterior sites (P4, PO1, PO2 O2 and Oz) where the direction of the effect was reversed.
Fig. 2.
Resting state (Eyes Closed) EEG amplitude spectra (0.5–45 Hz) averaged across all channels recorded during TETRA and sham exposure to the chest in Experiment 1. There was an overall significant difference between conditions (PLS P=0.017). The frequencies where there was a reliable difference between conditions in at least one channel and the direction of those differences are indicated by the coloured circles, with red circles indicating TETRA > sham and blue indicating TETRA < sham.
Fig. 3.
The SART grand average event-related potentials for a subset of channels recorded during TETRA and sham exposure to the chest in Experiment 1 from −50 ms to +800 ms around the time of stimulus presentation. There was an overall significant difference between conditions (P=0.037) and the times and locations where a reliable difference between conditions was seen are indicated by the black circles.
In contrast, in Experiment 2, we found no effect of TETRA signal on the EEG in the Eyes Open/Eyes Closed condition (see Supplementary Figs. 2 and 3 which graphically illustrates the effect of TETRA or sham exposure in the Eyes Closed, P=0.097, and the Eyes Open, P=0.556, conditions in Experiment 2) nor on the SART ERPs obtained from either the full sample (P=0.774) (see Supplementary Fig. 3, which graphically shows the Effect of TETRA on the SART event related potentials in Experiment 2) or from the sample excluding those with TETRA interference (P=0.963).
3.2. Heart-rate variability
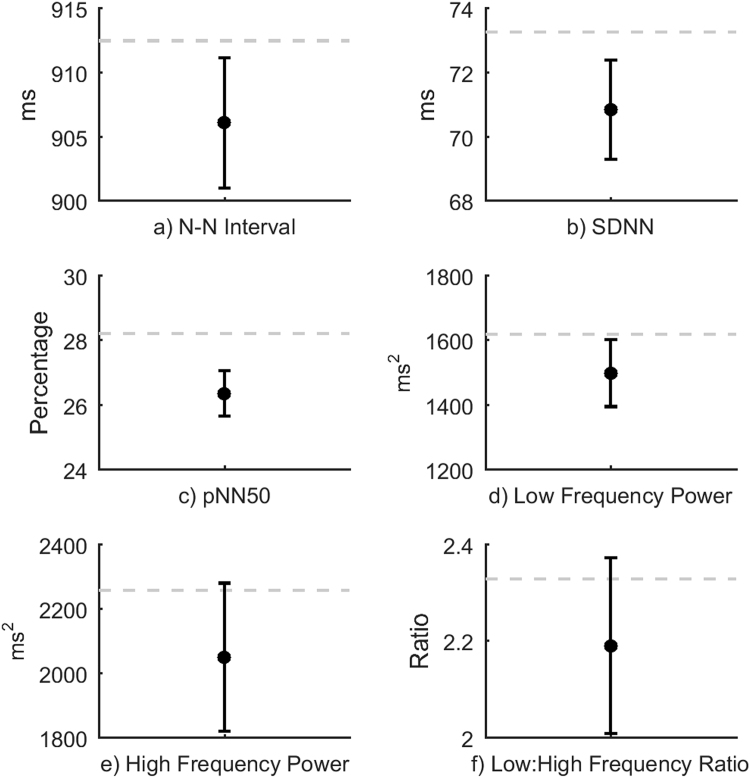
The results for the HRV analyses are summarized in Table 1 and Fig. 4. There was a significant difference between the TETRA and sham conditions (P=0.029) for all HRV indices combined (N-N Interval, SDNN, pNN50, Low Frequency power, High Frequency power and the ratio of Low to High Frequency power i.e. Low/High), reflecting mainly a significant decrease in pNN50 (P=0.010). Approximately 20% of the variance of the overall difference in HRV measures between the TETRA and sham conditions was attributed to the RF exposure (r2=0.20). Results remained significant when participants with TETRA interference on their EEG traces were excluded (P=0.015).
Table 1.
