Abstract
Although chronic widespread musculoskeletal pain is a significant health problem, the molecular mechanisms involved in developing and maintaining chronic widespread musculoskeletal pain are poorly understood. Central sensitization mechanisms maintained by stimuli from peripheral tissues such as muscle have been suggested. Lipid mediators with anti-inflammatory characteristics such as endogenous ligands of peroxisome proliferator activating receptor-α, oleoylethanolamide, and palmitoylethanolamide are suggested to regulate nociceptive transmission from peripheral locations on route towards the central nervous system. This case–control study investigates the levels of anti-inflammatory lipids in microdialysis samples collected during the first 2 h after microdialysis probe insertion and explores the association of these lipids with different pain characteristics in women with chronic widespread musculoskeletal pain (n = 17) and female healthy controls (n = 19). The levels of oleoylethanolamide, palmitoylethanolamide, and stearoylethanolamide were determined. During sampling of dialysate, pain ratings were conducted using a numeric rating scale. Pain thresholds were registered from upper and lower parts of the body. Oleoylethanolamide and stearoylethanolamide levels were significantly higher (p ≤ 0.05) in chronic widespread musculoskeletal pain at all time points. Numeric rating scale correlated with levels of stearoylethanolamide in chronic widespread musculoskeletal pain. Higher levels of lipid mediators could reflect an altered tissue reactivity in response to microdialysis probe insertion in chronic widespread musculoskeletal pain.
Keywords: Chronic widespread pain, microdialysis, trapezius muscle, tissue trauma, lipid mediators
Introduction
Chronic widespread musculoskeletal pain (CWP) is a significant health problem with a prevalence of approximately 10% in the population.1,2 Fibromyalgia syndrome (FMS) is an important subgroup with a prevalence of 2–4%. Central alterations in the processing of nociception (e.g., alterations in pain matrix together with alterations in the descending control of nociception) have been reported in CWP/FMS.3–8 Thus, CWP/FMS has been considered a central hyperexcitability pain condition.5,9,10 However, there are several recent indications that central alterations may be driven by peripheral alterations (e.g., in muscles), by nociceptive C-fibres, and/or by a chronic nociceptive input.11–17
There is a growing body of evidence indicating that various endogenous lipid mediators regulate the excitability of peripheral nociceptors18 (e.g., pro-algesic lipids such as prostaglandin E2 and analgesic lipids such as the endocannabinoids (ECs), N-acylethanolamines (NAEs)).19 The relatively well-studied NAE palmitoylethanolamide (PEA) has been proposed to modulate pain and inflammation mainly via peroxisome proliferator-activated receptor type-α (PPAR-α) activation.20–23 Oleoylethanolamide (OEA) is likewise signaling through PPAR-α and has upon its activation been associated with regulation of feeding and body weight.24 OEA is also related with both analgesic properties which has been suggested to occur independently of PPAR-α activation,25 and induction of visceral pain via transient receptor potential vanilloid-1 (TRPV-1) activation.26 Stearoylethanolamide (SEA) has been known to generate anti-inflammatory activity,27 not modulated via PPAR-α, but similar signalling pathways by inhibition of NF-κβ translocation and down-regulation of proinflammatory cytokines expression.28
Microdialysis (MD) is a method for sampling of unbound endogenous compounds in tissues.29 The insertion of a MD probe into muscle tissues leads to an acute inflammatory response such as increases in proinflammatory cytokines.30 To investigate the habitual situation of a certain tissue, the dialysate from the time immediately after probe insertion (1–2 h) is usually discarded.
Unlike other neurotransmitters (e.g., glutamate and GABA) that are stored in vesicles and released upon stimulation, lipid mediators have been considered to be synthesized and released ‘on demand’. Non-significant higher levels of PEA and SEA in trapezius microdialysate collected after the trauma phase (140–180 min) have previously been found in CWP.31
The question arises whether chronically painful muscles respond differently to acute trauma compared to non-painful muscle in regard to levels of lipid mediators. Hence, this study investigated the acute consequences (i.e., first 2 h) of MD probe insertion with respect to levels of PEA, SEA, and OEA in dialysate and to what extent differences exist between healthy subjects and patients with CWP. Within this aim, correlations between levels of these substances and pain characteristics (intensity and sensitivity) were investigated.
Methods
All methods were carried out following approved guidelines. All participants signed a consent form that was in accordance with the Declaration of Helsinki. All the experimental protocols were approved by the Linköping University Ethics Committee (Dnr: M10-08, M233-09, M233-09, Dnr: 2010/164-32).
Subjects
In this case–control study, women with CWP were identified and recruited via a fibromyalgia patient organization and a review of the medical reports of former out patients at the multidisciplinary Pain and Rehabilitation Centre, University Hospital, Linköping, Sweden. Inclusion criteria were female sex, age range 20–65 years, and widespread pain according to the American College of Rheumatology (ACR) 1990 classification criteria.32 Exclusion criteria were bursitis, disorders of the spine, tendonitis, capsulitis, post-operative conditions in neck/shoulder area, prior neck trauma, neurological disease, rheumatoid arthritis or any other systemic disease, metabolic disease, malignancy, severe psychiatric illness, pregnancy, and difficulties understanding the Swedish language. All participants underwent a standardized and validated clinical examination of the upper extremities according to Ohlsson et al.,33 as well as a standardized clinical examination of the lower extremities.
The control group (CON) were recruited via advertisements in the local daily newspaper. Inclusion criteria were female sex, age range 20–65 years, and pain-free. The exclusion criteria were the same as the CWP group as well as any pain lasting more than seven days during the past 12 months. Clinical examination was conducted as mentioned above.
Procedures
Primary screening was performed using the standardized Nordic Ministry Council Questionnaire for analysis of musculoskeletal symptoms34 together with a structured telephone interview. Subjects were then invited for a clinical examination as described above and recording of weight, height, and body mass index (BMI) in order to confirm eligibility. All eligible subjects went through pressure point measurements in upper and lower extremities. Pressure pain thresholds (PPT) from right and left side of trapezius and tibialis anterior muscles were determined. Heat pain thresholds (HPT) and cold pain thresholds (CPT) over right and left trapezius and tibialis anterior were also determined. MD was conducted in the trapezius muscle on the most painful side (CWP) or the dominant side (CON) and pain intensity was rated.
