Abstract
Recent evidence suggests that the development of Alzheimer’s disease (AD) and related cognitive loss is due to mutations in the Amyloid Precursor Protein (APP) gene on chromosome 21 and increased activation of eukaryotic translation initiation factor-2α (eIF2α) phosphorylation. The high level of misfolded and unfolded proteins loading in Endoplasmic Reticulum (ER) lumen triggers ER stress and as a result Unfolded Protein Response (UPR) pathways are activated. Stress-dependent activation of the protein kinase RNA-like endoplasmic reticulum kinase (PERK) leads to the significant elevation of phospho-eIF2α. That attenuates general translation and, on the other hand, promotes the preferential synthesis of Activating Transcription Factor 4 (ATF4) and secretase β (BACE1) - a pivotal enzyme responsible for the initiation of the amyloidogenic pathway resulting in the generation of the amyloid β (Aβ) variant with high ability to form toxic senile plaques in AD brains. Moreover, excessive, long-term stress conditions may contribute to inducing neuronal death by apoptosis as a result of the overactivated expression of pro-apoptotic proteins via ATF4. These findings allow to infer that dysregulated translation, increased expression of BACE1 and ATF4, as a result of eIF2α phosphorylation, may be a major contributor to structural and functional neuronal loss resulting in memory impairment. Thus, blocking PERK-dependent eIF2α phosphorylation through specific, small-molecule PERK branch inhibitors seems to be a potential treatment strategy for AD individuals. That may contribute to the restoration of global translation rates and reduction of expression of ATF4 and BACE1. Hence, the treatment strategy can block accelerated β-amyloidogenesis by reduction in APP cleaving via the BACE1-dependent amyloidogenic pathway.
Keywords: Alzheimer’s disease, amyloid β, APP, BACE1, eIF2α, ER stress, PERK inhibitors, PERK, unfolded protein response
1. INTRODUCTION
Alzheimer’s disease (AD), Parkinson’s disease (PD) and prion disease are individual clinical and pathological disease entities that through common mechanisms induce the death of neurons. AD is the most common progressive cause of dementia, is linked with the accumulation of misfolded proteins [1]. Alzheimer’s disease is a common illness worldwide, with an estimated approximately 24 million people affected [2].
An important risk factor of AD is advanced age [3]. The incidence rate is approximately 1% in the age group 60 – 64 years, but increases significantly up to 24 – 33% in the group of people over 85 years of age. Most cases of AD in elderly people occur sporadically and are not connected with genetic inheritance. Familial Alzheimer’s disease (FAD), which has a molecular genetic basis, accounts for approximately 5% of AD patients [4]. There are two different subtypes of AD based on the age of onset. The first is early-onset AD (EOAD) linked with all cases in the age group from 30 to 65 years. Late-onset AD (LOAD), occurring in patients over 65 years, is the most common subtype of AD. Both EOAD and LOAD cases occur in patients with a genetic predisposition for AD [5].
Alzheimer’s disease is named after Dr. Alois Alzheimer who in the early 1900s first noticed senile plaques in brain samples, which he analyzed in his research. Plaques are comprised mostly of Amyloid β (Aβ). Further research indicated that Aβ is derived from the amyloid β protein precursor (APP). Experiments in vitro showed that fibrils of Aβ play a fundamental role in signal transmission in synapses, plasticity and, most importantly, in memory processes and learning in AD. Afterwards, that significant evidence resulted in formulating the Amyloid Cascade Hypothesis which rapidly became a dogma after detailed investigations [6].
The Amyloid Cascade Hypothesis integrates histopathological and genetic aspects of Alzheimer’s disease. The main hallmark of Alzheimer’s disease is the generation of senile plaques and neurofibrillary tangles. The identification of Aβ as the major component of senile plaques and the latest genetic research, which described mutations in APP, PS1 and PS2, closely associated with the deposition of the pathological form of Aβ, allow to infer that the aggregation of pathological variants of Aβ in the brain parenchyma is the pivotal step leading to Alzheimer’s disease. Disturbances connected with the processing of APP lead to the increased generation of the longer form Aβ molecules consisting of 42 amino acids. Aβ42 is chemically stickier than other lengths and has a good ability to aggregate. Thus, Aβ42 molecules are part of toxic extracellular senile plaques. The Amyloid Cascade Hypothesis explains that pathogenesis of AD is associated with the presence of Aβ42 in senile plaques which are the results of the above-mentioned mutations that finally lead to cell death through the destruction of nerve cells and symptoms of dementia [7, 8].
The analysis of the pathogenesis of neurodegenerative diseases is of increasing importance, given the increasing age of the global population. Current estimates suggest that the prevalence of AD may quadruple by 2050 and dementia may become one of the principal public health issues globally [9], emphasing the need for effective therapies. Genetic mutations in APP cause <60% early-onset FAD. It is associated with about 5% of AD cases. However, up to 80% of AD cases involve inheritance and mutation in genes [3].
The general features of AD include memory loss and aggravation of cognitive function. These conditions have prominent influence on physical activities in the daily life of patients with AD as well as are associated with many kinds of neuropsychiatric disturbances [10]. Moreover, the hallmarks of AD involve language and visuospatial impairment and changes in personality, including, depression and social withdrawal [9]. Synaptic loss and extracellular accumulation of amyloid plaques composed of Aβ and intracellular neurofibrillary tangles consisting of Tau protein are typical of AD [11, 12]. The brain mass is significantly decreased as compared to the normal mass. Essentially, significant changes are associated with brain regions such as the hippocampus and the cerebral and entorhinal cortex. As a result, progressive dementia leads to mental and physical disablement and death [13].
2. GENETIC BASIS OF ALZHEIMER’S DISEASE
AD is characterized by deposition of Aβ plaques and neurofibrillary tangles among the neurons in the brain as well as synaptic degeneration [14], but the etiology of AD is not completely understood. The study of molecular mechanisms is a very effective way to learn more about the pathogenesis of AD [15], because the core of the disorder lies in genetic factors. The main rationale for AD symptoms is the occurrence of 305 mutations in all AD-related genes: 44 of with 30 are strictly associated with AD pathogenesis in a gene encoding Amyloid Precursor Protein (APP) on chromosome 21 (Table 1), 222 in a gene encoding presinilin-1 on chromosome 14 and 39 in a gene encoding presinilin-2 on chromosome 1 [14]. A significant number of the above-mentioned mutations may cause the increased formation of the pathological form of Aβ42 [11]. APP is a transmembrane glycoprotein encoded by a gene located on chromosome 21 consisting of 18 exons. Exons 16 and 17 encode the Aβ peptide. Alternative spilicing may generate variants of Aβ containing the Aβ sequence such as: APP695, APP751 and APP770 [16–18]. Genetic and biochemical investigations confirmed that mutations on chromosome 21 around the β- or γ- secretase cleavage site are the main reason for the production of longer, pathogenic form of Aβ42 specific for FAD, as compared to the shorter physiological variant of Aβ40 [11, 19]. Interestingly, BACE1 cleaves APP with the Swedish mutation 1-100-fold more efficiently than wild type APP [15]. While pathological Aβ can vary significantly in length from 37 to 43 amino acids, the physiological form of Aβ consists of 40 amino acids and is more soluble. In contrast, forms containing 42 to 43 amino acid peptides are characteristically deposited as senile plaques in the brain tissue [20].
Table 1.
AD pathogenic mutations in a gene encoding Amyloid Precursor Protein (APP) on chromosome 21.