Mean (SE) of Heart Rate Variability Indices for the TETRA and Sham Exposure Conditions. Overall difference between TETRA and sham was significant (P=0.029, multivariate analysis) accounting for 20% of the variance (effect size, r2=0.20).
|
TETRA Status |
|||||
|---|---|---|---|---|---|
|
TETRA On |
Sham |
||||
| Mean | (s.e.) | Mean | (s.e.) | p-Value | |
| N-N Interval/ ms | 906.1 | (17.5) | 912.5 | (17.5) | 0.214 |
| SDNN | 70.8 | (5.3) | 73.3 | (5.5) | 0.124 |
| pNN50 (%) | 26.4 | (2.9) | 28.2 | (2.89) | 0.010a |
| Median | (i.q.r) | Median | (i.q.r) | p-Value | |
| Low Frequency/ ms2 | 869.0 | (1168.1) | 912.9 | (1270.9) | 0.336b |
| High Frequency/ ms2 | 818.4 | (1404.0) | 796.7 | (1254.5) | 0.314b |
| Low : High Frequency ratio | 1.15 | (1.99) | 1.13 | (2.08) | 0.354c |
Analysis performed on the arcsine of the square root of the raw data.
Analysis performed on the normalised power values (n.u.) and not the raw values (ms2).
Analysis performed on the logarithm of the raw data.
Fig. 4.
Heart-rate Variability indices for the TETRA and sham exposure conditions in Experiment 2. In each panel (a–e), the dotted line indicates the mean value during sham exposure, ● indicates the mean value during TETRA exposure and the error bars indicate ±1 standard error of the difference between the TETRA and sham conditions.
4. Discussion
In this randomized provocation study of TETRA vs sham exposure to the head and chest, we found a significant effect of chest exposure on the EEG (Experiment 1 only) and HRV, but no effect on the EEG of head exposure. During chest exposure, SAR in the brain is much lower than for head exposure, suggesting an indirect rather than direct effect of the RF-signal on the brain. During chest exposure, the tip of the antenna was positioned against the collarbone on the left-hand side close to the left branch of the vagus. Vagal nerve stimulation (VNS) by electrical pulses is known to affect the EEG in animals (Bailey and Bremer, 1938), eliminate inter-ictal epileptic events in cats (Zanchetti et al. 1952), and control seizures in dogs (Zabara, 1992); it is licensed for the treatment of both epilepsy and depression in humans (Cyberonics, 2007, Schachter and Saper, 1998). The optimal frequency for VNS is 20–30 Hz (Labiner and Ahern, 2007) close to the pulse rate of TETRA. In addition, although the acute effects of VNS on the EEG in humans have been little reported, and early studies with small numbers of patients failed to find any effects (Hammond et al., 1992a, Hammond et al., 1992b, Salinsky and Burchiel, 1993), more recent studies have found chronic changes in the delta and gamma frequency ranges (Marrosu et al., 2005) and late components of ERPs (Brazdil et al., 2001), similar to those reported here. This pattern as well as localization of effects showing a maximum effect at frontal sites, as with the ERPs in our study, suggest that TETRA may induce physiological effects via stimulation of the vagus nerve.