Microdialysis
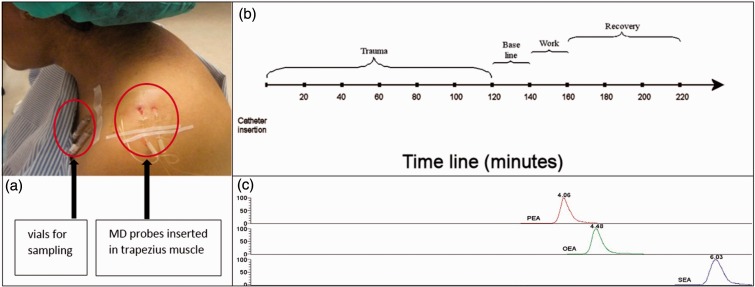
The MD procedure was accomplished as previously described.31 To guide the placement of catheters, ultrasonographic measurements of the trapezius muscle area were conducted. The skin and the subcutaneous tissue above where the catheter entered the trapezius muscle were anaesthetized with a 0.5-mL local injection of Xylocaine (20 mg/mL) without adrenaline, and care was taken not to anaesthetize the underlying muscle. The commercially available MD catheter (CMA 60; mDialysis, Stockholm, Sweden) with a 20-kDa cut-off was inserted parallel to the muscle fibres into the pars descendens of the trapezius muscle at half the distance between the processus spinosus of the seventh cervical spine and the lateral end of the acromion. After the insertion of catheters, participants rested comfortably in an armchair during the sample period. The set perfusion rate was 5 µL/min and dialysate was collected in 20–min intervals. The vials that were used for sampling of the dialysate were weighed before and after collection in order to confirm that sampling was working according to the set perfusion rate. All samples were kept on ice during the experiment and after the experiment the dialysate was stored in aliquots at −70℃ until day of analysis. Figure 1(a) shows the probes inserted in the trapezius muscle, and Figure 1(b) shows a timeline for the MD sampling.
Figure 1.
Muscle MD set up. (a) Two catheters (100 kDa and 20 kDa) inserted in trapezius muscle. (b): Timeline of the MD sampling. Samples were collected every 20 min. Subjects rested comfortably in an armchair during the trauma period (time point 20-120). After this period, subjects continued to rest for 20 min (baseline) followed by a 20-min period of standardized repetitive low-force exercise performed on a pegboard (work). The experiment ended with three recovery periods of 20 min, during which participants rested. Samples from the trauma period were analysed by LC-MS/MS. (c) Chromatograms of PEA, OEA, and SEA with retention times.
Pain intensity
The pain ratings collected every 20 min (same time points as dialysate sampling) during the MD procedure concerned pain in the trapezius muscle of the most painful side (CWP) or the dominant side (CON). The subjects were asked to rate their pain intensity on a numeric rating scale (NRS) with numbers (0–10) and with written descriptors at the two end points (0 = no pain and 10 = worst possible pain). The results concerning pain intensity have essentially (although not the same number of subjects) been published elsewhere.35
Pressure pain thresholds
The PPT measurements were performed as described earlier.31 A handheld electronic pressure algometer (Somedic, Hörby, Sweden) was used to assess PPT. Pressure was measured in kilopascal (kPa). PPT measurements were performed on the right and left side trapezius muscle and on the right and left side tibialis anterior muscle. Each PPT variable was determined as the mean of three measuring points from each muscle. The results concerning PPTs have essentially (although not the same number of subjects) been published elsewhere.31,35
HPT and CPT
Thermal sensory testing was performed using a modular sensory analyser from Somedic (Hörby, Sweden). The method has previously been described in detail.36,37 Thermal pain thresholds were measured using the method of limits with a baseline temperature of 32℃. HPT over right and left trapezius and tibialis anterior as well as CPT over the same regions were determined. A thermode with a stimulating surface of 25 × 50 mm, consisting of Peltier elements and a temperature change range of 1℃/s, was used for all tests. During the tests, the participants sat comfortably in a quiet room with an ambient temperature of approximately 22℃. The stimulator was applied to the skin and a constant current source was connected, giving a baseline temperature of 32℃. First, the ability to perceived changes in temperature was tested (not reported). Next, CPT and HPT were determined. During this procedure, the participants were instructed to activate the switch when they first perceived the stimulus as painful. The lowest and highest stimulation temperatures were 10 and 50℃, respectively. The time required for pain threshold measuring was about 15 min per site.
Analysis of NAE levels
A selected reaction monitoring (SRM) liquid chromatography tandem mass spectrometry (LC-MS/MS) method was used to analyse NAEs in human microdialysate samples using verified SRM transitions.38,39 An LC-MS/MS system containing a Thermo Scientific Accela AS auto sampler and Accela 1250 pump coupled to a Thermo Scientific TSQ Quantum Access max triple quadrupole mass spectrometer with an HESI II probe as ionization source was used. Liquid chromatography was performed using isocratic elution on a Xbridge C8 guard column (column dimensions 2.1 mm × 10 mm) coupled to a Xbridge C8 analytical column (column dimensions 2.1 mm × 150 mm) both with the particle size 2.5 µm; this set up was obtained from Waters (Dublin, Ireland). The mobile phase consisted of methanol-acetonitrile-Milli-Q water (60:25:15) (v/v/v), 0.1 % (v/v) formic acid, and 1 g/L ammonium acetate. The sample injection volume was 10 µl and the LC flow-rate was 300 µl/min. The electrospray interface was operated in positive ion mode and the spray voltage was set to 4.5 kV. The capillary temperature was set to 350℃ and sheath gas pressure to 40 arb units. The ion sweep gas pressure was set to 0.4 arb units. The SRM (m/z) transitions were 326.3/62.4 for OEA, 300.3/62.4 for PEA, and 328.3/62.4 for SEA.
MD samples were blinded and prepared before analysis as previously described.31,40 Briefly, on the day of analysis, 50 µl microdialysate and perfusate were dried by SpeedVacc vacuum concentration system (Savant, Farmingdale, NY, USA) and dissolved in 100 µl methanol, vortexed, and centrifuged.40 The supernatants were transferred to new tubes (0.65 ml) and dried. The residues were dissolved in 20 µl LC mobile phase, vortexed, and transferred to glass insert vials for LC-MS/MS.
The linearity of the measuring ranges was assessed with standard curves ranging from 0.1 to 20 nM for OEA and SEA and 0.5–20 nM for PEA in human muscle dialysate. The limit of detection (LOD) of the compounds (defined as the concentration at which a signal-to-noise ratio of greater than 3:1 was achieved following direct analyses from stock solutions) was ∼ 0.1 fmol for OEA, PEA, and SEA. Quantification was performed using external standards curves, linear regression, and equal weighting. Xcalibur® (version 2.1, Thermo Scientific) software was used for peak integration and quantification. Chromatograms of PEA, OEA, and SEA are shown in Figure 1(c).