| APP Mutation | Genomic Region |
Types of Mutation |
Mutation Type Codon Change |
Biological Effect | |
|---|---|---|---|---|---|
| E246K | Coding Exon 6 |
Point, missense | GAG | AAG | Unknown |
| K496Q | Coding Exon 12 |
Point, missense | AAG | CAG | Unknown |
| Y538H | Coding Exon 13 |
Point, missense | TAT | CAT | Unknown |
| P620L | Coding Exon 14 |
Point, missense | CCG | CTG | Unknown |
| KM670/671NL (Swedish) |
Coding Exon 16 |
Point, double | AAG.ATG | AAT.CTG | Increased total Aβ ; increased production and secre- tion of Aβ 40 and Aβ 42; no change in ratio. |
| A673V | Coding Exon 16 |
Point, missense | GCA | GTA |
In vitro, A673V shifts Secretace Β processing of APP toward the amyloidogenic pathway and in- creases Aβ aggregation; however, co-incubation of mutant and wild-type Aβ inhibits amyloidogenesis and toxicity |
| H677R (English) |
Coding Exon 16 |
Point, missense | CAT | CGT | Accelerated oligomerization kinetics and greater cytotoxicity than wild-type Aβ . |
| D678H (Taiwanesse) |
Coding Exon 16 |
Point, missense | GAC | CAC | Increased secreted Aβ 40 and Aβ 42 |
| D678N (Tottori) |
Coding Exon 16 |
Point, missense | GAC | AAC | Accelerated oligomerization kinetics and greater cytotoxicity than wild-type Aβ |
| E682K (Leuven, Italian) |
Coding Exon 16 |
Point, missense | GAA | AAA | Shifts BACE1 cleavage toward the β-site and causes a significant increase in total Aβ and in Aβ 42/ Aβ 40 levels. |
| K687N | Coding Exon 16 |
Point, missense | AAA | AAT | Reduces APP cleavage by secretase α. Reduced pro- duction of total APPs and especially APPsα. In- creased Aβ 40 and Aβ 42 |
| A692G (Flemish) |
Coding Exon 17 |
Point, missense | GCA | GGA | Increased secreted Aβ 42 and Aβ 40 in CHO, HEK- 293, and H4 cells line; altered APP processing. |
| E693del (Osaka, E693Δ, E693delta) |
Coding Exon 17 |
Deletion | GAA | --- | Reduced Aβ 42 and Aβ 40 with an unaffected ratio |
| E693G (Arctic) |
Coding Exon 17 |
Point, missense | GAA | GGA | Increased propensity of Arctic Aβ 40 to form proto- fibrils and at a faster rate |
| D694 (Iowa) |
Coding Exon 17 |
Point, missense | GAT | AAT | Increased fibrillogenesis of the Aβ peptide; greater Aβ -induced toxicity |
| A713T | Coding Exon 17 |
Point, missense | GCG | ACG | No change in the ratio of Aβ 42/Aβ 40 in postmor- tem brain |
| T714A (Iranian) |
Coding Exon 17 |
Point, missense | ACA | GCA | Unknown |
| T714I (Austrian) |
Coding Exon 17 |
Point, missense | ACA | ATA | Increased Aβ 42/ Aβ 40 ratio; increased Aβ 42, de- creased Aβ 40 |
| V715A (German) |
Coding Exon 17 |
Point, missense | GTG | GCG | Increased Aβ 42/Aβ 40 ratio. |
| V715M (French) |
Coding Exon 17 |
Point, missense | GTG | ATG | Reduced total Aβ production, especially Aβ 40; no change in Aβ 42 |
| I716F | Coding Exon 17 |
Point, missense | ATC | TTC | Increased Aβ 42; decreased Aβ 40 |
| I716T | Coding Exon 17 |
Point, missense | ATC | ACC | Unknown |
| I716V (Florida) |
Coding Exon 17 |
Point, missense | ATC | GTC | Increased Aβ 42(43) and increased Aβ 42(43)/ Aβ 40 ratio |
| V717F (Indiana) |
Coding Exon 17 |
Point, missense | GTC | TTC | Increased Aβ 42/ Aβ 40 ratio |
| V717G | Coding Exon 17 |
Point, missense | GTC | GGC | Increased Aβ 42/Aβ 40 ratio; increased Aβ 42; de- creased Aβ 40 |
| V717I (London) |
Coding Exon 17 |
Point, missense | GTC | ATC | Increased Aβ 42/Aβ 40 ratio by increasing Aβ 42 levels and decreasing Aβ 40 levels |
| V717L | Coding Exon 17 |
Point, missense | GTC | CTC | Increased Aβ 42/Aβ 40 ratio; increased Aβ 42; de- creased Aβ 40 |
| T719P | Coding Exon 17 |
Point, missense | ACC | CCC | Reduced total Aβ in CSF (Cerebro Spinal Fluid), especially Aβ 1–40 and Aβ 1–42 with a relative in- crease in Aβ 1–38. |
| L723P (Australian) |
Coding Exon 17 |
Point, missense | CTG | CCG | Increased Aβ 42 in CHO cells line |
| K724N (Belgian) |
Coding Exon 17 |
Point, missense | AAG | AAC | Increased Aβ 42/Aβ 40 ratio; increased Aβ 42; de- creased Aβ 40 |
Due to the fact that APP is a transmembrane protein, above-mentioned amyloidogenic components are derived from cleavage of APP by β and γ secretases in ER, Golgi apparatus or endosomal-lisosomal pathways [21, 22]. Localisation of these processes confirms the fact that BACE1 possess low pH optimum, and it is mainly localized in intracellular compartments with acidic pH e.g. ER, ER- Golgi intermediate compartment and endosomes, which consequently play the key role in production amyloidogenic Aβ42 [15, 23].
The model of APP processing confirms that post-transcriptional modifications of APP is strictly associated with its movement via the secretory pathway. APP possess signal peptide and it is firstly translocated into the ER where it undergoes maturation processes, where it may be cleaved by secretase β and γ to generate its N-terminally truncated Aβ peptides, which at insoluble form remains within ER lumen and trigger ER stress. Percentage of uncleaved APP molecules can be packed into transport vesicle and transported toward the Golgi network, where β and γ secretase generate Aβ peptide. Then, the molecules are transported to the cell membrane and secreted. Due to the fact that molecules of Aβ42 possess the ability to oligomerisation, Aβ plaque may be generated among the neurons in the AD brains. Alternatively, uncleaved APP can be transported to the cell membrane and cleaved by α secretase in non-amyloidogenic pathway [24].
Aβ42 was first detected in cerebral blood vessels from AD and Down’s syndrome (DS) patients [16]. Individuals with DS have the triplication of chromosome 21 which is strictly connected with the overexpression of APP. The fact may increase the risk of the development of early-onset AD [25]. The overexpression of the APP gene leading to the disruption of the endocytic system resulting in increased turnover from the cellular surface, which may be associated with the increased frequency of APP encountering enzyme BACE1 and being cleaved via the amyloidogenic pathway.
That results in large number of intracellular fragments of Aβ42 generated in the DS brain and formation of diffuse plaques. That theory confirms that the over-expression of APP is the main factor of AD pathology in the DS individuals [26]. Remarkably, that fact suggests that Aβ42 plays the central role in the pathogenesis of AD. The Aβ peptide is produced during the endoproteolysis of APP which is the type-I transmembrane glycoprotein located in numerous mammalian cell types [27]. In FAD, increased synthesis of the abnormal form of Aβ occurs many years before the symptoms of AD appear [11]. Moreover, this process contributes to neurodegeneration by increasing the risk of having Endoplasmic Reticulum stress by disrupting the signalling pathway of the Unfolded Protein Response (UPR) [14]. Medicinal therapies whose main aim is to reduce the quantity of Aβ42 in brain tissue are regarded as promising treatment and prevention of dementia [15].
3. GENERATION OF THE AMYLOID β PEPTIDES
The cleavage of APP is regulated by three proteases: α, β and γ secretases [28], which are all part of the family of proteolytic enzymes [27]. Secretase α belongs to the ADAM (disintegrin and metalloprotease domain) family of proteases [29]. Secretase β is commonly termed β-site APP - cleaving enzyme 1 (BACE1) [30]. It is the 501 amino acid type I transmembrane aspartic protease [15] which represents the most important enzyme associated with the production of the longer form of Aβ42 [31]. For a long time BACE1 was the subject of intense research because of its potential capability as a drug target for neurodegenerative diseases such as AD. Before the discovery of enzyme, the properties of BACE1 activity in cells had been precisely characterized [32]. Numerous studies were undertaken to gather knowledge to help in the identification of secretase β properties [15]. In 1999, five groups of researchers described the molecular cloning of secretase β which differed only in name: BACE, β - secretase, Asp2 or memapsin2 [32]. Many types of human tissue exhibit the activity of secretase β [33]. Moreover, the neuronal tissue demonstrates the highest activity [15]. Therefore, it could be assumed that secretase β preferentially cleaves membrane bound substrates [34]. Furthermore, BACE1 is mainly located in intracellular compartments with acidic pH, for instance, trans - Golgi and endosomes [15, 29].