Based on these findings, we hypothesized that the observed effects on the EEG were due to VNS by the TETRA signal in the chest position. As VNS is known to have an effect on HRV (Frei and Osorio, 2001), we undertook a second double-blind provocation experiment to compare the effects of TETRA and sham exposure on HRV as well as on the EEG. While we found the predicted effect of TETRA on HRV, we failed to replicate the EEG findings. One possibility for this discrepancy is a Type-2 error, as sample size in the second study (designed to detect an effect on HRV – see Supplementary Material) was less than a third that of the first study. There were also important differences between the two experiments that might explain our findings. First, different EEG recording equipment and different TETRA radios were used in the two experiments. While the EEG equipment in both experiments seemed adequate to detect any TETRA-induced changes in the EEG, there were important differences between the TETRA radios. The NPL system used in Experiment 2 was mains powered whereas the MTHR system used in Experiment 1 was battery powered. This meant that low frequency (1–2 Hz) signals generated by the battery current of the MTHR TETRA radio were not reproduced by the exposure system in Experiment 2. If the battery-generated low frequency signal were critical for stimulating the vagal nerve, then it could account for the failure to replicate the earlier EEG/ERP findings (though this would not explain why an effect on HRV was seen in Experiment 2). Furthermore, the frequency of the signal differed slightly in the two experiments, and there was greater interference between the TETRA radios and the EEG recording system in the second experiment. Although some TETRA-related interference on the EEG was seen during head exposure in Experiment 1, none was seen during chest exposure. Even when interference was seen, it typically affected only a few channels (about 2% of the total) and was intermittent. In contrast, interference in Experiment 2 was found in 14 cases (25%) and when it occurred it was seen across all channels and was prolonged. This suggests that there were important differences in the way that the TETRA signal interacted with the EEG recording system in the two experiments, which might have relevance to the failure to replicate the original findings.
There were also important demographic differences between participants in the two experiments. In Experiment 1, the cohort consisted predominantly of white British male (85%) Police Officers aged between 32 and 46 years of age, whereas the participants in Experiment 2 were recruited from inner-city Birmingham, were ethnically diverse, had a higher proportion of women (62%) and a broader age range 20-47 years). Of these differences, age and sex are known to affect HRV and EEG, and anatomical differences between men and women, most obviously the presence or otherwise of breast tissue and associated clothing, mean that the relative position of the radio with regard to the vagal nerve and its exposure to the RF-signal will tend to vary between the sexes. Breasts and clothes would be expected to reduce exposure to the signal (meaning that any effect would typically be less in women), but the orientation of the antenna might increase or decrease exposure.
Another methodological limitation of our study was the partial failure to maintain the double-blind component of the experimental design. Whilst the participants were fully blind to the exposure condition, in a minority of cases the experimenters were un-blinded by the presence of TETRA-induced artefact on the EEG recordings. As the data analysis was largely automated, the scope for introducing experimenter bias in this way was limited. Furthermore, the results of the study were unaffected when participants with TETRA-artefact were excluded from the analysis which suggests that the un-blinding was not a critical feature.
Other evidence has suggested that pulsed-RF signals may have a physiological effect on humans not seen with continuous wave signals of comparable power. There have, for example, been multiple reports that exposure to GSM mobile phones induces an increase in alpha power on the EEG (Croft et al., 2002, Croft et al., 2008, Croft et al., 2010, Curcio et al., 2005). GSM or GSM-like phone signals have a much higher carrier wave frequency (900 MHz) than TETRA but they are also pulsed, albeit at different frequencies (2.1, 8.3 and 217 Hz). It has been suggested that amplitude modulation of the RF-signal is critical for any effect. For example, Huber et al (2002). reported that exposure to pulse modulated RF-EMF during waking had an effect on cerebral blood flow and the EEG during both sleep and waking states that was not seen with unmodulated signals or sham exposure. Furthermore, the magnitude of effect appears to depend upon the strength of the signal to which volunteers were exposed (Regel et al., 2007) and the frequency of pulsing, with modulation at 14 Hz, close to the TETRA pulse modulation rate, being more potent than 217 Hz (Schmid et al., 2012). This would seem to suggest that the modulation frequency rather than the carrier frequency is critical. Additional evidence from the effects of pulsed magnetic fields on the EEG (Cook et al., 2004, Cook et al., 2005, Cook et al., 2009) would seem to point to the importance of ELF-EMF modulation.
The above findings however are not consistent with results from a large and well-controlled study by Perentos et al. (2013) who investigated the effects on the EEG of GSM-like signals (900 Mz, pulsed at 2.1,8.3 and 217 Hz), continuous RF with the same carrier frequency, ELF-EMF with the same pulsing rates as the GSM signal and sham exposure. They found that both GSM-like RF and continuous RF had significant and comparable effects on the EEG but ELF did not. Their conclusion was that neither the pulsing of the RF-signal nor the ELF magnetic field is necessary for GSM-like signals to affect the EEG.