Statistics
The IBM SPSS version 22.0 (IBM Corporation, Route 100, Somers, NY) and the GraphPad Prism computer programme (GraphPad Software Inc, San Diego, CA) were used for the traditional statistical analyses. The number of patients needed in order to achieve sufficient power was based on the concentration of interstitial lactate of the trapezius in healthy controls and in patients with chronic trapezius myalgia reported in one of our previous studies.41 Hence, using Power and Sample Size Calculation, ver. 3.0.229 based on the following parameters: a = 0.05, power = 0.8, difference between groups = 1.7, and SD = 1.7, it was found that 17 patients in each group were needed. Hence, this power analysis can be considered as post hoc calculation assuring that the number of subjects in the present study has the power to reveal relevant differences.
T-test of independent samples was used for group comparisons and one-way repeated measures ANOVA was used for pairwise comparisons within groups. Pearson’s correlation analyses were used for investigations of associations between variables. Tables 1–3 present data as means ± SD values, and Figures 2 and 3 present data as mean ± one standard error of the mean ( ± 1 SEM). SIMCA 14 (Umetrics) was used for the multivariate data analysis (MVDA), and a p ≤ 0.05 was used as level of significance in all analyses.
Table 1.
Age, height, weight, and BMI in CWP and CON.
| CWP (n = 17) Mean (SD) | CON (n = 19) Mean (SD) | Statistics (p-value) | |
|---|---|---|---|
| Age (years) | 48.8 (10.0) | 41.8 (10.7) | 0.04* |
| Height (cm) | 167.5 (5.2) | 169.5 (6.2) | 0.30 |
| Weight (kg) | 77.4 (17.7) | 70.6 (10.7) | 0.17 |
| BMI (kg/m2) | 27.5 (5.7) | 24.5 (2.9) | 0.05* |
Values are denoted as means ± SD (in brackets). Furthest to the right are shown the results of the group comparisons (p-values). * denotes statistically significant difference between groups. BMI: body mass index.
Table 2.
Concentrations of the three NAEs investigated.
| NAE | Time | CWP | CON | Statistics |
|---|---|---|---|---|
| (nM) | (min) | Mean (SD) | Mean (SD) | (p-value) |
| 20 | 1.64 (1.64) | 0.32 (0.69) | 0.003* | |
| 40 | 2.36 (3.21) | 0.44 (0.65) | 0.018* | |
| OEA | 60 | 2.11 (2.04) | 0.45 (0.67) | 0.002* |
| 80 | 1.95 (1.37) | 0.39 (0.54) | 0.000* | |
|
|
120 |
2.22 (1.78) |
0.34 (0.48) |
0.000* |
| 20 | 0.68 (0.69) | 0.38 (0.64) | 0.193 | |
| 40 | 1.34 (2.47) | 0.56 (1.05) | 0.252 | |
| PEA | 60 | 0.85 (0.57) | 0.48 (0.95) | 0.164 |
| 80 | 0.87 (0.67) | 0.21 (0.17) | 0.000* | |
|
|
120 |
0.93 (0.50) |
0.55 (0.95) |
0.148 |
| 20 | 2.76 (1.84) | 0.57 (0.37) | 0.000* | |
| 40 | 2.95 (3.00) | 0.50 (0.25) | 0.001* | |
| SEA | 60 | 3.96 (5.07) | 0.73 (0.83) | 0.019* |
| 80 | 4.03 (4.73) | 0.64 (0.75) | 0.004* | |
| 120 | 3.67 (3.54) | 0.74 (0.69) | 0.001* |
Values are denoted as mean ± SD. Furthest to the right shows the results of the group comparisons (p-values) from each time point in (0–120 min). * denotes statistically significant differences between groups; p-values are corrected for inequality of variance using Leven’s test for equality of variance, when needed.
Table 3.
PPT, CPT, and HPT values from the most painful or the dominant (A) or non-painful or non-dominant (B) side of trapezius (Trap) and tibialis (Tib) anterior muscles.
| Pain thresholds Measure | Region | CWP (n = 17) Mean (SD) | CON (n = 19) Mean (SD) | Statistics (p-value) |
|---|---|---|---|---|
| PPT (kPa) | Trap-A | 227.7 (134.2) | 535.5 (100.8) | 0.000* |
| Trap-B | 219.5 (122.6) | 539.0 (94.3) | 0.000* | |
| Tib-A | 293.2 (144.3) | 576.2 (49.1) | 0.000* | |
| Tib-B | 310.8 (151.3) | 583.8 (45.9) | 0.000* | |
| CPT (℃) | Trap-A | 16.5 (6.3) | 12.5 (4.9) | 0.042* |
| Trap-B | 16.5 (5.4) | 12.6 (5.1) | 0.031* | |
| Tib-A | 14.1 (5.9) | 11.6 (4.3) | 0.151 | |
| Tib-B | 14.5 (5.1) | 11.3 (3.9) | 0.043* | |
| HPT(℃) | Trap-A | 46.7 (2.5) | 48.4 (1.5) | 0.015* |
| Trap-B | 46.6 (1.8) | 46.9 (4.7) | 0.825 | |
| Tib-A | 46.8 (2.2) | 48.1 (1.2) | 0.024* | |
| Tib-B | 46.3 (2.1) | 47.6 (1.6) | 0.043* |
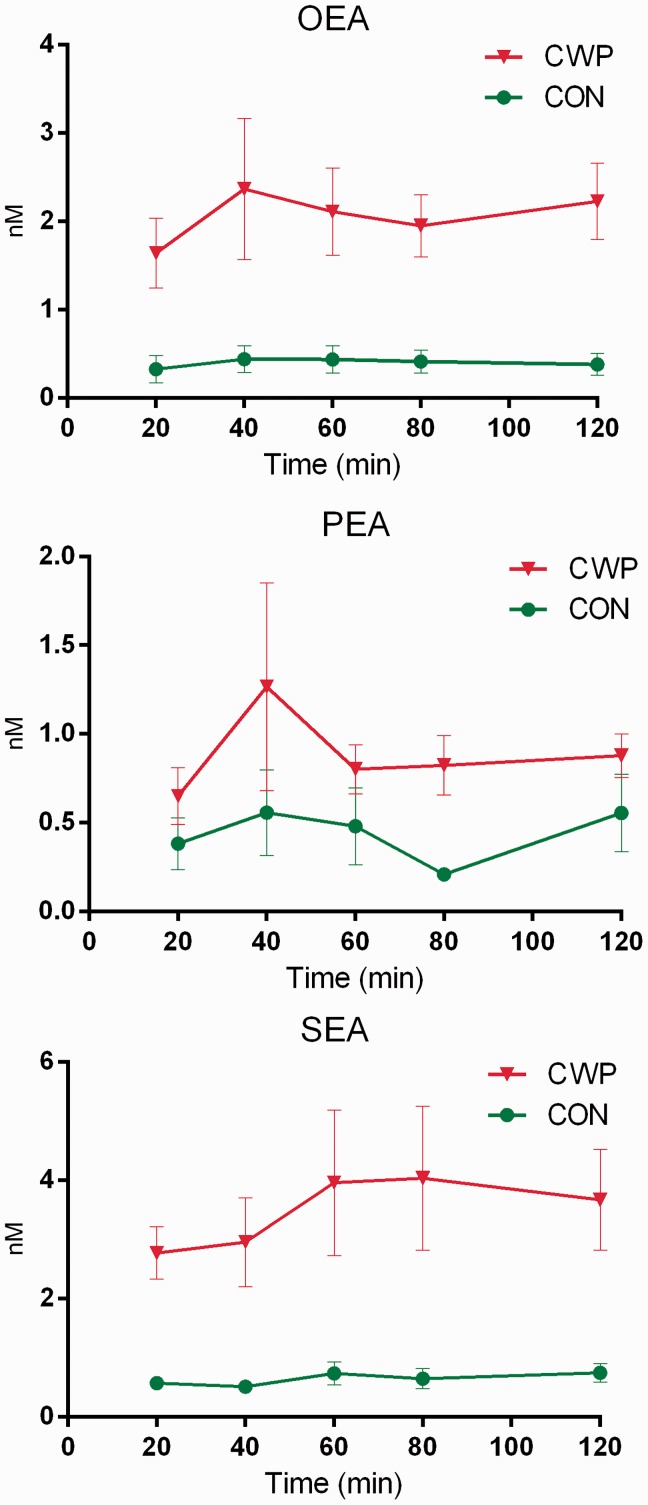
Figure 2.