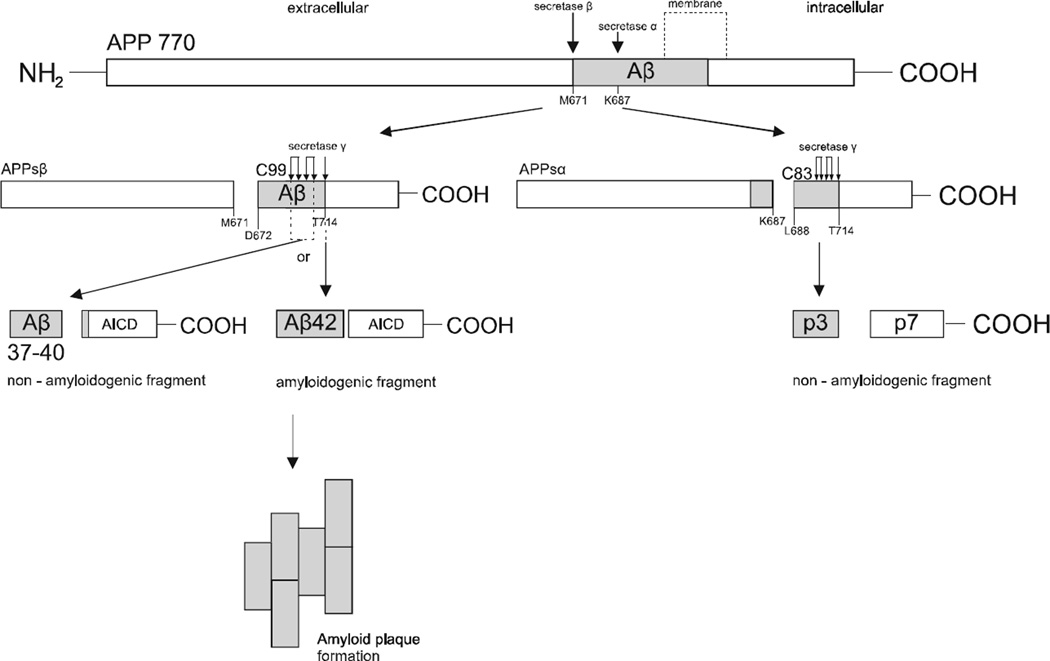
There are two different pathways involved in the cleavage of APP. Before cleaving by secretase γ, APP is first processed by the α or β secretase [29]. The physiological molecular pathway responsible for the endoproteolysis of APP is closely connected with cleaving APP by secretase α in the middle of the Aβ sequence which prevents the generation of full-length Aβ42 [27]. It occurs specifically at K687/L688 and releases two molecular products [35]: the soluble N-terminal part of APP, called APPsα, consisting of 105 −125 kDA, which is transferred to the extracellular environment [27], and the C-terminal fragment C83 (688–770), which is next cleaved by secretase γ. As a result, fragment p7 and non-amyloidogenic fragment called p3 are generated and released [35], thereby resulting in cleavage by secretase α in the non-amyloidogenic pathway, which precludes the generation of the pathological form of Aβ42 and thus cannot lead to AD [27].
Since it was first recognized that Aβ is the product of cleavage by APP proteolysis, significant data has been gathered concerning the properties of secretase β activity in cells and tissues [11]. Generally, nowadays it is clear that the second way is strictly connected with secretase β, which is responsible for cleaving APP at M671/D672 into two different parts. The process releases ectodomain APPsβ and membrane-bound C99 (672–770). In turn, the second fragment is the substrate for secretase γ which imprecisely cleaves C99 fragment at specific sites such as: G708/G709 (Aβ37 generation), G709/V710 (Aβ38 generation), V711/I712 (Aβ40 generation), A713/T714 (Aβ42 generation) [21, 36]. Consequently, it undergoes cleaving which releases Aβ peptides and an additional fragment - the carboxyl terminus of Aβ called AICD (APP intracellular domain) [35, 37]. Cleaving by secretase γ is essential for the genesis of AD, since it may generate Aβ peptides of different lengths, including the pathogenic form of Aβ42 (Fig. 1) [15].
Fig. (1).
Processing of Amyloid Precursor Protein (APP) by α, β and γ secretases. The proteolytic cleavage in non-amyloidogenic pathway by α secretase within the Aβ sequence and γ secretase resulting in the formation of soluble APP fragments: APPsα, p3 and p7. The APP processing by secretases β and γ in the amyloidogenic pathway leads to the formation of C-terminal APP domain AICD and soluble Aβ37–40 or insoluble Aβ42, which depends on the γ secretase cleaving site. Aβ42 aggregates into amyloid plaques in the brain tissue.
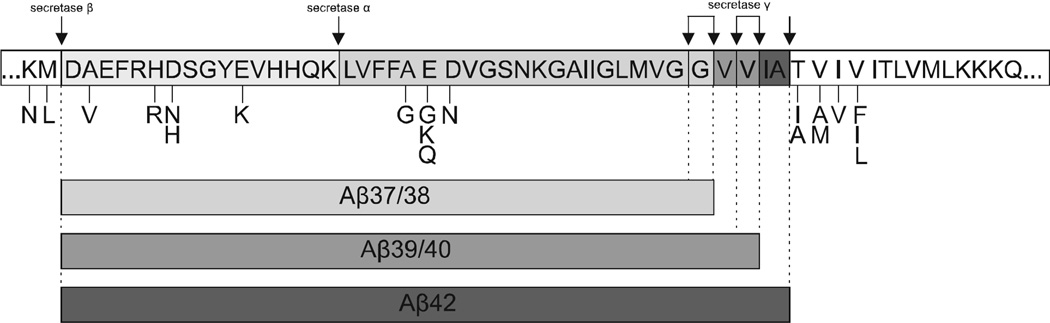
Mutations connected with chromosome 21, where the APP gene is located, are the crucial cause of increasing the longer, abnormal type of Aβ whose characteristic feature is its ability to extracellularly aggregate in the brain [35]. Changes in the nucleotide sequence which lead to FAD include mutations which lie within or near the Aβ domain for instance: Dutch, Flemish, London, Indiana, Swedish, Florida, Iowa and Arctic among others [8]. The mutation at the cleavage site for secretase β leads to the increased number of APP cleavage via the amyloidogenic pathway. Mutations near the cleavage site for secretase γ alter the APP processing, which results in the higher generation of the Aβ42 variant with ability to aggregate. Moreover, mutations located in the middle of Aβ domain are closely associated with a significant decrease in APP processing by secretase α and thereby promote the amyloidogenic pathway. These mutations can also promote Aβ aggregation and modify degradation by other proteases [38–40].
For example, the Swedish double mutation (KM670/671NL) near the cleavage site resulting in substitution of two amino acids: lysine [41] and methionine [42] to asparagine [43] and leucine [44], which as a consequence leads to the enhanced efficiency of secretase β cleaving. In turn, many kinds of other substitutions at those surrounding positions reduce APP cleaving in the non-amyloidogenic pathway and indicate that BACE1 is a very specific enzyme [45], since its processing occurs mainly at M671/D672 (between position 0 and 1 in Aβ) and G680/Y681 (between position 10 and 11 in Aβ) [15, 46]. The London point, missense mutation (V717I), is linked to significant alterations in γ secretase cleaving. As a result, levels of senile plaques composed of Aβ42 and neurofibrillary tangles are significantly higher [8] Pike et al. indicate that Aβ42 becomes highly toxic for neurons during its aggregation [47] (Fig. 2, Table 2).
Fig. (2).
Schematic representation of APP point mutations sequence affecting secretase activities. Pathogenic amino acids substitutions, which are identified near or within the APP cleavage site by secretases, shift the APP molecular processing toward the amyloidogenic pathway. That leads to overall increase in total Aβ generation and/or alterations in the ratio of specific Aβ peptides.
Table 2.
Common mutations of APP affecting secretase activities with giving the site of mutation and amino acid change.
| APP Mutation | Amino Acid Change | Site of Mutation (APP Amino Acid Number) |
|
|---|---|---|---|
| Swedish | K > N M > L |
Lysine > Asparagine Methionine >Leucine | 670 671 |
| DN | A > V | Alanine > Valine | 673 |
| English | H > R | Histidine > Arginine | 677 |
| Tottori | D > N | Aspartic acid > Asparagine | 678 |
| Taiwanese | D > H | Aspartic acid > Histidine | 678 |
| Leuven (Italian) | E > K | Glutamic acid > Lysine | 682 |
| Flemish | A > G | Alanine >Glycine | 692 |
| Arctic | E > G | Glutamic acid > Glycine | 693 |
| Italian | E > K | Glutamic acid > Lysine | 693 |
| Dutch | E > Q | Glutamic acid > Glutamine | 693 |
| Iowa | D > N | Aspartic acid > Asparagine | 694 |
| Austrian | T > I | Threonine >Isoleucine | 714 |
| Iranian | T > A | Threonine > Alanine | 714 |
| German | V > A | Valine > Alanine | 715 |
| French | V > M | Valine > Methionine | 715 |
| Florida | I > V | Isoleucine > Valine | 716 |
| Indiana | V > F | Valine > Phenylalanine | 717 |
| London | V > I | Valine > Isoleucine | 717 |
| --------- | V > L | Valine > Leucine | 717 |
4. ENDOPLASMIC RETICULUM STRESS AS AN ACTIVATOR OF THE UPR SIGNALLING
The endoplasmic reticulum (ER) is a dynamic membrane system of enclosed sacs and tubules and is the primary site for the maturation of secretory proteins. Structurally, ER is made up of different domains that include the nuclear envelope (NE), rough and smooth ER, and parts of which contact other organelles. The ER has many functions including: synthesis and export of proteins across the ER membrane, embedding proteins into other membranes and synthesis of phospholipids, storage of calcium ions within the lumen and regulating the ions release. The mechanism of folding proteins and modification within the ER is significant in the pathogenesis of AD [48]. The lumen of the ER is crowded with chaperones and enzymes that are specialized in protein processing. Furthermore, ER has a control system which plays a vital role in providing the selective transport of only correctly folded proteins and export of misfolded proteins for ubiquitin-dependent proteolytic degradation in the cell cytosol. The recognition of substrates by molecular chaperones and associated factors and their retrotransolocation to the cytoplasm is known as the ER - associated protein degradation (ERAD) pathway [49].