In the light of inconsistencies in the evidence to date, the importance or otherwise of pulse modulation of RF-EMF remains an open question. The only study that has compared TETRA signals with continuous wave signals, with the same carrier frequency and power, has come from Nieto-Hernandez et al. (2011) who investigated the effects of TETRA on symptom reporting in volunteers, some of whom self-identified as being sensitive to EMF-RF. They found no significant effect of exposure to TETRA but there was a small decrease in the ratings of symptoms of skin sensations (e.g. itching) when participants were exposed to the unmodulated TETRA-frequency signal.
There have been a few studies investigating the effects of head exposure to TETRA on the EEG. In a series of four experiments, Butler (2005) investigated the effects of TETRA on evoked potentials (visual, auditory and somaesthetic), on the power spectrogram of resting-state EEG (Eyes Open and Eyes Closed) and the time-locked response of the EEG to TETRA pulses but found no evidence of any detectable effect in any of these studies. Similarly, Eggert et al. (2015) found no evidence of any effect of TETRA on EEG slow potentials including the Contingent Negative Variation and the Bereitschafts potential. These findings are consistent with our observations that head exposure has no effect on the EEG.
Barker et al. (2007) investigated the effects of TETRA exposure to the head on blood pressure, catechol levels and HRV but found no evidence of any acute effects for any of these measures. Other studies have investigated the effects of TETRA on cognitive performance. Smith (2007) investigated performance on a wide range of cognitive tests (mostly tests of working memory, reaction time and attention) but found no effect of TETRA in 19 out of 22 tests. Of the three tests where a difference was seen, only one remained significant (Semantic Recognition) once a correction for multiple comparisons had been applied. Smith (2007) concluded that there was no robust effect of TETRA on cognitive functioning as did Riddervold et al. (2010) in a subsequent study.
If the effects of TETRA on the EEG and HRV in our own study represent a true physiological finding, it is important to consider the possible implications for health. We found a significant difference between the TETRA and sham conditions for all HRV indices combined, especially for pNN50. These changes are consistent with changes in vagal enervation of the heart. However, we found no evidence that TETRA exposure induced symptoms specifically associated with vagal nerve stimulation. It is known that stimulation of the vagus can have important physiological effects; left-sided hyperstimulation may cause atrioventricular block and right-sided hyperstimulation may cause bradycardia; current VNS therapies are licensed for left-sided stimulation only. Reviews of long-term outcome studies of VNS conclude that the procedure is safe (Daban et al., 2008, Milby et al., 2009), and with more than 50,000 vagal nerve stimulators implanted (Cyberonics, 2007), any clinically relevant cardiac effects appear to be rare (Tatum and Vale, 2009).
Given the evidence from VNS, it seems unlikely, even if TETRA does stimulate the vagus, that it will have serious side effects as the size of the TETRA effect on HRV is small, accounting for 11% of the variance in pNN50. In fact, the magnitude of the changes in HRV due to TETRA exposure was comparable with those seen between the Eyes Open and Eyes Closed conditions. On the other hand, regardless of the magnitude of the changes seen, the fact that there were statistically significant changes in HRV is noteworthy. The intensity of exposure to TETRA used in this study complied with the International Council on Non-Ionizing Radiation Protection guidelines (NRPB, 1999) and is comparable to TETRA exposure in normal use. The guidelines define conservative levels of EMF exposure set at levels designed to ensure that TETRA should have no significant biological effects on its users.
5. Conclusion
We found evidence for a significant TETRA-induced change in the EEG in our first experiment and the hypothesis arising from this that TETRA might affect HRV was supported in our second experiment by a measurable reduction in pNN50, a marker of parasympathetic (i.e. vagal) influence, during chest exposure , although the EEG effect was not replicated. The change in pNN50 was small, comparable to the difference seen between opening and closing the eyes, and as such, it seems unlikely that it poses any serious health risk. Nevertheless, the fact that physiological effects were found in humans during short-term exposures to levels of TETRA signal that were conservatively defined to exclude such influences is a novel finding worthy of further investigation and further studies with chest exposure to TETRA-RF should be undertaken. Long-term monitoring of the health of the police force in relation to TETRA use is on-going (Elliott et al., 2014).