Mean concentrations (nM) with error bars (SEM) for OEA, PEA, and SEA in microdialysate sampled from the trapezius muscle of women with CWP and healthy female controls during the acute tissue trauma phase (20–120 min) immediately after the insertion of the MD probe.
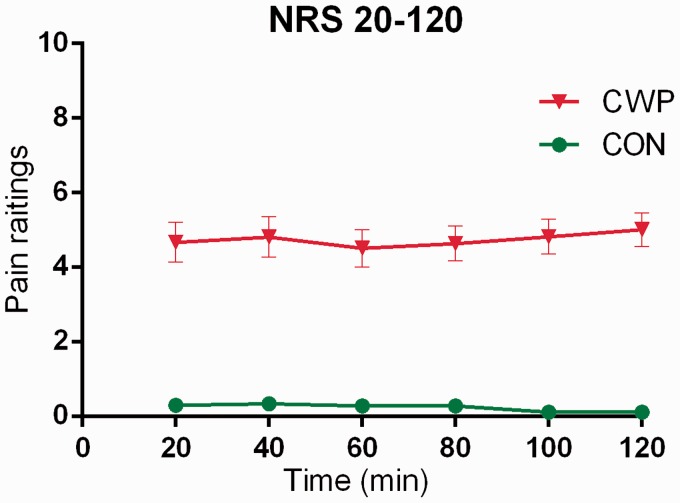
Figure 3.
Pain intensity ratings (0–10; mean ± SEM) during the acute tissue trauma phase immediately after the insertion of the MD probe in women with CWP and CON.
For all MVDA analyses, data were log-transformed when needed (using the SIMCA-P+ function) and scaling to unit variance was applied.42,43 The present study uses principal component analysis to identify multivariate outliers using two powerful methods available in SIMCA-P+. No multivariate outliers were identified.
For MVDA, the obtained components are per definition not correlated and are arranged in decreasing order with respect to explained variation. R2 describes the goodness of fit—the fraction of sum of squares of all the variables explained by a principal component.44
Orthogonal projections to latent structures-discriminant analysis (OPLS-DA) was used for the multivariate regression analysis of group membership (CWP or CON)44 using the mean levels of the investigated lipids alone or in combination with other variables. The variable influence on projection (VIP) indicates the relevance of each X-variable pooled over all dimensions and the Y-variables—the group of variables that best explains Y. VIP ≥ 1.0 was considered significant. Coefficients (OPLS scaled and centred regression coefficients) were used to note the direction of the relationship (positive or negative; in the text reported immediately after the VIP value). Moreover, analysis of variance of cross-validated predictive residuals (CV-ANOVA), which is a diagnostic tool for assessing model reliability, was also computed. CV-ANOVA measures the significance of the observed group separation.42
Results
A total of 43 participants (19 CWP and 24 CON) were recruited in the original study; 17 CWP and 19 CON had sufficient MD volume for analysis and were included in the data analysis. Sample volumes deviating more than 20% from the actual flow set in the MD experiment, were excluded from the analysis. In addition, the number of data points from each subject had to be ≥ 3 (out of five), which resulted in 36 samples from time point 20 min, 34 samples (40 min), 36 samples (60 min), 34 samples (80 min), and 35 samples (120 min) with sufficient volume for the analysis.
The number of subjects in each group, their age, height, weight and body mass index (BMI) are tabulated in Table 1. There were statistically significant (p ≤ 0.05) differences in age and BMI between CWP and CON.