Disturbances in function or loss of integrity of the ER lead to ER stress. There are ample evidences which show that ER stress in neurons may be a pivotal contributor to AD pathogenesis. Stress conditions are strictly link to deposition of misfolded and unfolded proteins and changes in calcium homeostasis within the ER lumen, which are the characteristic hallmarks in many neurodegenerative disease. The native monomeric proteins are generally composed of α-helix, since the main feature of misfolded proteins is the occurrence of β-sheet confirmation [50, 51]. The increased deposition of pathological forms of proteins occurs as a result of transcription and translation disturbances, genetic mutations and cellular stresses [50], for instance, dysregulation of cellular redox caused by hypoxia, deficiency of cellular glucose level strictly associated with N-linked proteins glycosylation within the ER, alterations in calcium homeostasis, viral infections and high-fat diet [15]. Furthermore, the intracellular calcium concentration and calcium release form the ER lumen are significant in interactions between ER and mitochondrion as well as play the pivotal role in cell death controlling [52].
Research by O’Connor et al. confirms that longer, toxic variants of Aβ possess the ability to induce ER stress and eIF2α phosphorylation. APP transgenic mice without any cellular stress had increased levels of phosphorylated eIF2α and BACE1 which confirms the fact that disturbances in metabolism may induce UPR branches and as a result eIF2α phosphorylation, and increase the BACE1 level, which may initiate AD pathogenesis. However, when the critical concentration of Aβ with ability to aggregate is reached, the reaction becomes self-sustaining and AD progression is enhanced [15, 53].
The characteristic feature of the intrinsic environment of the ER is the occurrence of numerous calcium-dependent proteins, for instance, glucose-regulated proteins, which belong to the family of GRP molecules. Due to that fact, disturbances connected with calcium concentration may contribute to unfolded and misfolded protein accumulation within the ER lumen [54] which reduces chaperon activity, hence leading to the misfolding and unfolding of proteins and activation of specific cell adaptive molecular branches [50]. Interestingly, APP may affect calcium ion channels. Mutations in the APP gene are known as calcium oscillations due to reduction in APP translocation to the outer membrane. Moreover, the high concentration of calcium in the cell cytosol, which is a specific hallmark of AD, leads to the attenuation of non-amyloidogenic cleavage of APP by secretase α and as a result, aggregation of the neurotoxic variant of Aβ42 [55, 56]. Moreover, Aβ molecules may contribute to release of calcium ions into the cytoplasm from ER stories [57].
When the ER homeostasis is disrupted, as a response to ER stress, cells activate signalling pathways called as the Unfolded Protein Response (UPR). The primary function of the UPR is to restore secretory and cellular homeostasis by the inhibition of protein translation, refolding and/or degradation of the misfolded protein molecules and increase in the synthesis of chaperones which first activates the UPR pro-survival branch to rebalance the ER function by activating the adaptive programs which allow the cells to deal with the accumulation of abnormal proteins via the attenuation of general protein synthesis and switching on the expression of specific genes responsible for enhancing protein folding mechanisms as well as genes for ER-assisted degradation (ERAD). The response to ER stress stimuli allows to prevent the accumulation of pathological proteins within the ER lumen through exporting them to cytosol for degradation [50, 57]. In contrast, intensive or prolonged ER stress will evoke apoptosis [58]. Recent research results indicate that ER stress is linked to genetic and neurodegenerative diseases. Besides, huge progress has been made in science which focuses on the association of FAD and the activation of pathways involving the Unfolded Protein Response inside the neurons [59].
There is a body of evidence suggesting that the occurrence of AD mutations is closely linked to ER stress and as a result, an increased amount of phosphorylated eukaryotic initiation factor-2α (eIF2α), BACE1 and ATF4 in the brain tissue of AD patients [60]. Furthermore, study by Hoozemans et al. shows that UPR is activated in AD individuals, since the presence of misfolded proteins and its aggregation in ER has ability to trigger the UPR signalling pathways, since the levels of GRP proteins, which are up-regulated in stress conditions, was increased especially in AD temporal cortex and hippocampus. Levels of phosphorylated PERK, and eIF2α,was increased in the AD brains, mainly in hippocampal neurons. Altogether, that research indicate that UPR signalling can play the neuroprotective role in early AD, but long-term activation of UPR branches can lead to initiate or mediate AD pathogenesis [61]. Hoshino et al. reported that increased levels of chaperones may suppress the generation of Aβ peptides. Moreover, ER stress causes increased expression of BACE1, hence triggers APP processing in ER [62].
After the accumulation of misfolded and unfolded proteins within the ER lumen that triggers ER stress and UPR activation, the general protein synthesis is attenuated, but the translation of specific proteins such as ATF4 and BACE1 is enhanced. The transition between AD-related mutations and eIF2α phosphorylation is confirmed by the significantly increased levels of BACE1 and the fact that its activity is elevated roughly twofold in AD individuals. Several lines of evidence suggest a pivotal role of BACE1 in AD, since level and activity in the post mortem AD brains are significantly increased [63]. Occurrence of Swedish mutation, which promotes APP cleavage through the amyloidogenic pathway suggests that the higher level of phosphorylated eIF2α and, as a result, BACE1 may be a significant cause of the initiation or acceleration of AD pathogenesis. Moreover, other stress stimuli, such as disturbances in glucose metabolism, closely associated with AD pathogenesis, have ability to activate the UPR signalling pathways elevate eIF2α and BACE1 levels in AD brains. Velliquette et al. reported that disrupted glucose metabolism in APP transgenic mice resulted in an increased level of BACE1 and as a result elevated toxic form of Aβ around twofold [64]. Furthermore, O’Connor et al. reported that the glucose deficiency caused the increased level of BACE1 as a result of the activation of PERK-dependent signalling branches. Treatment with AD model mice inhibitors of energy metabolism triggered ER stress conditions, activated the UPR and contribute to increased levels of phosphorylated eIF2α, BACE1 and the toxic form of Aβ which are the main components of senile plaques accumulated in the AD brains [53].
5. THREE PRIMARY EFFECTORS OF THE UPR
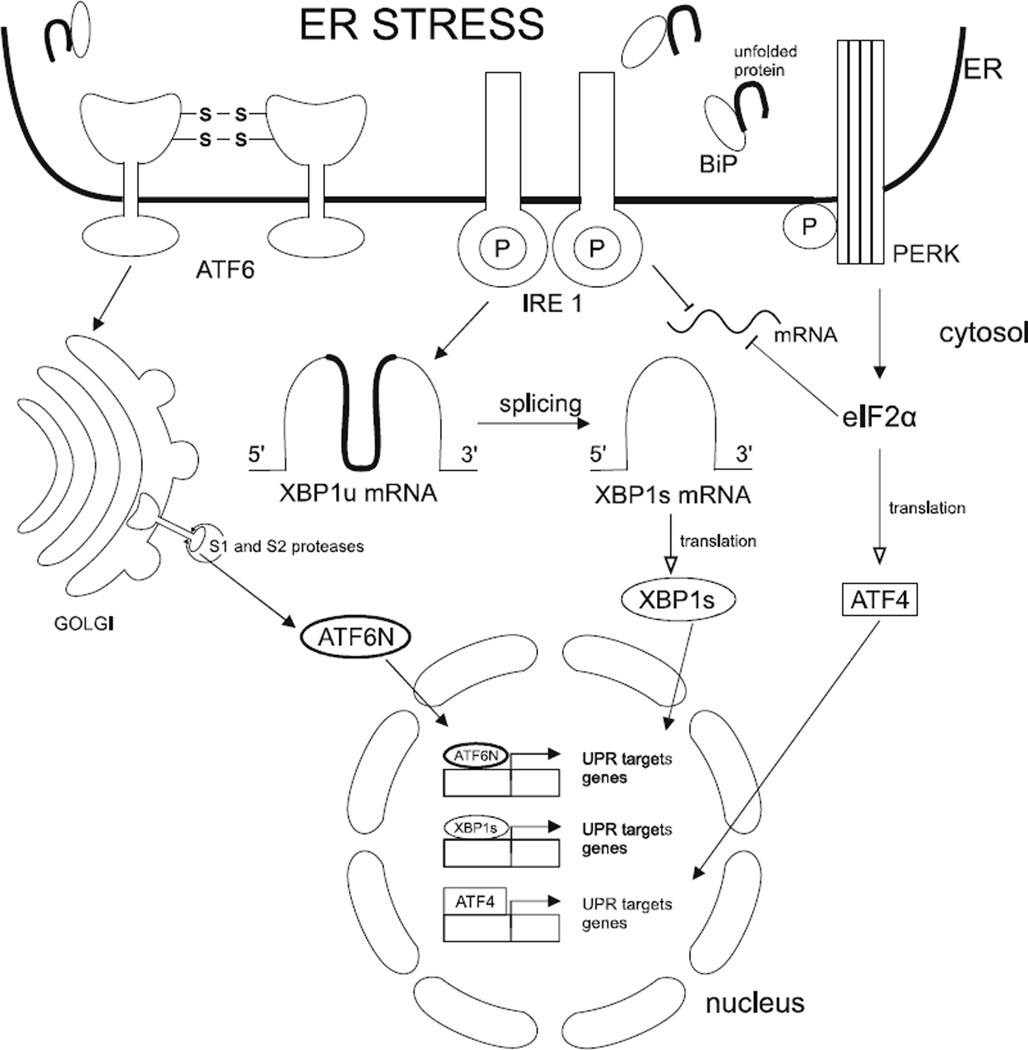
Mammalian cells have three transmembrane ER stress signalling molecules that are responsible for regulating the UPR pathways [59]. These include: protein kinase RNA - like endoplasmic reticulum kinase (PERK), which is closely associated with the pathogenesis of AD; Inositol Regulating Enzyme 1 (IRE1); Activating Transcription Factor 6 (ATF6). All of them are activated in response to high levels of unfolded and misfolded proteins within the lumen of the ER [65]. During cell homeostasis, the above-mentioned sensors remain inactive, thanks to binding to, among others, the molecular chaperones termed Binding immunoglobulin protein (BiP). As a result of high levels of unfolded and misfolded proteins within the ER lumen, BiP dissociates from the transmembrane receptors and allows them to oligomerisation and autophosphorylation and, as a result, they become active [66].