Conflicts of interest
The authors certify that their freedom to design, conduct, interpret, and publish the research presented here was not compromised by the funders. The authors declare they have no actual or potential competing financial interests.
Source of funding
This work was supported by the UK Home Office [780-TETRA; Experiment 1] and the UK Department of Health Policy Research Programme [RDD/091/210; Experiment 2]. P.E. is an NIHR senior investigator and is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Imperial College Healthcare NHS Trust and Imperial College London, the Medical Research Council - Public Health England (MRC-PHE) Centre for Environment and Health and the NIHR Health Protection Research Unit on Health Impact of Environmental Hazards.. The views expressed in this publication are those of the authors and not necessarily those of the Home Office, the Department of Health or the NIHR.
Ethical approval
Ethical approval for the Airwave Health Monitoring Study was provided by the National Health Service Multi-Site Research Ethics Committee reference MREC/13/N/0588 and also by the Aston University Ethics Committee REC ethics application 503 (10 April 2013).
Acknowledgements
We thank the volunteers who took part in the two studies and the Police forces who permitted their officers to participate in Experiment 1. We also thank the Airwave Health Monitoring team for their help in recruitment, Prof Patrick Haggard in his role as Project Monitor and for his advice during Experiment 1, Roger Blackwell of the National Radiation Protection Board (NRPB) for his advice on shielding the EEG equipment from TETRA RF during Experiment 1 and to Benjamin Loader of National Physical Laboratory for technical advice and assistance during Experiment 2.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2016.06.031.
Contributor Information
Adrian P. Burgess, Email: a.p.burgess@aston.ac.uk.
Paul Elliott, Email: p.elliott@imperial.ac.uk.
Appendix A. Supplementary material
Supplementary material
.
References
- Bailey P., Bremer F.A. Sensory cortical representation of the vagus nerve. J. Neurophysiol. 1938;1:405–412. [Google Scholar]
- Barker A.T., Jackson P.R., Parry H., Coulton L.A., Cook G.G., Wood S.M. The effect of GSM and TETRA mobile handset signals on blood pressure, catechol levels and heart rate variability. Bioelectromagnetics. 2007;28:433–438. doi: 10.1002/bem.20333. [DOI] [PubMed] [Google Scholar]
- Bawin S.M., Kaczmarek L.K., Adey W.R. Effects of modulated vhf fields on the central nervous system. Ann. N.Y. Acad. Sci. 1975;247:74–81. doi: 10.1111/j.1749-6632.1975.tb35984.x. [DOI] [PubMed] [Google Scholar]
- BESA, 2014. Brain electrical source analysis. Available: 〈http://www.besa.de/〉.
- Bianchi L., Quitadamo L.R., Marciani M.G., Maraviglia B., Abbafati M., Garreffa G. How the npx data format handles EEG data acquired simultaneously with fMRI. Magn. Reson. Imaging. 2007;25:1011–1014. doi: 10.1016/j.mri.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Brazdil M., Chadim P., Daniel P., Kuba R., Rektor I., Novak Z. Effect of vagal nerve stimulation on auditory and visual event-related potentials. Eur. J. Neurol. 2001;8:457–461. doi: 10.1046/j.1468-1331.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Butler, S.R., 2005. Detection of effects of microwave radiation on the electrical activity of the brain. Available: 〈http://www.mthr.org.uk/research_projects/home_office_funded_projects/documents/RUM21FinalReport.pdf〉 (accessed 28.10.14).
- Challis, L., 2007. Mobile Telecommunications Health Research (MTHR) Committee Report (Chairman Prof Lawrie Challis), London: MTHR.