NAE concentrations
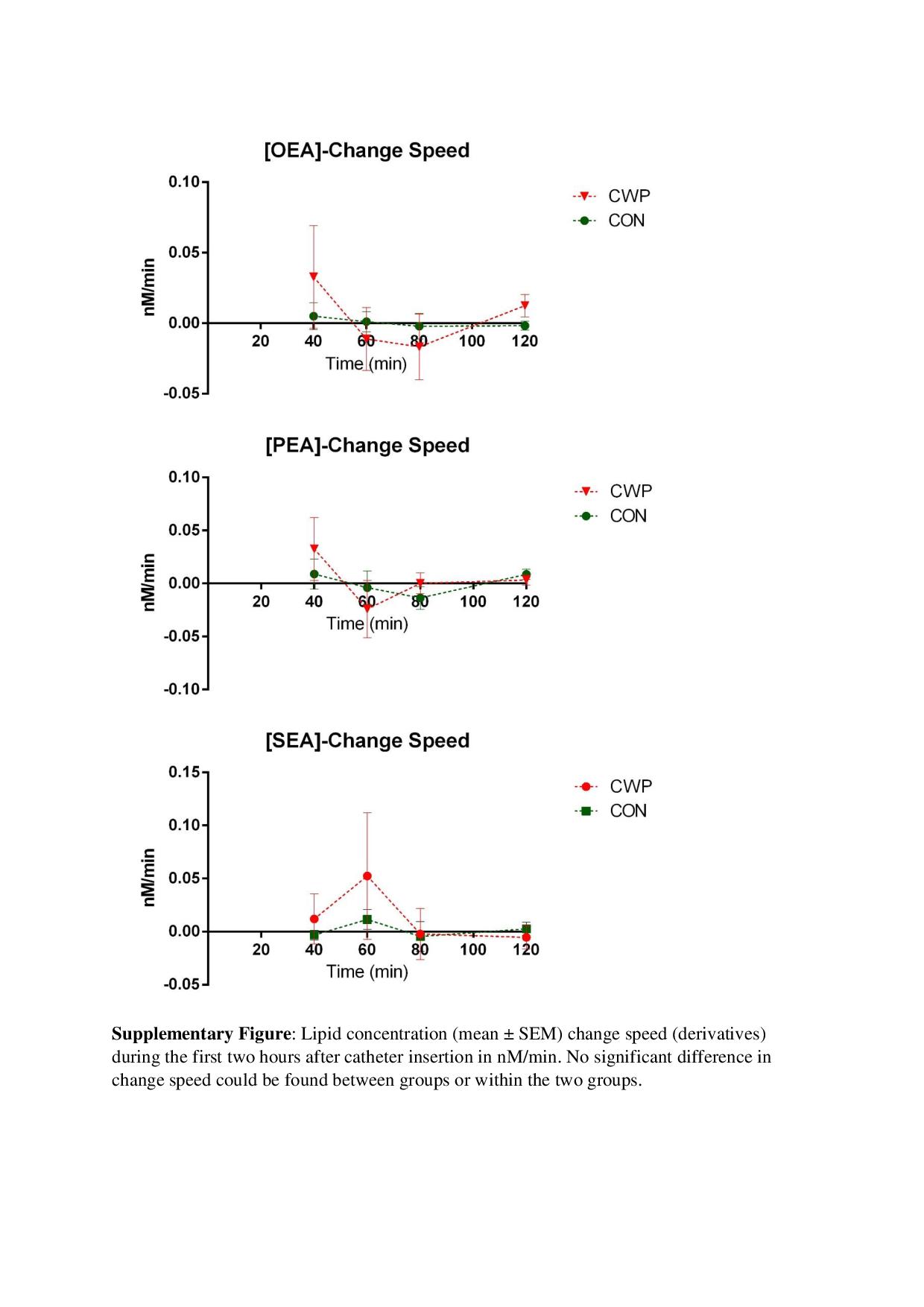
OEA and SEA levels were significantly higher in CWP compared to CON during the tissue trauma period at all time points (Table 2 and Figure 2). CWP also had significantly higher levels of PEA at one time point (80 min) (Table 2 and Figure 2). To confirm these results, a regressions analysis (OPLS-DA) of group membership (CWP or CON) was obtained using the mean values of the lipids as regression parameters (R2 = 0.69, Q2 = 0.65, and CV-ANOVA: p < 0.001). VIP values for the different lipids in this model were 1.08, 1.04, and 0.86 for SEA, OEA, and PEA, respectively. Hence, MVDA confirmed the traditional statistics. No significant changes in levels of NAEs over time could be found in the two groups. In order to evaluate if the rate of change of the lipid concentrations between time points were different, derivatives were calculated and means of derivatives were compared between groups. No significant difference in the rate of change could be found, nor any significant difference in the rate of change was found within the two groups. (Figure of the lipids rate of change exists as supplementary material.)
Pain intensity
Significantly higher ratings of pain intensity during the trauma phase were as expected and, as presented elsewhere35, found for CWP compared to CON (all p < 0.001) (Figure 3). No significant changes in pain intensity over time were found within the two groups.
Pain thresholds
In the pain thresholds comparison, the most painful side in CWP and the dominant side in CON were compared (denoted A in Table 3). Vice versa, the least painful side in CWP was compared with the non-dominant side in CON (denoted B in Table 3). Significantly lower PPTs in both sides of trapezius (Trap) and tibialis (Tib) were found in CWP compared to CON (all p < 0.001) (Table 3). These data have essentially been presented elsewhere.31,35 Significantly higher CPTs were found in three anatomical areas in CWP compared to CON (Trap-A, Trap-B, and Tib-B), p < 0.05) (Table 3). Significantly lower HPT were found in CWP in three areas compared to CON (Trap-A, Tib-A, and Tib-B) (p < 0.05) (Table 3).
Correlation analyses
Correlation analyses were performed between mean levels of the NAEs and pain characteristics (intensity and pain thresholds, i.e., PPT, CPT, and HPT), BMI, and age. When all participants (CWP = CON) were included, significant correlations existed between lipid levels and pain intensity (OEA: r = 0.61, p < 0.01; SEA: r = 0.72, p < 0.01; and PEA: r = 0.41, p < 0.05). Significant negative correlations also existed between OEA and PPTs (Trap-A: r = −0.34; Trap-B: r = −0, 37; Tib-B: r = −0.33, p < 0.05) and between SEA and PPTs (Trap-A: r = −0.37; Trap-B: r = −0.35; Tib-A: r = −0.36; Tib-B: r = −0.33, p < 0.05).
When the two groups were tested separately, no significant correlations were found in CON. In CWP, a significant correlation existed between SEA and pain intensity (r = 0.55, p < 0.05). No significant correlations were found between the three lipid levels and age and BMI in all subjects taken together or in the two groups separately.
The relative importance of the lipids – Multivariate analyses
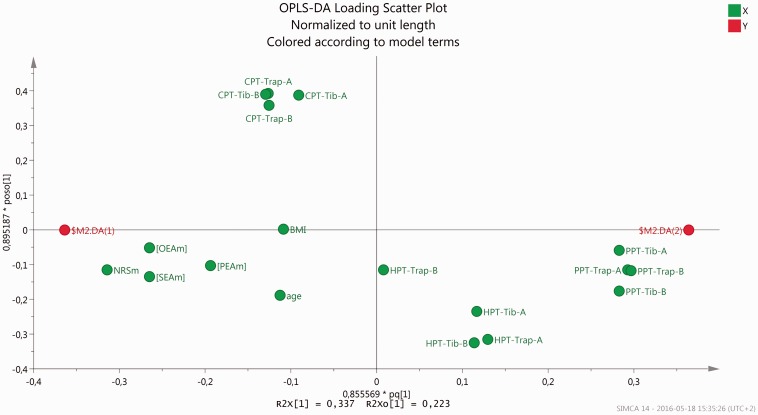
In the following step, mean levels of the NAEs and NRS together with PPT, HPT, CPT, age, and BMI were used for OPLS-DA regression of group membership. A significant regression was obtained (R2 = 0.91, Q2 = 0.88, and CV-ANOVA: p < 0.001). As expected pain intensity was the most important regressor (VIP = 1.40 (+)). Of the pain thresholds the PPTs of the four anatomical sites were the most important regressors (VIP: 1.26–1.33 (−)), but SEA (VIP = 1.20 (+)) and OEA (VIP = 1.18 (+)) were also significant regressors in this context. PEA (VIP = 0.87 (+)), HPTs (VIP: 0.22–0.82 (−)), and CPTs (VIP: 0.83–0.93 (+)) of the four anatomical sites, age (VIP = 0.61 (+)), and BMI (VIP = 0.48 (+)) were not important regressors. Figure 4 shows a loading scatter OPLS-DA plot of mean concentrations of NAEs and NRS together with PPT, HPT, CPT, age, and BMI.