IRE1 is responsible for the rapid repression of mRNAs located in the ER, which causes decreased protein loads within the ER. IRE1 also catalyzes the splicing of XBP1u mRNA into XBP1s mRNA. XBP1s and ATF6 are responsible for controlling the adaptive response. The main aim of this process is to increase the transcription of chaperone proteins residing in the lumen of the ER. Chaperones enable modification of other protein molecules. Moreover, that leads to increased lipid biosynthesis to enhance the capacity for folding and degradation activities of the ER [59].
PERK is a transmembrane protein located in the ER membrane. There is a strong resemblance between PERK and the IRE1: cytoplasmic kinase domain - the serine/threonine kinase and the N-terminal luminal domain [59]. Serine/threonine kinase is a part of the cytoplasmic domain which is responsible for receptor’s activation associated with high catalytic activity [65].
Moreover, PERK and IRE1 are activated through similar mechanisms [67]. The process requires oligomerization of monomers into dimers or higher structures. Oligomerization occurs when the level of unfolded and misfolded proteins within the ER is sufficiently high to titrate the ER chaperone, BiP, away from PERK/IRE1/ATF6 monomers, thereby permitting oligomerization and activation, which initiates the process of protein-folding homeostasis restoration [59]. (Fig. 3)
Fig. (3).
Three branches of UPR signalling from the Endoplasmatic Reticulum. In response to accumulation unfolded and misfolded proteins within ER lumen that trigger ER stress BiP chaperones release the luminal domain of ER transmembrane transducers such as PERK, IRE1, ATF6. That allows PERK and IRE1 to oligomerise and trans-autophosporylate, which result in their activation. Also ATF6 is regulated by chaperones BiP which hinder its relocation to the Golgi, where it is processed by specific proteases Site-1 (S1P) and Site-2 (S2P) and, as a result, becomes active as a monomeric ATF6N. Activated PERK phosphorylates eIF2α resulting in global translation attenuation apart from selective mRNAs e.g. ATF4. ATF6N, XBP1s and ATF4 translocates to the nucleus where upregulates expression of UPR target gene to restore cells homeostasis.
6. MECHANISM OF ACTIVATION PERK-DEPENDENT UPR SIGNALLING PATHWAY
It has been reported that PERK and eukaryotic initiation factor-2α (eIF2α) are closely associated with pathological mechanisms occurring within neurons in patients afflicted with AD [68]. eIF2α consists of three general parts; they are subunits α, β and γ. eIF2α binds two elements like guanosine-5’-triphosphate (GTP) and initiator methionyl - tRNA (Met-tRNAiMet) and is responsible for transferring Met-tRNAiMet to the 40S ribosomal subunit.
After the initiation of the translation process, GTP is hydrolysed to GDP. Then guanine nucleotide exchange factor −2β (eIF2β) plays the crucial role during the exchange of GDP for GTP. This exchange is required to rebuild a new complex of eIF2α and subsequently activates the next round of translation initiation [45]. The presence of diverse stress conditions, which includes ER stress, activates the survival pathway known as the UPR pathway and is connected with the modulation of factor eIF2α [69]. PERK, activated after the accumulation of abnormal proteins within the lumen of the ER, gains the ability to phosphorylate the eIF2α on its subunit α at serine 51. From this moment, the exchange from eIF2α - GDP to eIF2α - GTP is impossible, since this process arrests the formation of the 43S translation initiation complex [65]. This results in the repression of general translation initiation and activates the transient attenuation of cellular protein synthesis [46].
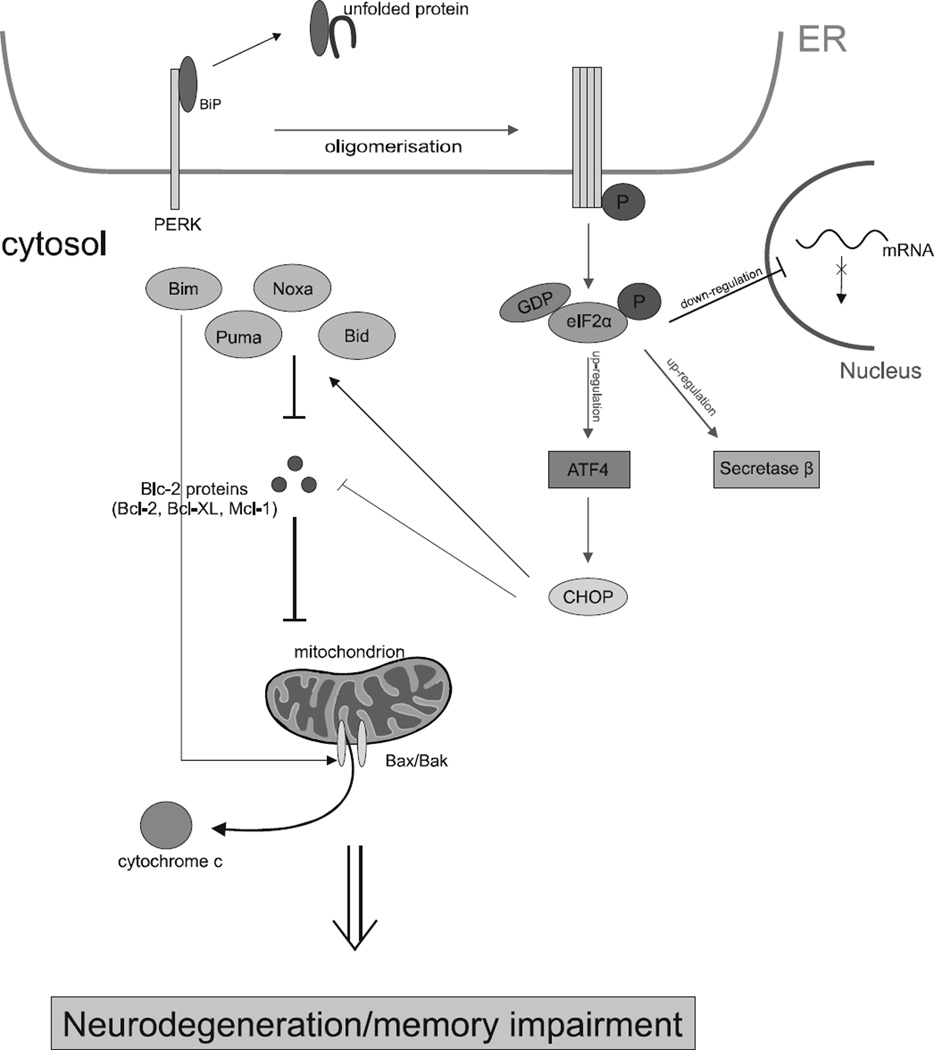
In opposition to the phosphorylation of eIF2α supressing the general translation, it also causes an increase in the preferential translation of a subset of messenger RNAs that encode a few short upstream open reading frames (uORFs) which include secretase β [70, 71]. This is the most important enzyme associated with the production of the longer form of Aβ [60] as well as ATF4 that is associated with the development of AD [72]. The levels of eIF2α with phosphorylated subunit α are significantly higher in the AD brains and positively correlate with expression levels of BACE1 and Aβ plaque aggregation [53]. These phenomena reveal that overactivated phosphorylation of eIF2α and may be the main cause of memory impairment associated with AD pathogenesis not only by arresting the general protein synthesis, but also by increasing the production of Aβ42 through secretase β [60] (Fig. 4).