- Cook C.M., Saucier D.M., Thomas A.W., Prato F.S. Changes in human EEG alpha activity following exposure to two different pulsed magnetic field sequences. Bioelectromagnetics. 2009;30:9–20. doi: 10.1002/bem.20434. [DOI] [PubMed] [Google Scholar]
- Cook C.M., Thomas A.W., Keenliside L., Prato F.S. Resting EEG effects during exposure to a pulsed ELF magnetic field. Bioelectromagnetics. 2005;26:367–376. doi: 10.1002/bem.20113. [DOI] [PubMed] [Google Scholar]
- Cook C.M., Thomas A.W., Prato F.S. Resting EEG is affected by exposure to a pulsed ELF magnetic field. Bioelectromagnetics. 2004;25:196–203. doi: 10.1002/bem.10188. [DOI] [PubMed] [Google Scholar]
- Croft R.J., Chandler J.S., Burgess A.P., Barry R.J., Williams J.D., Clarke A.R. Acute mobile phone operation affects neural function in humans. Clin. Neurophysiol. 2002;113:1623–1632. doi: 10.1016/s1388-2457(02)00215-8. [DOI] [PubMed] [Google Scholar]
- Croft R.J., Hamblin D.L., Spong J., Wood A.W., McKenzie R.J., Stough C. The effect of mobile phone electromagnetic fields on the alpha rhythm of human electroencephalogram. Bioelectromagnetics. 2008;29:1–10. doi: 10.1002/bem.20352. [DOI] [PubMed] [Google Scholar]
- Croft R.J., Leung S., McKenzie R.J., Loughran S.P., Iskra S., Hamblin D.L. Effects of 2G and 3G mobile phones on human alpha rhythms: resting EEG in adolescents, young adults, and the elderly. Bioelectromagnetics. 2010;31:434–444. doi: 10.1002/bem.20583. [DOI] [PubMed] [Google Scholar]
- Curcio G., Ferrara M., Moroni F., D'Inzeo G., Bertini M., De Gennaro L. Is the brain influenced by a phone call? An EEG study of resting wakefulness. Neurosci. Res. 2005;53:265–270. doi: 10.1016/j.neures.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cyberonics, 2007. VNS therapy procedure. Available: 〈http://eu.cyberonics.com/en/vns-therapy/procedure/〉.
- Daban C., Martinez-Aran A., Cruz N., Vieta E. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J. Affect. Disord. 2008;110:1–15. doi: 10.1016/j.jad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Dimbylow P., Khalid M., Mann S. Assessment of specific energy absorption rate (SAR) in the head from a TETRA handset. Phys. Med. Biol. 2003;48:3911–3926. doi: 10.1088/0031-9155/48/23/008. [DOI] [PubMed] [Google Scholar]
- Eggert T., Dorn H., Sauter C., Marasanov A., Hansen M.L., Peter A. Terrestrial trunked radio (TETRA) exposure and its impact on slow cortical potentials. Environ. Res. 2015;143:112–122. doi: 10.1016/j.envres.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Elliott P., Vergnaud A.C., Singh D., Neasham D., Spear J., Heard A. The Airwave health monitoring study of police officers and staff in Great Britain: rationale, design and methods. Environ. Res. 2014;134C:280–285. doi: 10.1016/j.envres.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Fouquet N.C., Hawken M.B., Elliott P., Burgess A.P. TETRA mobile radios interfere with electroencephalography recording equipment. Med. Eng. Phys. 2013;35:1688–1691. doi: 10.1016/j.medengphy.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Frei M.G., Osorio I. Left vagus nerve stimulation with the neurocybernetic prosthesis has complex effects on heart rate and on its variability in humans. Epilepsia. 2001;42:1007–1016. doi: 10.1046/j.1528-1157.2001.0420081007.x. [DOI] [PubMed] [Google Scholar]
- Green A.C., Scott I.R., Gwyther R.J., Peyman A., Chadwick P., Chen X. An investigation of the effects of TETRA RF fields on intracellular calcium in neurones and cardiac myocytes. Int. J. Radiat. Biol. 2006;81:869–885. doi: 10.1080/09553000600555389. [DOI] [PubMed] [Google Scholar]