Figure 4.
Loading scatter plot of mean levels of pain intensity and of lipids ([PEAm], [OEAm],] and [SEAm]), PPT, CPT, and HPT values for trapezius (Trap) and tibialis anterior (Tib) (A = most painful/dominant side and B = least painful/non-dominant side) together with age, and BMI (these variables marked in green). $M2.DA (1) (in red) represent the predicted value for the CWP group and $M2.DA (2) (in red) for CON. The approximation to prediction values in the plot is proportional to the contribution of the normalized levels of the parameters in the model. NRS, PPTs, OEA, and SEA contribute more to the model (i.e., graphically located near $M2.DA (1) or $M2.DA (2)) than PEA, age, BMI, and thermal pain thresholds.
Trauma NAE levels in comparison with post trauma
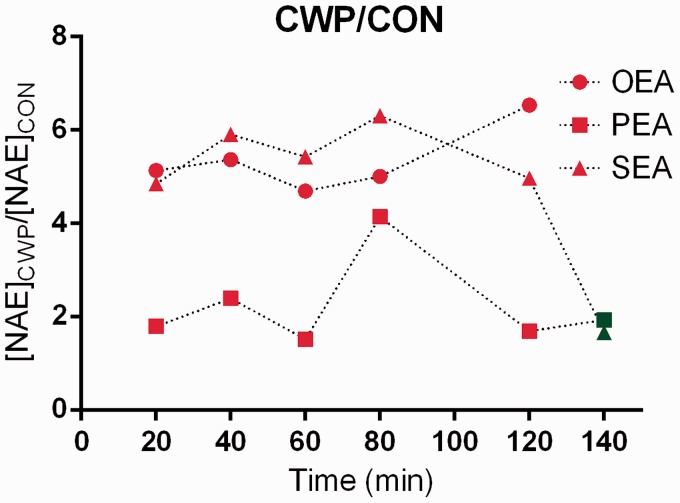
Our novel results clearly indicate that there are differences in the levels of two NAEs in CWP. The results primarily indicate a tonic situation (i.e., no significant changes during the investigated trauma period) with higher levels of SEA and OEA in CWP. However, a different interpretation of the NAE response during the trauma period could not be entirely ruled out. For the same group of subjects, we earlier have reported the levels of SEA and PEA for the time after the trauma period and compared these levels before and after a brief repetitive work period of the shoulders (20 min);31 both substances decreased significantly in CWP. When comparing the trauma period levels with the 140 min level, considerably higher SEA levels were found during the trauma period. However, different analytical methods were used to measure NAE levels in our previous study and this study, so the validity of such a comparison can certainly be questioned. Presumably it is more valid to compare the relative differences (quota: CWP/CON) of the lipid levels throughout the trauma period and after the trauma period (i.e., at 140 min reported in our previous study31) (Figure 5).
Figure 5.
Group quota (CWP/CON) of means of OEA, PEA, and SEA from trauma period (red) and post trauma point (green; data obtained for SEA and PEA from31). A straight line would indicate a static situation during the time period. Time point 80 min for PEA deviates substantially from the other time points which (most likely) suggests it to be an outlier in the context or (less likely) to reflect a different response. The post trauma point (140 min) for SEA deviates substantially from the trauma points (20–120 min) which could indicate it to be an outlier or to reflect a different tissue response to the insertion of MD probe between groups.
According to these calculations, SEA quotas are relatively stable (4.96–6.30) during the trauma period but are substantially lower at time point 140 min, indicating a different response to the insertion of MD probe in combination with higher tonic concentration in CWP. Except for the substantially higher quota at 80 min (4.14), the PEA quotas are stable during trauma and at time point 140 min (1.69–2.39). This finding indicates that PEA responds differently to MD probe insertion compared to SEA in combination with habitually higher levels in CWP. OEA quotas are stable during trauma and no data exist for the following time point for this substance as it was not investigated in our previous study.31
Discussion
This study has two major findings:
Significantly higher levels of OEA and SEA were found in CWP during the first 2 h after the MD probe insertion.
A significant positive correlation existed between SEA and pain intensity in CWP.
We earlier reported from this cohort of CWP (although not with the same number of subjects) significantly lower PPTs both in upper and lower parts of the body.35 The pattern with respect to CPT and HPT shows less prominent group differences and only partly significant differences (Table 3). Thermal pain thresholds mainly reflect nociceptive skin properties while PPT also and mainly reflects deeper tissues, i.e., muscles. Hence, the more substantial differences in PPT compared to thermal pain thresholds differences between groups may indicate that pain input from muscle tissues has a higher impact in CWP than input from skin tissues. As expected both from the inclusion criteria and from earlier studies including these groups of subjects (CWP and CON), we found significantly higher pain intensity but without significant changes over time during the trauma period after MD probe insertion in CWP compared to CON.35
In muscle studies, dialysate samples from the trauma period (1–2 h) have usually been discarded due to the trauma and the subsequent acute inflammation.41,45,46 The dialysate obtained after the trauma period is supposed to reflect the tonic situation of the muscle tissue. The development of catheters has progressed enabling easier and less traumatic catheter insertion; in studies from our group in 2004–2010, we used custom made catheters that required another technique to insert these larger needles. Few studies have investigated the acute tissue effects after probe insertion of certain molecules as in the present study and none have investigated NAEs. Increased serotonin levels in the superficial masseter muscle of healthy subjects were found during the initial 20 min.47 An increase in interstitial interleukin-6 (IL-6) in the trapezius muscle during the first hour has been reported.46 Carson and co-workers reported that for healthy subject’s three amino acids, glucose, and lactate decreased and stabilized within 2 h after probe insertion, while dramatic increases in IL-6 and IL-8 still were present 6 h post MD probe insertion.30
To the best of our knowledge, there are no other studies comparing the molecular responses between chronic muscle pain patients and healthy subjects during the acute trauma period. Although the acute tissue trauma in this study is induced experimentally by MD insertion and is not a natural trauma, it is clinically interesting to study the potential differences in the peripheral response in patients with CWP compared to healthy subjects.