Fig. (4).
Mechanism of UPR-induced apoptosis in Alzheimer’s disease. Multiple signalling pathways of the UPR are activated by PERK kinase by dissociation of BiP proteins after the accumulation of unfolded proteins within the lumen of the ER. This dissociation causes PERK to undergo homo-oligomerization and trans-autophosphorylation within its cytosolic kinase domain. The activated UPR network results in the phosphorylation of eIF2α and, consequently, in the sustained inhibition of general translation, while it leads to up-regulation of mRNAs BACE1 and ATF4. Long-term stress conditions may induce neuronal death by mitochondrial apoptosis regulated by the family of the anti-apoptotic and pro-apoptotic proteins. This results in neurodegeneration and memory impairment associated with AD.
7. NEURONAL DEATH CASCADES DURING APOPTOSIS
On the contrary, in response to genetic disturbances and environmental risks for AD, ER stress may activate the UPR pathways as an adaptive cellular reaction and during chronic stress conditions, induces neuronal loss by apoptosis [73]. Taken together, in non-physiological conditions, the UPR mechanism is insufficient to sustain homeostasis within the lumen of the ER. This is the main consequence of cell death by apoptosis [59]. This was thoroughly investigated by researchers and, since it is crucial for tissue homeostasis, it provides a lot of information about disease pathogenesis. This also offers clues on how to treat many kinds of illnesses such as neurodegenerative diseases and cancer [74].
Apoptosis is characterized by three general biochemical changes. These are: caspase activation, destruction of DNA, synthesis of intracellular proteins and outer mitochondrial membrane changes which are the essential cause of the release of proapoptotic molecules, like cytochrome c in the cytoplasm, which is responsible for activating caspases [75]. It is strictly associated with the intracellular protein family Bcl-2, which is divided into two different groups. The first is the proapoptotic molecules containing the apoptosis promoters. The crucial members of this group are Bax (Bcl-2 associated protein X) and Bak (Blc-2 antagonist killer) [74]. The latter group is comprised of antiapoptotic proteins such as Bcl-2, Bcl-W, Bcl-1 and Mcl-1. The difference between them is that antiapoptotic molecules inhibit the release of cytochrome c from the mitochondrion to the cell cytosol and the proapoptotic proteins promote that process [76]. The long-term activation of the PERK/eIF2α/ATF4 signalling pathway resulted in the upregulation of the proapoptotic transcription factor CHOP, which down-regulates the antiapoptotic Blc-2 proteins and, on the other hand, induces the expression of BH3-only proteins. Inversely, during ER stress, the expression of CHOP is upregulated by ATF4 [77] after the phosphorylation of eIF2α by PERK [72]. There is crucial evidence that ATF4 has a negative influence on memory processes. It allows to conclude that high levels of phosphorylated eIF2α by PERK and, as a result, ATF4 and CHOP are closely correlated with one another in AD and in disease conditions that can account for cognitive impairment [78].
In addition, some researchers indicate that there is a third group of proteins activated in response to disturbances in ER homeostasis - BH3-only proteins for instance: Bid, Bad, Bim, Noxa and Puma [76]. They might bind to anti-apoptotic Bcl-2 proteins and repress the death-inhibiting molecules. Proteins that belong to the first group such as Bax and Bak cause the flow of cytochrome c to the cytosol from mitochondria through the outer destroyed membrane [75]. They may also by activated by other proteins like Bid, which belongs to BH3-only proteins [79]. ER stress, initiated by stress inducers, for instance tunicamycin, is the stimulus for the BH3-domain-only which causes the homooligomerization process of multidomain proapoptotic proteins: Bak and Bax. Those homodimers are formed on the outer membrane of the mitochondrion, which triggers permeabilization resulting in cytochrome c being released. Consequently, the process described above leads to rapid neurodegeneration and, as a result, it is significantly decreased in AD brains [80].
8. PERK AS A THERAPEUTIC TARGET FOR ALZHEIMER’S DISEASE
Neurodegenerative diseases represent the principal public health problems in nations worldwide, since there is no available cure for this kind of diseases [81]. Currently, there is no known effective treatment other than symptom-based, which includes administration of acetylocholinesterase inhibitors that can slow down the progression of AD but not completely arrest it [82]. Although the molecular mechanisms of AD are not totally understood, recent evidence suggests that molecular alteration in translational machinery through phosphorylation subunit α in eIF2α may play the key role in the pathogenesis of neurodegenerative diseases [59, 68].
There is abundant evidences which show that eIF2α plays a pivotal role in the regulation of translation and overactivation of the eIF2α phosphorylation signalling pathway, which can account for the deposition of senile plaques and memory impairments in AD through accelerating β-amyloidogenesis. Except PERK, there are three protein kinases, i.e.: GCN2 (general control non-derepressible-2 kinase), PKR (double-stranded RNA-activated protein kinase), HRI (heme-regulated inhibitor kinase), which have the ability of phosphorylation at the serine51 α subunit of eIF2α, which results in the attenuation of general protein synthesis and, on the other hand, promotes the translation of selective mRNAs such as BACE1 and ATF4, which is known as a memory and synaptic plasticity repressor [83–87].
Due to the fact that nowadays protein kinases are regarded to be popular, novel drug targets research by L. Devi and M. Ohno was focused on the relationship between the activation of PERK and another eIF2α kinase - GCN2. The aim of that research was to examine whether the GCN2-dependent signalling pathway leads to AD development and AD-related memory impairments. L. Devi and M. Ohno reported that under physiological conditions in wild type mice the deletion of GCN2 reduced the level of phosphorylated eIF2α. Interestingly, GCN2 deficiency in AD model mice contributed to BACE1, ATF4 elevations, thus the increased formation of senile plaques. Moreover, they found that PERK is overactivated under GCN2 deletion in AD model mice. In response to GCN2 deficiency, in AD model mice, phosphorylated PERK was significantly elevated. This evidence confirms that PERK activation is closely associated with AD pathology and suggests that PERK might be a pivotal mediator of eIF2α phosphorylation, which results in the dysregulation of the protein synthesis process as well as the deposition of senile plaques in AD brains [88].
There is ample evidences which shows that PERK activity is closely associated with ER stress involved in AD pathogenesis. Due to that fact, the study by T. Ma et al. was focused on supressing eIF2α kinases during the activation of the UPR molecular pathway. They reported that PERK deletion may be a potential therapeutic target to prevent eIF2α phosphorylation and aberrations connected with protein synthesis, synaptic plasticity and spatial memory in AD model mice. They showed that PERK deletion and, as a consequence, decreased level of phosphorylated eIF2α, prevents deficits in protein synthesis, protects AD mice from AD-associated synaptic plasticity and spatial memory impairment. Besides, T. Ma et al. reported the decreased formation of AD-related senile plaques in the AD brains. Interestingly, T. Ma et al. also emphasized that PERK-dependent eIF2α phosphorylation is dominant in the AD brain. Only PERK deletion alone caused a decreased level of phospho-eIF2α. The deletion of only GCN2 kinase had no significant impact on the level of eIF2α in the AD brain [89].
The above-mentioned results indicate that pharmacologically decreasing phosphorylation of eIF2α by blocking PERK-dependent molecular pathways may by a potential therapy for AD. In the study by Moreno et al. on neurodegenerative diseases using a mouse model, a specific inhibitor of PERK kinase called GSK2606414 was used. Moreno et al. reported that the therapeutic effect of the newly characterized inhibitor eliminates the attenuation of translation and prevents neurodegeneration in the mouse brain. That evidence suggests that the inhibition of the PERK-eIF2α pathway might be a crucial therapeutic strategy against neurodegenerative diseases [90]. The GSK2606414 inhibitor seems to be a promising avenue to pursue for neurodegenerative disease treatment. It is highly specific for PERK and possesses the ability to penetrate the blood-brain barrier [91].
Moreover, a recent study by Sidrauski et al. shows that other small molecules termed ISBIR (Integrated Stress Response inhibitor) have ability to reverse the effects of PERK activation under sustained ER stress. It has been reported that ISRIB selectively attenuates only the PERK pathway, but IRE 1 and ATF6 branches of UPR remain intact. Interestingly, ISRIB small molecules cause a significant decrease in the ATF4 level under ER stress conditions without any alterations in the level of phosphorylated eIF2α. Due to the fact that CHOP is a transcriptional target of ATF4, its level was also significantly reduced after ER stress induction which means that ISRIB arrests only effects of ER stress-induced PERK activation and eIF2α phosphorylation. ISRIB also contributes to reverse protein synthesis under ER stress conditions. The study conducted by Sidrauski et al. also shows that ISRIB arrests ATF4 expression after the activation of two other eIF2α kinases, such as, GCN2 and HRI [41]. UPR activation, which results in increased levels of both phosphorylated PERK and eIF2α, is strictly associated not only with Alzheimer’s disease, but also with other protein misfolding neurodegenerative diseases and prion disorders. Recent studies by Halliday et al. confirmed that treatment with ISRIB prion-infected mice reduces ATF4 expression without unwanted side effects, as well as does not lead to the attenuation of PERK activation and eIF2α phosphorylation [92]. ATF4 is known as a repressor of memory, long-term synaptic plasticity and synthesis of new proteins, and is necessary for memory consolidation. Sidrauski et al. showed that treatment with ISRIB plays the pivotal role in memory and learning processes, since it markedly enhances memory consolidation in wild type mice. Furthermore, those small molecule inhibitors of the PERK signalling pathway can be used for the treatment of cognitive disorders, since investigations by Sidrauski et al. showed their good pharmacokinetic properties without toxicity [41].
First inhibitor of UPR signalling pathway with potential ability to prevention the phosphorylation of eIF2α was Guanabezen (GBZ). GBZ prolongs adaptive response, but due to the fact that it is hypotensive drug as well as possess nanomolar affinity for the α-adrenergic receptor can not be used as a selective in-fibitor of UPR signalling branches. The latest data indicate that pharmacological manipulations of eIF2α phosphorylation by Sephin1, the small molecule selective inhibitor of holophosphatase, contribute to prolonged eIF2α phosphorylation without adverse effects which results in promoting the cellular adaptive phosphor-signalling cascade to support cell survival under stress conditions, which is closely associated with numerous pathological conditions involving neurodegenerative diseases, e.g. AD [44].
There are two eIF2α holophosphatases within mammalian cells composed of PP1 catalytic subunit and one or two regulatory subunits: PPP1R15A also termed GADD34 and PP1R15B. The expression of PPP1R15A occurs only in stressed cells due to the fact that downstream of ATF4 and CHOP, as a result of eIF2α phosphorylation, causes the transcriptional activation of PPP1R15A. Generally, the main aim of eIF2α holophosphatases is to dephosphorylate eIF2α in stressed cells and renew the translation process [42, 43, 93].
Sephin 1 can bind to the subunit PPP1R15A, which causes in the selective inhibition of holophosphatase activity. That causes prolonged eIF2α phosphorylation, inhibition of general protein synthesis and consequently the attenuation of expression of pro-apoptotic proteins like CHOP, since gene expression requires translation recovery. Moreover, there are no alterations in ATF4 translation in stressed cells treated with Sephin-1 [44].
CONCLUSION
Data suggest that nowadays more than 24 million people suffer from AD whose main hallmark is the deposition of Aβ plaques in the brain. Although the aetiology of AD is not completely clear, abundant experimental evidence suggests that mutation in the APP gene initiates the amyloidogenic pathway where the key role is played by enzyme BACE1. As a result, the level of the non-physiological form of Aβ is increased. Because those disturbances during ER stress activate the protective cellular response, the phosphorylation of eIF2α causes attenuation of global translation as well as, conversely, promotes the synthesis of BACE1 and ATF4. Moreover, recent molecular and genetic investigations present a new point of view on the therapeutic strategy for AD. Deactivation of PERK kinase via small-molecule inhibitors has been identified as a potential therapeutic target. It is highly possible that the inhibition of PERK activity may contribute to preventing the excessive accumulation of Aβ42 among the neurons and, as a result, neuronal loss and memory impairment in AD.
Acknowledgments
This work was supported by grant HARMONIA no. 2013/10/M/NZ1/00280 from the Polish National Science Centre.
Biography

Ireneusz Majsterek
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J, Dinsdale D, Ortori CA, Barrett DA, Tsaytler P, Bertolotti A, Willis AE, Bushell M, Mallucci GR. Sustained translational repression by eIF2alpha-P mediates prion neurode-generation. Nature. 2012;485(7399):507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayeux R. Epidemiology of neurodegeneration. Ann. Rev. Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 3.Blacker D, Tanzi RE. The genetics of Alzheimer disease: current status and future prospects. Arch. Neurol. 1998;55(3):294–296. doi: 10.1001/archneur.55.3.294. [DOI] [PubMed] [Google Scholar]
- 4.Philipson O, Lord A, Gumucio A, O’Callaghan P, Lannfelt L, Nilsson LN. Animal models of amyloid-beta-related pathologies in Alzheimer’s disease. FEBS J. 2010;277(6):1389–1409. doi: 10.1111/j.1742-4658.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- 5.Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, Raux G, Camuzat A, Penet C, Mesnage V, Martinez M, Clerget-Darpoux F, Brice A, Frebourg T. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 1999;65(3):664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobow T, Flirski M, Liberski PP. Amyloid-beta and tau proteins as biochemical markers of Alzheimer’s disease. Acta Neurobiol. Exp. 2004;64(1):53–70. doi: 10.55782/ane-2004-1491. [DOI] [PubMed] [Google Scholar]
- 7.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 8.Schaeffer EL, Figueiro M, Gattaz WF. Insights into Alzheimer disease pathogenesis from studies in transgenic animal models. Clinics. 2011;66(Suppl 1):45–54. doi: 10.1590/S1807-59322011001300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci. Trans. Med. 2011;3(77) doi: 10.1126/scitranslmed.3002369. 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C. Natural compounds that modulate BACE1-processing of amyloid-beta precursor protein in Alzheimer’s disease. Discov. Med. 2012;14(76):189–197. [PubMed] [Google Scholar]
- 11.Harrison SM, Harper AJ, Hawkins J, Duddy G, Grau E, Pugh PL, Winter PH, Shilliam CS, Hughes ZA, Dawson LA, Gonzalez MI, Upton N, Pangalos MN, Dingwall C. BACE1 (beta-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol. Cell Neurosci. 2003;24(3):646–655. doi: 10.1016/s1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 12.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 13.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neuribiol. Aging. 2004;25(3):303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 14.Katayama T, Imaizumi K, Honda A, Yoneda T, Kudo T, Takeda M, Mori K, Rozmahel R, Fraser P, George-Hyslop PS, Tohyama M. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer’s disease-linked presenilin-1 mutations. J. Biol. Chem. 2001;276(46):43446–43454. doi: 10.1074/jbc.M104096200. [DOI] [PubMed] [Google Scholar]
- 15.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J. Neurosci. 2009;29(41):12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuchi K, Hart M, Li L. Alzheimer’s disease and heparan sulfate proteoglycan. Front. Biosci. 1998;3:d327–d337. doi: 10.2741/a277. [DOI] [PubMed] [Google Scholar]
- 17.Revest P, Longstaff A. Molecular neuroscience. Oxford, UK: Bios Scientific Pub.; Springer; 1998. [Google Scholar]
- 18.Davis KL. Neuropsychopharmacology : the fifth generation of progress. Philadelphia: Lippincott/Williams & Wilkins; 2002. American College of Neuropsychopharmacology. [Google Scholar]
- 19.Armstrong RA. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathol. [Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences] 2009;47(4):289–299. [PubMed] [Google Scholar]
- 20.Walker ES, Martinez M, Brunkan AL, Goate A. Pre-senilin 2 familial Alzheimer’s disease mutations result in partial loss of function and dramatic changes in Abeta 42/40 ratios. J. Neurochem. 2005;92(2):294–301. doi: 10.1111/j.1471-4159.2004.02858.x. [DOI] [PubMed] [Google Scholar]
- 21.Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J. Biol. Chem. 1999;274(27):18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- 22.Selkoe DJ. Amyloid beta-protein precursor: new clues to the genesis of Alzheimer’s disease. Curr. Opin. Neurobiol. 1994;4(5):708–716. doi: 10.1016/0959-4388(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 23.Imaizumi K, Miyoshi K, Katayama T, Yoneda T, Ta-niguchi M, Kudo T, Tohyama M. The unfolded protein response and Alzheimer’s disease. Biochim. Biophys. Acta. 2001;1536(2–3):85–96. doi: 10.1016/s0925-4439(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 24.Welsh EW. Focus on Alzheimer’s Disease Research. New York: Nova Science Pub Inc; 2003. [Google Scholar]
- 25.Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van Broeckhoven C. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain. 2006;129(Pt 11):2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- 26.Webb RL, Murphy MP. beta-Secretases, Alzheimer’s Disease, and Down Syndrome. Curr. Gerontol. Geriatr. Res. 2012;2012:362839. doi: 10.1155/2012/362839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA. 1999;96(7):3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010;6(2):99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lichtenthaler SF, Multhaup G, Masters CL, Beyreuther K. A novel substrate for analyzing Alzheimer’s disease gamma-secretase. FEBS Lett. 1999;453(3):288–292. doi: 10.1016/s0014-5793(99)00730-9. [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Lesne S, Kotilinek L, Steidl-Nichols JV, Sherman M, Younkin L, Younkin S, Forster C, Sergeant N, Delacourte A, Vassar R, Citron M, Kofuji P, Boland LM, Ashe KH. Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2007;104(19):8167–8172. doi: 10.1073/pnas.0609521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J. Neurosci. 2007;27(14):3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassar R, Kandalepas PC. The beta-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimer’s Res. Ther. 2011;3(3):20. doi: 10.1186/alzrt82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 34.Citron M, Teplow DB, Selkoe DJ. Generation of amyloid beta protein from its precursor is sequence specific. Neuron. 1995;14(3):661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- 35.Vassar R. BACE1: the beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004;23(1–2):105–114. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- 36.Tian Y, Bassit B, Chau D, Li YM. An APP inhibitory domain containing the Flemish mutation residue modulates gamma-secretase activity for Abeta production. Nat. Struct. Mol. Biol. 2010;17(2):151–158. doi: 10.1038/nsmb.1743. [DOI] [PubMed] [Google Scholar]
- 37.Hoke DE, Tan JL, Ilaya NT, Culvenor JG, Smith SJ, White AR, Masters CL, Evin GM. In vitro gamma-secretase cleavage of the Alzheimer’s amyloid precursor protein correlates to a subset of presenilin complexes and is inhibited by zinc. FEBS J. 2005;272(21):5544–5557. doi: 10.1111/j.1742-4658.2005.04950.x. [DOI] [PubMed] [Google Scholar]
- 38.De Jonghe C, Esselens C, Kumar-Singh S, Craessaerts K, Serneels S, Checler F, Annaert W, Van Broeckho-ven C, De Strooper B. Pathogenic APP mutations near the gamma-secretase cleavage site differentially affect Abeta secretion and APP C-terminal fragment stability. Hum. Mol. Gen. 2001;10(16):1665–1671. doi: 10.1093/hmg/10.16.1665. [DOI] [PubMed] [Google Scholar]
- 39.Van Dam D, De Deyn PP. Drug discovery in dementia: the role of rodent models. Nat. Rev. Drug Dis. 2006;5(11):956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Walter J. Phosphorylation of amyloid beta (Abeta) peptides - a trigger for formation of toxic aggregates in Alzheimer’s disease. Aging. 2011;3(8):803–812. doi: 10.18632/aging.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonen-berg N, Walter P. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 2003;163(4):767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J. Biol. Chem. 2009;284(11):6661–6673. doi: 10.1074/jbc.M806735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’An-tonio M, Barry N, Sigurdardottir A, Bertolotti A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348(6231):239–242. doi: 10.1126/science.aaa4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hershey JW. Protein phosphorylation controls translation rates. J. Biol. Chem. 1989;264(35):20823–20826. [PubMed] [Google Scholar]
- 46.Hoozemans JJ, Veerhuis R, Van Haastert ES, Roze-muller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110(2):165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 47.Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563(1–2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 48.Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Reports. 2002;3(10):944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, Samali A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J. Cell. Mol. Med. 2011;15(10):2025–2039. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65(2):184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 52.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 2005;85(1):201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 53.O’Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hebert SS, De Strooper B, Haass C, Bennett DA, Vassar R. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60(6):988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7(12):1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 55.Santos SF, Pierrot N, Morel N, Gailly P, Sindic C, Octave JN. Expression of human amyloid precursor protein in rat cortical neurons inhibits calcium oscillations. J. Neurosci. 2009;29(15):4708–4718. doi: 10.1523/JNEUROSCI.4917-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierrot N, Ghisdal P, Caumont AS, Octave JN. Intraneuronal amyloid-beta1-42 production triggered by sustained increase of cytosolic calcium concentration induces neuronal death. J. Neurochem. 2004;88(5):1140–1150. doi: 10.1046/j.1471-4159.2003.02227.x. [DOI] [PubMed] [Google Scholar]
- 57.Leissring MA, LaFerla FM, Callamaras N, Parker I. Subcellular mechanisms of presenilin-mediated enhancement of calcium signaling. Neurobiol. Dis. 2001;8(3):469–478. doi: 10.1006/nbdi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 58.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Meth. Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornejo VH, Hetz C. The unfolded protein response in Alzheimer’s disease. Semin. Immunopathol. 2013;35(3):277–292. doi: 10.1007/s00281-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 60.Devi L, Ohno M. PERK mediates eIF2alpha phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2014;35(10):2272–2281. doi: 10.1016/j.neurobiolaging.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoozemans JJ, van Haastert ES, Nijholt DA, Roze-muller AJ, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am. J. Pathol. 2009;174(4):1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoshino T, Nakaya T, Araki W, Suzuki K, Suzuki T, Mizushima T. Endoplasmic reticulum chaperones inhibit the production of amyloid-beta peptides. Biochem. J. 2007;402(3):581–589. doi: 10.1042/BJ20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neo-cortex in Alzheimer disease. Arch. Neurol. 2002;59(9):1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 64.Velliquette RA, O’Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer’s disease pathogenesis. T.J. Cogn. Neurosci. 2005;25(47):10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol. Biol. Cell. 2006;17(7):3095–3107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Y, Hendershot LM. The mammalian endoplasmic reticulum as a sensor for cellular stress. Cell Stress Chaperones. 2002;7(2):222–229. doi: 10.1379/1466-1268(2002)007<0222:tmeraa>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui W, Li J, Ron D, Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr. [Section D, Biological Crystallography] 2011;67(Pt 5):423–428. doi: 10.1107/S0907444911006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohno M. Roles of eIF2alpha kinases in the pathogenesis of Alzheimer’s disease. Front. Mol. Neurosci. 2014;7:22. doi: 10.3389/fnmol.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille JC, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436(7054):1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hinnebusch AG. Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. J. Biol Chem. 1997;272(35):21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 71.De Pietri Tonelli D, Mihailovich M, Di Cesare A, Co-dazzi F, Grohovaz F, Zacchetti D. Translational regulation of BACE-1 expression in neuronal and non-neuronal cells. Nucleic Acids Res. 2004;32(5):1808–1817. doi: 10.1093/nar/gkh348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 73.Duran-Aniotz C, Martinez G, Hetz C. Memory loss in Alzheimer’s disease: are the alterations in the UPR network involved in the cognitive impairment? Front. Aging Neuro-sci. 2014;6:8. doi: 10.3389/fnagi.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009;122(Pt 4):437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002;318(5):1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 78.Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J. Biol. Chem. 2009;284(2):1106–1115. doi: 10.1074/jbc.M807325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merksamer PI, Papa FR. The UPR and cell fate at a glance. J. Cell Sci. 2010:1003–1006. doi: 10.1242/jcs.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei MC, Zong WX, Cheng EH, Lindsten T, Panout-sakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aprahamian I, Stella F, Forlenza OV. New treatment strategies for Alzheimer’s disease: is there a hope? Indian J. Med. Res. 2013;138(4):449–460. [PMC free article] [PubMed] [Google Scholar]
- 82.Georgi S. Nicotinic acetylcholine receptors and Alzheimer’s disease therapeutics: a review of current literature. J. Young Investig. 2005 [Epub ahead of print] [Google Scholar]
- 83.Fujii S, Matsumoto M, Igarashi K, Kato H, Mikoshiba K. Synaptic plasticity in hippocampal CA1 neurons of mice lacking type 1 inositol-1,4,5-trisphosphate receptors. Learn. Memory. 2000;7(5):312–320. doi: 10.1101/lm.34100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408(6812):584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- 85.Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129(1):195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn. Memory. 2004;11(4):365–372. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- 87.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34(Pt 1):7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 88.Devi L, Ohno M. Deletion of the eIF2alpha Kinase GCN2 fails to rescue the memory decline associated with Alzheimer’s disease. PloS One. 2013;8(10):e77335. doi: 10.1371/journal.pone.0077335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat. Neurosci. 2013;16(9):1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, Mallucci GR. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013;5(206) doi: 10.1126/scitranslmed.3006767. 206ra138. [DOI] [PubMed] [Google Scholar]
- 91.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, Atkins C, Liu Q, Rabindran S, Kumar R, Hong X, Goetz A, Stanley T, Taylor JD, Sigethy SD, Tomberlin GH, Hassell AM, Kahler KM, Shew-chuk LM, Gampe RT. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J. Med. Chem. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 92.Halliday M, Radford H, Sekine Y, Moreno J, Verity N, le Quesne J, Ortori CA, Barrett DA, Fromont C, Fischer PM, Harding HP, Ron D, Mallucci GR. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015;6:e1672. doi: 10.1038/cddis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22(5):1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]