- Hammond E.J., Uthman B.M., Reid S.A., Wilder B.J. Electrophysiological studies of cervical vagus nerve stimulation in humans: I. EEG effects. Epilepsia. 1992;33:1013–1020. doi: 10.1111/j.1528-1157.1992.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Hammond E.J., Uthman B.M., Reid S.A., Wilder B.J. Electrophysiologic studies of cervical vagus nerve stimulation in humans: II. Evoked potentials. Epilepsia. 1992;33:1021–1028. doi: 10.1111/j.1528-1157.1992.tb01753.x. [DOI] [PubMed] [Google Scholar]
- Huber R., Borbely A.A., Gottselig J.M., Kuster N., Landolt H.P., Schuderer J. Effects of electromagnetic fields of mobile phones on the human sleep EEG. Sleep. 2002;25:A291–A292. doi: 10.1046/j.1365-2869.2002.00314.x. [DOI] [PubMed] [Google Scholar]
- IEGMP . Health Protection Agency; Chilton: 2000. Mobile Phones and Health: Report of an Independent Expert Group on Mobile Phones (Chairman Sir William Stewart)〈http://www.Iegmp.Org.Uk/report/text.Htm〉 [Google Scholar]
- Ille N., Berg P., Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J. Clin. Neurophysiol. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Labiner D.M., Ahern G.L. Vagus nerve stimulation therapy in depression and epilepsy: therapeutic parameter settings. Acta Neurol. Scand. 2007;115:23–33. doi: 10.1111/j.1600-0404.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Loader, B., 2013. TETRA handset exposure system for Volunteer Studies. 〈http://www.police-health.org.uk/sites/default/files/useful-resources/tetra-npl-test-rep.pdf〉. (Accessed 13.04.16)
- Lobaugh N.J., West R., McIntosh A.R. Spatiotemporal analysis of experimental differences in event-related potential data with partial least squares. Psychophysiology. 2001;38:517–530. doi: 10.1017/s0048577201991681. [DOI] [PubMed] [Google Scholar]
- Marrosu F., Santoni F., Puligheddu M., Barberini L., Maleci A., Ennas F. Increase in 20–50 Hz (gamma frequencies) power spectrum and synchronization after chronic vagal nerve stimulation. Clin. Neurophysiol. 2005;116:2026–2036. doi: 10.1016/j.clinph.2005.06.015. [DOI] [PubMed] [Google Scholar]
- MatLab, 2014a. Matlab: The language of technical computing. Available: 〈http://www.mathworks.co.uk/products/matlab/〉.
- MatLab, 2014b. Matlab: The language of technical computing: Spectral analysis - multitaper method. Available: 〈http://www.mathworks.co.uk/help/signal/ug/spectral-analysis.html#f12–18397〉.
- Milby A.H., Halpern C.H., Baltuch G.H. Vagus nerve stimulation in the treatment of refractory epilepsy. Neurotherapeutics. 2009;6:228–237. doi: 10.1016/j.nurt.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTHR, 2001. MCL MTHR GSM and TETRA handset exposure systems for human volunteer studies. Available: 〈http://www.police-health.org.uk/sites/default/files/useful-resources/mthr-radio-spec.pdf〉 (Accessed13.04.16).
- MTHR, 2007. Mobile Telecommunications Health Research (MTHR) Committee Report (Chairman Prof Lawrie Challis), London: MTHR.
- National Physics Laboratory. Test Report Serial No: UL-SAR RPA93703JD01A V1.0.〈http://www.police-health.org.uk/sites/default/files/useful-resources/tetra-radio-test-rep.pdf〉 (Accessed 13.04.16).
- National Radiological Protection Board, Board statement, 1999. Advice on the 1998 ICNIRP Guidelines for Limiting Exposure to Time-varying Electric, Magnetic and Electromagnetic Fields (up to 300 GHz): National Radiological Protection Board.
- Nieto-Hernandez R., Williams J., Cleare A.J., Landau S., Wessely S., Rubin G.J. Can exposure to a terrestrial trunked radio (TETRA)-like signal cause symptoms? A randomised double-blind provocation study. Occup. Environ. Med. 2011;68:339–344. doi: 10.1136/oem.2010.055889. [DOI] [PubMed] [Google Scholar]
- Niskanen J.P., Tarvainen M.P., Ranta-Aho P.O., Karjalainen P.A. Software for advanced HRV analysis. Comput. Methods Prog. Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- NRPB, 2001. Possible health effects from terrestrial trunked radio (TETRA). Report of an Advisory Group on Non-ionising Radiation. Doc NRPB. 12, 2, pp. 1–80.
- Percival D.B., Walden A.T. Spectral Analysis for Physical Applications. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- Perentos N., Croft R.J., McKenzie R.J., Cosic I. The alpha band of the resting electroencephalogram under pulsed and continuous radio frequency exposures. IEEE Trans. Biomed. Eng. 2013;60:1702–1710. doi: 10.1109/TBME.2013.2241059. [DOI] [PubMed] [Google Scholar]
- Regel S.J., Tinguely G., Schuderer J., Adam M., Kuster N., Landolt H.P. Pulsed radio-frequency electromagnetic fields: dose-dependent effects on sleep, the sleep EEG and cognitive performance. J. Sleep. Res. 2007;16:253–258. doi: 10.1111/j.1365-2869.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso G.A., Langewitz W., Mulder L.J., van Roon A., Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- Riddervold I.S., Kjaergaard S.K., Pedersen G.F., Andersen N.T., Franek O., Pedersen A.D. No effect of TETRA hand portable transmission signals on human cognitive function and symptoms. Bioelectromagnetics. 2010;31:380–390. doi: 10.1002/bem.20571. [DOI] [PubMed] [Google Scholar]
- Salinsky M.C., Burchiel K.J. Vagus nerve stimulation has no effect on awake EEG rhythms in humans. Epilepsia. 1993;34:299–304. doi: 10.1111/j.1528-1157.1993.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Saul J.P., Rea R.F., Eckberg D.L., Berger R.D., Cohen R.J. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am. J. Physiol. 1990;258:H713–H721. doi: 10.1152/ajpheart.1990.258.3.H713. [DOI] [PubMed] [Google Scholar]
- Schachter S.C., Saper C.B. Progress in epilepsy research: vagus nerve stimulation. Epilepsia. 1998;39(7):677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Schlogl A., Keinrath C., Zimmermann D., Scherer R., Leeb R., Pfurtscheller G. A fully automated correction method of EOG artifacts in EEG recordings. Clin. Neurophysiol. 2007;118:98–104. doi: 10.1016/j.clinph.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Schmid M.R., Loughran S.P., Regel S.J., Murbach M., Grunauer A.B., Rusterholz T. Sleep EEG alterations: effects of different pulse-modulated radio frequency electromagnetic fields. J. Sleep. Res. 2012;21:50–58. doi: 10.1111/j.1365-2869.2011.00918.x. [DOI] [PubMed] [Google Scholar]
- Smith, S.J., 2007. RF effects on human cognitive performance. Paper presented at the TETRA industry group seminar London. Available: 〈http://www.tetrahealth.info/presentations/BAPCO_Sarah_Smith_slides_2007.pdf〉 (accessed 28.10.15).
- Shaffer F., McCraty R., Zerr C.L. A healthy heart is not a metronome: An integrative review of the heart's anatomy and heart rate variability. Front. Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen M.P., Niskanen J.P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. Kubios HRV-heart rate variability analysis software. Comput. Methods Prog. Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Tatum W.O., Vale F.L. Letter: vagus nerve stimulation and cardiac asystole. Epilepsia. 2009;50:2671–2672. doi: 10.1111/j.1528-1167.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005–1012. doi: 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- Zanchetti A., Wang S.C., Moruzzi G. The effect of vagal afferent stimulation on the EEG pattern of the cat. Electro. Clin. Neurophysiol. 1952;4:357–361. doi: 10.1016/0013-4694(52)90064-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material