Undoubtedly, there are central alterations in central nervous system in patients with CWP (cf. Introduction). However, there is a growing interest in peripheral factors in CWP and to what extent such factors contribute to maintaining central mechanisms. The level of SEA correlated significantly positively with pain intensity but the explained variation was low (R2: 30.3 %), indicating that other factors (e.g., molecular alterations) also are important when explaining group belonging using peripheral biochemical factors. Thus, measurement of NAE levels alone in a given pain condition will not give a complete picture unless information about other molecules are also provided. As mentioned above, recent studies have reported small fibre pathology in C-fibres in FMS.13,15,16,48 The muscle interstitium (i.e., extra cellular fluid), where nociceptor free nerve endings terminate, can provide information on the local biochemical milieu. Indeed, studies have shown muscle interstitial alterations of the trapezius in CWP (e.g., increased interstitial concentrations of lactate,35,49 pyruvate,49s and glutamate35). The concentrations of glutamate and lactate correlated with pressure pain thresholds (PPTs) of trapezius and pain intensity in CWP.35 Also proteomic studies of the interstitium50 and biopsies51 of trapezius in CWP have shown marked alterations that may indicate activated peripheral nociceptive processes. The causal relationship between such muscle tissue alterations and recently reported alterations in C-fibres is not known and warrants further investigations.
Numerus studies associate various NAEs with analgesia and anti-inflammation. Moreover, these lipids are also associated with feeding behaviour, energy metabolism, and diet. OEA has been associated with feeding and bodyweight24 as well as SEA.52 Both PEA and OEA are associated with energy metabolism.53 A majority of NAEs, including OEA, PEA, and SEA, have been reported to be influenced by diet.54
Here we report levels of lipid mediators associated with pain and inflammation as well as feeding behaviour and energy metabolism. The present study gives support to the conclusion that NAE levels are altered in painful muscle tissue and it also gives some support for a different tissue response to MD probe insertion for SEA and PEA. Although age and BMI were significantly higher in CWP, only very weak non-significant correlations existed between the lipids and these two factors (r = 0.07–0.14), both according to the bivariate correlations and the MVDA (Figure 4). Hence, although dietary factors cannot be ruled out as causal factors for the levels of SEA and OEA, pain characteristics appear to be more important in the cross-sectional perspective of this study.
A strength of the present study is that the levels of lipid mediators were investigated at five time points. Using MVDA, we confirmed the traditional statistical analyses and determined the relative importance of the investigated variables when regressing group membership. One limitation is that dietary factors were not captured. Another limitation is that the levels of inflammatory cytokines were not measured due to limited sample volume. Even though several previous investigations of muscle MD cytokine levels during chronic pain conditions not have revealed any differences on group level (between trapezius myalgia patients and controls55 (IL-6), or between fibromyalgia patients versus controls56 (IL-1β, IL-6, IL-8, TNF)), the MD technique could be a useful tool to investigate the relationship between NAEs and cytokines in vivo. A third limitation is that levels of the lipids during the tissue trauma and after the trauma period were not analysed in the same study.
In conclusion, high levels of NAEs in CWP are probably a result of habitually higher levels compared to CON; however, an altered tissue reactivity in response to MD probe insertion cannot be entirely ruled out. Pain intensity correlated with SEA, but age and BMI did not seem to be important factors for NAE levels. A better understanding of the interplay between these lipids and peripheral pain signaling might provide new therapeutic opportunities for patients with CWP.
Supplementary Material
Acknowledgements
The authors would like to thank Eva-Britt Lind for her technical expertise in conducting MD procedures, Professor Christopher Fowler for his advice and comments, and Håkan Träff for a fruitful discussion of the manuscript.
Author contributions
Design of the study: all authors; microdialysis: NS and NG; biochemical analyses: NS and BGh; statistical analyses: NS and BGe; first draft of the manuscript: NS; comments on drafts of the manuscript: all authors; approval of the final draft: all authors.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Swedish Research Council (K2015-99x-21874-05-4), Swedish Council for Working Life and Social Research (2010-0913), the County Council of Östergötland, and AFA Insurance.
References
- 1.Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17: 547–561. [DOI] [PubMed] [Google Scholar]
- 2.Mansfield KE, Sim J, Jordan JL, et al. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain 2016; 157: 55–64. [DOI] [PMC free article] [PubMed]
- 3.Jensen KB, Loitoile R, Kosek E, et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain 2012; 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijs J, Kosek E, Van Oosterwijck J, et al. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician 2012; 15: ES205–ES213. [PubMed] [Google Scholar]
- 5.Petersel DL, Dror V, Cheung R. Central amplification and fibromyalgia: disorder of pain processing. J Neurosci Res 2011; 89: 29–34. [DOI] [PubMed] [Google Scholar]
- 6.Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 2003; 48: 1420–1429. [DOI] [PubMed] [Google Scholar]
- 7.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep 2008; 12: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain 2002; 99: 49–59. [DOI] [PubMed] [Google Scholar]
- 9.Bradley LA. Pathophysiology of fibromyalgia. Am J Med 2009; 122: S22–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith H, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 2011; 14: E217–E245. [PubMed] [Google Scholar]
- 11.Staud R. The role of peripheral input for chronic pain syndromes like fibromyalgia syndrome. J Musculoskelet Pain 2008; 16: 67–74. [Google Scholar]
- 12.Gerdle B, Hilgenfeldt U, Larsson B, et al. Bradykinin and kallidin levels in the trapezius muscle in patients with work-related trapezius myalgia, in patients with whiplash associated pain, and in healthy controls – a microdialysis study of women. Pain 2008; 139: 578–587. [DOI] [PubMed] [Google Scholar]
- 13.Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain: a journal of neurology 2013; 136: 1857–1867. [DOI] [PubMed] [Google Scholar]
- 14.Staud R, Nagel S, Robinson ME, et al. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain 2009; 145: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oaklander AL, Herzog ZD, Downs HM, et al. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154: 2310–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra J, Collado A, Sola R, et al. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2014; 75: 196–208. [DOI] [PubMed] [Google Scholar]
- 17.Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain 1996; 64: 415–423. [DOI] [PubMed] [Google Scholar]
- 18.Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci 2014; 17: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piomelli D, Hohmann AG, Seybold V, et al. A lipid gate for the peripheral control of pain. J Neurosci 2014; 34: 15184–15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Verme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 2005; 67: 15–19. [DOI] [PubMed] [Google Scholar]
- 21.Solorzano C, Zhu C, Battista N, et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Natl Acad Sci U S A 2009; 106: 20966–20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khasabova IA, Xiong Y, Coicou LG, et al. Peroxisome proliferator-activated receptor alpha mediates acute effects of palmitoylethanolamide on sensory neurons. J Neurosci 2012; 32: 12735–12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LoVerme J, Russo R, La Rana G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Therap 2006; 319: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 24.Fu J, Gaetani S, Oveisi F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 2003; 425: 90–93. [DOI] [PubMed] [Google Scholar]
- 25.Suardiaz M, Estivill-Torrus G, Goicoechea C, et al. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 2007; 133: 99–110. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Miyares RL, Ahern GP. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol 2005; 564: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalle Carbonare M, Del Giudice E, Stecca A, et al. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: a neglected story. J Neuroendocrinol 2008; 20(Suppl 1): 26–34. [DOI] [PubMed] [Google Scholar]
- 28.Berdyshev AG, Kosiakova HV, Onopchenko OV, et al. N-Stearoylethanolamine suppresses the pro-inflammatory cytokines production by inhibition of NF-kappaB translocation. Prostaglandins Other Lipid Mediat 2015; 121: 91–96. [DOI] [PubMed] [Google Scholar]
- 29.de la Pena A, Liu P, Derendorf H. Microdialysis in peripheral tissues. Adv Drug Deliv Rev 2000; 45: 189–216. [DOI] [PubMed] [Google Scholar]
- 30.Carson BP, McCormack WG, Conway C, et al. An in vivo microdialysis characterization of the transient changes in the interstitial dialysate concentration of metabolites and cytokines in human skeletal muscle in response to insertion of a microdialysis probe. Cytokine 2015; 71: 327–333. [DOI] [PubMed] [Google Scholar]
- 31.Ghafouri N, Ghafouri B, Larsson B, et al. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 2013; 154: 1649–1658. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 33.Ohlsson K, Attewell RG, Johnsson B, et al. An assessment of neck and upper extremity disorders by questionnaire and clinical examination. Ergonomics 1994; 37: 891–897. [DOI] [PubMed] [Google Scholar]
- 34.Kuorinka I, Jonsson B, Kilbom A, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 1987; 18: 233–237. [DOI] [PubMed] [Google Scholar]
- 35.Gerdle B, Larsson B, Forsberg F, et al. Chronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasma. Clin J Pain 2014; 30: 409–420. [DOI] [PubMed] [Google Scholar]
- 36.Wallin MK, Raak RI. Quality of life in subgroups of individuals with whiplash-associated disorders. Eur J Pain 2008; 12: 842–849. [DOI] [PubMed] [Google Scholar]
- 37.Raak R, Wallin M. Thermal thresholds and catastrophizing in individuals with chronic pain after whiplash injury. Biol Res Nurs 2006; 8: 138–146. [DOI] [PubMed] [Google Scholar]
- 38.Balvers MG, Verhoeckx KC, Witkamp RF. Development and validation of a quantitative method for the determination of 12 endocannabinoids and related compounds in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 39.Zoerner AA, Gutzki FM, Batkai S, et al. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochim Biophys Acta 2011; 1811: 706–723. [DOI] [PubMed] [Google Scholar]
- 40.Ghafouri N, Ghafouri B, Larsson B, et al. High levels of N-palmitoylethanolamide and N-stearoylethanolamide in microdialysate samples from myalgic trapezius muscle in women. PloS ONE 2011; 6: e27257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosendal L, Larsson B, Kristiansen J, et al. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain 2004; 112: 324–334. [DOI] [PubMed] [Google Scholar]
- 42.Wheelock AM, Wheelock CE. Trials and tribulations of ‘omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol Biosyst 2013; 9: 2589–2596. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson L, Byrne T, Johansson E, et al. Multi- and megavariate data analysis: Basic principles and applications, Third revised edition Malmö: MKS Umetrics AB, 2013. [Google Scholar]
- 44.Eriksson L, Johansson E, Kettaneh-Wold N, et al. Multi- and megavariate data analysis; part I and II, 2 ed Umeå: Umetrics AB, 2006. [Google Scholar]
- 45.Vissing J, MacLean DA, Vissing SF, et al. The exercise metaboreflex is maintained in the absence of muscle acidosis: insights from muscle microdialysis in humans with McArdle's disease. J Physiol 2001; 537: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosendal L, Sogaard K, Kjaer M, et al. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol (Bethesda, Md: 1985) 2005; 98: 477–481. [DOI] [PubMed] [Google Scholar]
- 47.Ernberg M, Hedenberg-Magnusson B, Alstergren P, et al. The level of serotonin in the superficial masseter muscle in relation to local pain and allodynia. Life Sci 1999; 65: 313–325. [DOI] [PubMed] [Google Scholar]
- 48.Doppler K, Rittner HL, Deckart M, Sommer C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain 2015; 156: 2319–2325. [DOI] [PubMed] [Google Scholar]
- 49.Gerdle B, Soderberg K, Salvador Puigvert L, et al. Increased interstitial concentrations of pyruvate and lactate in the trapezius muscle of patients with fibromyalgia: a microdialysis study. J Rehabil Med 2010; 42: 679–687. [DOI] [PubMed] [Google Scholar]
- 50.Olausson P, Gerdle B, Ghafouri N, et al. Identification of proteins from interstitium of trapezius muscle in women with chronic myalgia using microdialysis in combination with proteomics. PLoS ONE 2012; 7: e52560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olausson P, Gerdle B, Ghafouri N, et al. Protein alterations in women with chronic widespread pain–An explorative proteomic study of the trapezius muscle. Sci Rep 2015; 5: 11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terrazzino S, Berto F, Dalle Carbonare M, et al. Stearoylethanolamide exerts anorexic effects in mice via down-regulation of liver stearoyl-coenzyme A desaturase-1 mRNA expression. FASEB J 2004; 18: 1580–1582. [DOI] [PubMed] [Google Scholar]
- 53.Hansen HS. Role of anorectic N-acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharmacol Res 2014; 86: 18–25. [DOI] [PubMed] [Google Scholar]
- 54.Artmann A, Petersen G, Hellgren LI, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta 2008; 1781: 200–212. [DOI] [PubMed] [Google Scholar]
- 55.Rosendal L, Kristiansen J, Gerdle B, et al. Increased levels of interstitial potassium but normal levels of muscle IL-6 and LDH in patients with trapezius myalgia. Pain 2005; 119: 201–209. [DOI] [PubMed] [Google Scholar]
- 56.Christidis N, Ghafouri B, Larsson A, et al. Comparison of the levels of pro-inflammatory cytokines released in the vastus lateralis muscle of patients with fibromyalgia and healthy controls during contractions of the quadriceps muscle – a microdialysis study. PloS ONE 2015; 10: e0143856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials