Abstract
Purpose
To demonstrate the feasibility of using dual-energy computed tomography to accurately quantify uric acid and non-uric-acid components in urinary stones having mixed composition.
Materials and Methods
A total of 24 urinary stones were analyzed with microCT to serve as the reference standard for uric acid and non-uric-acid composition. These stones were placed in water phantoms to simulate body attenuation of slim to obese adults and scanned on a third-generation dual-source scanner using dual-energy modes adaptively selected based on phantom size. CT number ratio, which is distinct for different materials, was calculated for each pixel of the stones. Each pixel was then classified as uric acid and non-uric-acid by comparing the CT number ratio with preset thresholds ranging from 1.1 to 1.7. Minimal, maximal and root-mean-square errors were calculated by comparing composition to the reference standard and the threshold with the minimal root-mean-square-error was determined. A paired t-test was performed to compare the stone composition determined with dual-energy CT with the reference standard obtained with microCT.
Results
The optimal CT number ratio threshold ranged from 1.27 to 1.55, dependent on phantom size. The root-mean-square error ranged from 9.60% to 12.87% across all phantom sizes. Minimal and maximal absolute error ranged from 0.04% to 1.24% and from 22.05% to 35.46%, respectively. Dual-energy CT and the reference microCT did not differ significantly on uric acid and non-uric-acid composition (P from 0.20 to 0.96, paired t-test).
Conclusion
Accurate quantification of uric acid and non-uric-acid composition in mixed stones is possible using dual-energy CT.
Introduction
In addition to detecting stone location and quantifying stone size, dual-energy computed tomography (DECT) has recently been used to differentiate the chemical composition of urinary stones to assist with patient management [1–10]. Multiple techniques have been used to achieve dual-energy exams, including the use of dual source CT, fast kV switching, dual layer detectors, and 2 consecutive scans with different tube potentials [1, 6, 8, 11, 12]. The most successful application of DECT is to differentiate uric acid (UA) from non-uric acid (NUA) stones due to the substantial difference in the effective atomic number of these stones. This information is critical because UA stones can be treated with urinary alkalization rather than surgical procedures. Accurate differentiation of UA from NUA stones has been reported in multiple in vitro phantom studies and in vivo patient studies [1–4, 13]. Researchers have also investigated methods to further differentiate among different types of NUA stones [5, 11, 14–16].
Most studies in the literature have focused on pure stones that are composed of a single material. Use of such stone samples simplifies data analysis and has led to demonstration that DECT can differentiate different types of stones. However, the majority of urinary stones are mixed stones that contain 2 or more materials [17, 18]. Therefore, it is essential to identify and quantify individual components inside each stone to ensure proper management. Several studies using DECT to differentiate stone materials have included a few mixed stones in addition to pure stones [2–5]. However, none of these studies quantified each component within a mixed stone.
To determine whether stone composition can be differentiated and quantified by using DECT, a reference standard of stone composition is needed. Multiple techniques have been used as standards, such as X-ray diffraction crystallography, infrared spectroscopy, and wet chemical analysis [19]. Infrared spectroscopy (IR) is the most widely used method for in vitro stone composition analysis and has been used as the reference standard in the majority of the published papers [1, 5, 7, 9, 13, 14, 16, 20]. However, IR samples only part of the stone, limiting its ability to accurately report the compositions of mixed stones [21]. Recently, microCT has been shown to provide accurate determination of mixed mineral components without destroying the stone (as IR does) [21–23]. Unlike IR, microCT provides 3-dimensional images of the whole stone and is therefore well suited for determining the heterogeneity in mixed stones. However, this is a research technique that requires an isolated stone, and therefore is not a substitute for clinical techniques such as DECT. Calcium oxalate and uric acid have dramatically different x-ray attenuation values by micro CT [22, 24], allowing mineral percentages to be measured for each stone by grayscale segmentation of the image stacks [23]. Measurement of mineral percentages on a given stone was quite reproducible, with coefficients of variation for the material having the larger fraction averaging 1.9±0.8%. The combination of microscopic localization of minerals [24] with the dramatically different x-ray attenuation of uric acid and calcium salts in micro CT [22] allowed this process to provide a reference standard for the actual percentage of uric acid in each stone used in this study.
The purpose of this study was to demonstrate the feasibility of using dual-energy computed tomography (DECT) to accurately quantify UA and NUA components in urinary stones having mixed composition.
Materials and Methods
Kidney Stone Selection and microCT Scans
A total of 24 urinary stones with previously acquired microCT data showing mixed UA/NUA composition were selected for this study. Stones with diameters of 5 mm and larger were included in this study. No internal review board approval was required, as all stones were obtained without any information related to patient identifiers. All of the stones were first scanned with microCT to determine the chemical composition; the result was used as the reference standard of stone composition. Micro CT scans were performed using a Skyscan 1172 system (Bruker, Billerica, MA) with a tube potential of 60 kV and a 0.5 mm aluminum filter at the source. Stones were scanned to obtain image stacks with cubic voxels between 6 and 10 µm in size. Chemical composition based on microCT (and backed up with infrared spectroscopy of cohort stones) was used as the true composition for each stone (Figure 1).
Fig. 1.
MicroCT. Example of the ability to distinguish uric acid and calcium salts using micro CT. (A) Photo of stone, on mm background. (B) Micro CT slice through the stone, showing UA (which has a characteristically low x-ray attenuation value) and calcium oxalate monohydrate (COM). The identity of these minerals was verified in cohort stones using infrared spectroscopy. This stone was scanned at 9 µm voxel size. Segmentation of the UA and NUA portions of this stone yielded 32% UA/68% NUA. Note that the distinction between UA and NUA in micro CT is so obvious that accurate measurement of the proportion of UA in the stone is very easy, and very accurate.
All stones were hydrated in distilled water for 24 hours before the DECT experiments to mimic the clinical scenario, where stones are surrounded by urine. Each stone was placed in an individual water-filled vial, and air bubbles eliminated from around the stones. All of the vials were then placed into a water phantom (Fig. 2) using a plastic grid to hold the vials in place.
Fig. 2.
Experimental setup. Each stone was placed in an individual water-filled vial (a) and then the vials were all attached to a plastic stand and placed into the center of a water phantom (b) for the CT scans.
To investigate the impact of patient size on image quality and accuracy of stone composition quantification, 6 phantoms with lateral widths of 30, 35, 40, 45, 50, and 55 cm were used to simulate different patient habitus. Each of the 24 stones was scanned in each of the 6 phantoms and all data were analyzed for each phantom. To minimize variability in positional configuration, stones remained fixed in the plastic grid (Fig. 2b) when transferred between phantoms, and were centered in each phantom.
CT Scans and Image Reconstruction
Scans were performed on a 192-slice (96 detector rows with flying focal spot technique) dual-source CT scanner (Somatom Force, Siemens Healthcare, Forchheim, Germany) using our clinical dual-energy urinary stone composition protocol. The dual-energy scan modes (tube potential pairs) were selected based upon phantom size. A 0.6-mm tin (Sn) filter was added to the high tube potential (150 kV) to increase the spectral separation and consequently material decomposition capability [15, 25–28]. Key scanning parameters, such as tube potentials and tube current time products, are summarized in Table 1. These parameters were selected so that the volume CT dose index (CTDIvol) would be the same for phantoms of the same size, even with use of different tube potential pairs. CTDIvol was 18 mGy for a standard-sized adult (with attenuation equivalent to a 33-cm-diameter water phantom), approximately 70 to 80 kg. Tube current modulation (CareDose 4D, Siemens Healthcare) was used to adapt the tube current according to phantom size and to optimize dose delivery within the scan plane. This improves image quality consistency along the z axis relative to scans performed without tube current modulation [29].
Table 1.
Key Scanning and Reconstruction Parameters Used in the Dual-Energy CT Exams
| Scan Type | Spiral / Dual Energy | |||||
| Rotation Time | 0.5 s | |||||
| Collimation | 128 × 0.6 mm | |||||
| Pitch | 0.6 | |||||
|
kV pair at specific phantom sizes |
30 cm | 35 cm | 40 cm | 45 cm | 50 cm | 55 cm |
| 70/Sn150 | 70/Sn150 | 80/Sn150 | 90/Sn150 | 100/Sn150 | 100/Sn150 | |
|
Quality reference tube- current time product for low and high energy tubes |
30 cm | 35 cm | 40 cm | 45 cm | 50 cm | 55 cm |
| 875/219 | 875/219 | 500/250 | 350/219 | 300/150 | 300/150 | |
| CARE Dose 4D | ON | |||||
| Recon Kernel | Qr40 | |||||
|
Image Thickness |
1 mm | |||||
|
Image Increment |
0.8 mm | |||||
Images were reconstructed with a medium-smooth dual-energy kernel (Qr40) at 1-mm image thickness and 0.8-mm increment for both low- and high-energy data sets.
Dual-Energy Processing
The reconstructed images were post-processed using a custom urinary stone analysis software programmed in Matlab (Math-Works, Natick, MA). The locations of the stones were automatically detected by the software after loading of the DICOM images. Stones were then segmented using a threshold-based method, with the threshold adapted to the Hounsfield units of each stone [30]. The CT number ratio (CTR), which is defined as the ratio of the CT number at the low tube potential to the CT number at the high tube potential, was calculated for each pixel of the stone, and each pixel of the stone was classified as UA or NUA by comparing the CTR at the pixel to a pre-determined CTR threshold. Pixels with CTR lower than the threshold were classified as UA and those with CTR higher than the threshold were classified as NUA. The percentage of UA and NUA for each stone was then calculated from the number of UA and NUA pixels in the whole stone.
Statistical Analysis
The UA percentage stone composition obtained from the DECT images at a given CTR threshold was compared to that of the reference standard obtained from microCT. The error for the UA component was calculated for each stone and CTR threshold value. Because the stones contained only UA and NUA, the NUA error was the same in magnitude but opposite sign as the UA error. Therefore, the UA error was used in all following data analyses. The root mean square error (RMSE) over all stones was calculated as:
where N was the number of stones (24 in this study), UADECT was the UA percentage determined from DECT images, and UAmicroCT was the UA percentage determined from the microCT, which was used as the reference standard.
As stone composition (UA or NUA) was determined by comparing the measured CTR values to the CTR threshold used to separate UA from NUA, the specific value of the selected CTR threshold affected the RMSE. In this study a series of CTR threshold values, ranging from 1.10 to 1.70 and incremented by 0.01, was used to determine the optimal threshold for each phantom size and dual energy scan mode. The range was selected so that it was wide enough to cover all reasonable threshold values. Since the CTR of UA stones is around 1.0 and the CTR of NUA stones is between 1.4 and 2.0, and the optimal threshold for differentiating UA from NUA is expected to be between the CTRs of UA and NUA [15], the thresholds investigated (1.10 to 1.70) were considered sufficient to include all reasonable threshold values. RMSE was calculated for each value of CTR threshold, and the threshold associated with the minimal RMSE was defined as the optimal threshold. This was repeated for each phantom size and dual-energy scan mode because previous studies showed that the optimal threshold varies with phantom size and tube potentials [15]. The error in UA percentage composition for each stone was then calculated using this optimal threshold. Minimal and maximal errors (in absolute values) from all stones were also calculated.
For each phantom size, a paired t-test was performed to compare the UA stone composition from microCT and DECT, with P < 0.05 considered as statistically significant.
Results
Stone Volume and Composition from microCT
The volumes and compositions of each stone (24 total) determined with microCT are listed in Table 2. Stone volume ranged from 75.3 to 319.1 mm3 (mean = 170.2 mm3, standard deviation = 61.9 mm3), which covered the range of stone sizes typically seen in patients. Among these stones, 1 was pure UA, 1 was pure NUA, and the remaining 22 were mixed stones, with the percentage of UA ranging from 12% to 93% and the percentage of NUA ranging from 88% to 7%, respectively.
Table 2.
Volume and Composition of Each Stone as Measured with MicroCT. Stones are presented in order of increasing volume; stone 1 is the smallest and stone 24 the largest.
| Stone Volume and Composition Determined by MicroCT | |||
|---|---|---|---|
| Stone # | Volume (mm3) | UA (%) | NUA (%) |
| 1 | 75.3 | 100 | 0 |
| 2 | 89.4 | 43 | 57 |
| 3 | 99.2 | 50 | 50 |
| 4 | 103.2 | 12 | 88 |
| 5 | 115.2 | 60 | 40 |
| 6 | 117.6 | 78 | 22 |
| 7 | 130.1 | 0 | 100 |
| 8 | 132.9 | 77 | 23 |
| 9 | 133.8 | 92 | 8 |
| 10 | 135.6 | 29 | 71 |
| 11 | 138.2 | 36 | 64 |
| 12 | 139.1 | 51 | 49 |
| 13 | 158.5 | 49 | 51 |
| 14 | 183.7 | 93 | 7 |
| 15 | 187.4 | 93 | 7 |
| 16 | 188.8 | 15 | 85 |
| 17 | 191 | 77 | 23 |
| 18 | 211.4 | 13 | 87 |
| 19 | 230.3 | 18 | 82 |
| 20 | 238.4 | 35 | 65 |
| 21 | 239.2 | 49 | 51 |
| 22 | 239.4 | 26 | 74 |
| 23 | 251.5 | 36 | 64 |
| 24 | 319.1 | 38 | 62 |
Stone Composition from DECT
Example DECT images of a mixed stone scanned with the 70/Sn150 kV dual-energy mode in a 30-cm phantom are shown in Figure 3(a, b). The measurement from microCT indicated that this mixed stone was 49% UA and 51% NUA. The CTR map is shown in Figure 3(c). Composition maps at different CTR thresholds, with UA pixels in red and NUA pixels in blue, and the corresponding calculated errors of the UA estimation, are shown in Fig 3(d)–(f). For a very low CTR threshold (e.g., 1.10, as shown in panel d), more pixels were classified as NUA, which underestimated the UA components (by −46% for the example shown in panel d). Conversely, for a very high CTR threshold (e.g., 1.70, as shown in panel f), more pixels were classified as UA, which overestimated the UA component (by 26% for the example shown in panel f). A midrange CTR threshold (e.g., 1.55 in panel e) produced minimal error (6% for the example shown in panel e). Figure 4 shows two examples of DECT quantification of stone composition. Micro CT images (Fig. 4a) showed clear mixed stone composition (49% UA and 51% NUA and 43% UA and 57% NUA, respectively). Mixed images of DECT (Fig. 4b) showed the same outline of the stones as that of microCT images, but with much fewer details of the inner structure. The CTR map showed that the UA and NUA composition determined by DECT (54% UA and 46% NUA and 53% UA and 47% NUA, respectively) was close to that of the corresponding microCT results (error of 5% and 10%, respectively).
Fig. 3.
Example of stone analysis. Low and high energy CT images of a mixed stone (a, b) respectively, with 49% UA and 51% NUA as identified with microCT, and the corresponding CTR map (c). (d–f) Composition maps show UA in red and NUA in blue with CTR thresholds of 1.10 (d), 1.55 (e), and 1.70 (f). The error of UA estimation was 46% (d), 6% (e), and 26% (f) in comparison with the values obtained from microCT.
Fig. 4.
Two examples (top and bottom rows) of DECT quantification of stone composition with micro CT images (a), mixed DECT images (b) and composition maps from DECT (c). UA and NUA composition values from microCT and DECT were displayed together with the corresponding images.
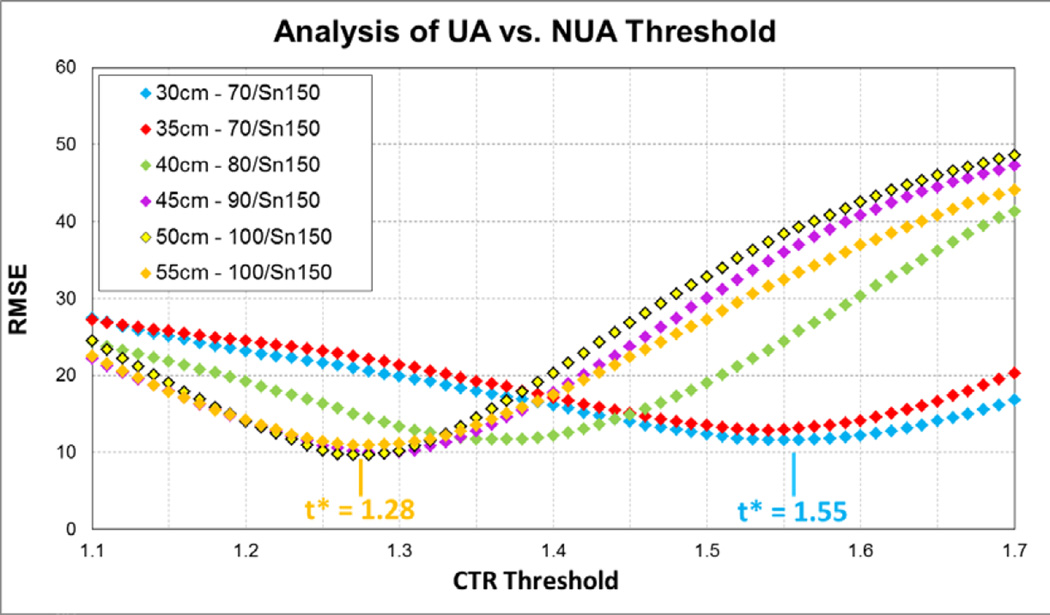
For all CTR thresholds, at different phantom sizes and dual-energy scan modes, RMSE first decreased as CTR threshold increased from 1.10, and then increased as CTR approached 1.70 (Fig 5, Table 3). The optimal CTR threshold, which corresponded to the minimal RMSE, depended on phantom size and dualenergy scan mode. For example, it was 1.55 for the 30-cm phantom scanned with the 70/Sn150 scan mode and 1.28 for the 55-cm phantom scanned in the 100/Sn150 scan mode. In general, the optimal CTR was higher for DECT scan modes with lower tube potentials (i.e. 70 or 80 kV) for the “low-energy” beam compared to those with higher tube potentials (i.e. 90 and 100 kV) for the “low-energy” beam. This is due to the fact that CT number and hence CTR strongly depend on tube potential. For the same urinary stone, the CT number is higher at lower tube potentials. Because the high-energy beam was the same for all DECT scan modes (i.e. 150 kV with Sn filtration), the CTR of each stone was higher for DECT modes with lower tube potential on the “low-energy” beam..
Fig. 5.
Variation of root mean square error (RMSE) as CTR threshold is varied. CTR thresholds are shown for different phantom sizes and dual-energy scan modes. The optimal CTR threshold corresponding to the minimal RMSE depends on phantom size and dual-energy mode. The optimal thresholds for the 30-cm and 55-cm phantoms are indicated.
Table 3.
Measurement Parameters and Results of the Comparison of Stone Composition Quantification with DECT and microCT.
| Phantom Size (cm) |
DE Mode (kV Pair) |
Optimal CTR Threshold |
RMSE (%) |
Minimum Absolute Error (%) |
Maximum Absolute Error (%) |
t-test: microCT vs. DECT (P) |
|---|---|---|---|---|---|---|
| 30 | 70/Sn150 | 1.55 | 11.55 | 0.07 | 32.67 | 0.26 |
| 35 | 70/Sn150 | 1.54 | 12.87 | 0.84 | 35.46 | 0.20 |
| 40 | 80/Sn150 | 1.37 | 11.65 | 1.24 | 32.45 | 0.22 |
| 45 | 90/Sn150 | 1.29 | 9.81 | 0.61 | 28.46 | 0.30 |
| 50 | 100/Sn150 | 1.27 | 9.60 | 0.34 | 22.94 | 0.96 |
| 55 | 100/Sn150 | 1.28 | 10.87 | 0.04 | 22.05 | 0.83 |
DECT, dual-energy computed tomography; CTR, CT number ratio; RMSE, root mean square error across all 24 stones.
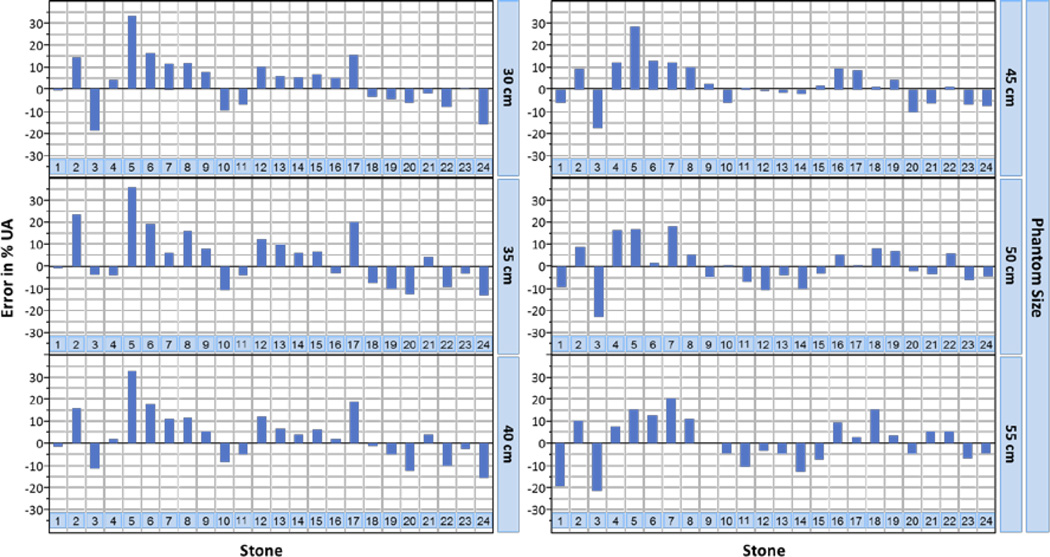
The RMSE ranged from 9.60% to 12.87%. The minimum absolute UA errors ranged from 0.04% to 1.24%, and the maximum absolute UA errors ranged from 22.05% to 35.46% (Fig 6, Table 3). Both positive and negative errors were observed, indicating some UA components were estimated as NUA and vice versa. No clear bias was observed. Paired t-tests showed no significant difference in the UA percentages estimated with clinical DECT and microCT (P values ranged from 0.20 to 0.96, Table 3).
Fig. 6.
Error of UA estimation for each stone at different phantom sizes. Stones are presented in order of increasing volume; stone 1 is the smallest and stone 24 the largest.
Discussion
Most studies in the literature have focused on pure stones, with very few studies including mixed stones. Graser et al investigated stone composition differentiation using a first-generation dual-source scanner operated at 80 and 140 kV [2]. Most stones in their study were pure stones, although 4 mixed stones were included. In the study performed by Boll et al, more mixed stones were included, together with pure UA and NUA stones. Each stone was treated as a whole, and the main goal was to differentiate among pure UA, pure NUA, and mixed stones. As a consequence, individual components inside the mixed stones were neither differentiated nor quantified [5]. Stolzmann et al used color coding by commercial DECT software to detect UA and NUA components in both pure and mixed stones. Stones were considered to have UA components if any red color was observed and to have NUA components if any blue color was observed. There were no quantitative data presented on the percentage of UA and NUA in each stone [3, 4]. In this study, we performed dual-energy analysis on a pixel-by-pixel basis. Each pixel inside a stone was classified as either UA or NUA by comparing its CTR to a predetermined threshold. This allowed us to quantify UA and NUA percentage inside the mixed stones.
For first generation dual source DECT, 80 and 140 kV beams were used without additional filters, which may be problematic for large patients because the accuracy of stone composition differentiation decreases in large patients, mainly due to the limited penetration of the 80-kV beam [1, 15]. Thus, there was an upper limit on the size of patients that could undergo dual-energy exams with the first-generation dual-source scanners [2–5, 15]. The introduction of a tin filter in the second-generation dual-source scanners and the availability of the 100 kV/Sn140kV scan mode improved the ability to perform DECT on large patients [15, 25–28]. In this study, we used a third-generation dual-source scanner with a total of 5 dual-energy scan modes available, 4 of which have tin filters on the high (150 kV) beam. We covered a wide range of body sizes in this study, using phantoms with lateral width from 30 to 55 cm, representing slim to very obese adult patients. We selected dual-energy scan modes based on phantom sizes: 70 and 80 kV were used for phantoms representing small and medium patients, respectively, because these modes have better spectral separation yet still provide sufficient penetration for patients of this size. Ninety and 100 kV were used for phantoms representing large and obese patients, respectively, to provide sufficient penetration. Varying the scan modes based on phantom size matched our clinical work flow, which was designed to take advantage of wider spectral separation on slim patients, while utilizing better penetration on large patients. The varying scan modes, however, added complexity to the data analysis and required the CTR threshold to be adjusted for each dual energy (kV) mode. In other words, a single UA/NUA cutoff cannot be applied to all scan modes, as shown in the results. Our results indicated that DECT can provide accurate quantification of UA and NUA components in mixed stones at all body sizes, with RMSE ranging from 9.60% to 12.87%. However, even though the overall RMSE was similar for different sized phantoms, the error of individual stones depended on the phantom size (Fig. 6).
In this study, we investigated the selection of the CTR threshold, which has a substantial impact on stone composition differentiation and quantification. CTR not only depends on the dual-energy scan mode used, but may also depend on patient size [20]. In this study, we used RMSE, averaged over all 24 stones, as the figure of merit to determine the optimal threshold for a given dual-energy scan mode and phantom size. As expected, the optimal CTR was lower for dual-energy modes with higher tube potentials for the low-energy beam. The RMSE-versus-CTR-threshold curves in Figure 5 showed relatively wide and flat valleys around the optimal CTR threshold, indicating that RMSE will not dramatically increase when the CTR selected is slightly different from the optimal values. This makes the selection of CTR stable in non-ideal scenarios, such as when image noise is present.
One potential source of error in the dual-source, dual-energy data is the partial volume effect, which is due to the limited pixel size and resolution (~0.5 mm) achieved with the evaluated commercial CT scanner. The microCT data, however, provide highly accurate quantification due to the very high spatial resolution (0.006 to 0.010 mm) and hence greatly reduced partial volume effect. The good agreement observed between the whole-body CT results and the micro-CT results indicate that for the task of assessing percentage UA composition, the spatial resolution of the evaluated scanner was sufficient.
A range of stone sizes were included in this study, such as are typically seen in patients. Visual observation of the data showed no clear relationship between the magnitude of errors and stone size (Fig. 6). Statistical testing for a potential relationship (e.g. a correlation analysis) was not performed due to the limited sample size (n=24).
There are several limitations to this study. First, this was an in vitro phantom study. Multiple stones were placed in vials and scanned at the same time. Arrangement does not emulate the stone location and perhaps orientation relative to patient cases. We do not believe that this presents a major concern, as CT number accuracy and uniformity were routinely tested and found to meet or exceed regulatory requirements. Establishing the accuracy of CT numbers ensured the consistency of CTR, which was used to determine percent stone composition. Further, similar phantom designs have been used in several previous studies in the literature, which were found to agree with clinical studies [2–4, 15, 20]. Nonetheless, in vivo patient studies are warranted to fully confirm the clinical accuracy and utility. Second, the number of stones was relatively small. This was due to the limited availability of stones that had been scanned by microCT. Third, this study only focused on differentiation and quantification of UA from NUA components. It might be possible to further differentiate and quantify NUA components in a study with a larger sample size. Fourth, our study focused on the quantification of only UA and NUA components in mixed stones. The substantial difference between the effective atomic numbers of UA and NUA enabled the quantification of each component. It is of clinical interest to further differentiate and quantify different NUA components. However, this will be a more challenging task as the difference of effective atomic numbers between NUA components is smaller than that between UA and NUA. Finally, the results of our study can only be applied to the evaluated dual-energy scan modes and scanner, i.e. the third generation dual-source scanner. Quantification accuracy on scanners that do not use a tin filter or the evaluated tube potential combinations (i.e. the 1st and 2nd generation dual-source scanners), or on scanners that use different dual energy acquisition techniques (e.g. kV switching or dual-layer detector), requires further investigation.
In conclusion, we have demonstrated in phantom studies that accurate quantification of UA and NUA components in mixed stones is possible using DECT.
Acknowledgments
Dr. McCollough receives research funding from Siemens Healthcare.
Grant Support: This study was supported by grant DK100227 from the National Institute of Diabetes and Digestive and Kidney Diseases, and training grant R25 DK101405 for Mayo Clinic Summer Undergraduate Research in Nephrology & Urology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure: No other authors have anything to disclose.
IRB: No IRB approval was required for this phantom study.
References
- 1.Primak AN, Fletcher JG, Vrtiska TJ, et al. Noninvasive differentiation of uric acid versus non-uric acid kidney stones using dual-energy CT. Acad Radiol. 2007;14:1441–1447. doi: 10.1016/j.acra.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graser A, Johnson TR, Bader M, et al. Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Invest Radiol. 2008;43:112–119. doi: 10.1097/RLI.0b013e318157a144. [DOI] [PubMed] [Google Scholar]
- 3.Stolzmann P, Kozomara M, Chuck N, et al. In vivo identification of uric acid stones with dual-energy CT: diagnostic performance evaluation in patients. Abdom Imaging. 2009;19:2896–2903. doi: 10.1007/s00261-009-9569-9. [DOI] [PubMed] [Google Scholar]
- 4.Stolzmann P, Scheffel H, Rentsch K, et al. Dual-energy computed tomography for the differentiation of uric acid stones: ex vivo performance evaluation. Urol Res. 2008;36:133–138. doi: 10.1007/s00240-008-0140-x. [DOI] [PubMed] [Google Scholar]
- 5.Boll DT, Patil NA, Paulson EK, et al. Renal stone assessment with dual-energy multidetector CT and advanced postprocessing techniques: improved characterization of renal stone composition--pilot study. Radiology. 2009;250:813–820. doi: 10.1148/radiol.2503080545. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni NM, Eisner BH, Pinho DF, Joshi MC, Kambadakone AR, Sahani DV. Determination of renal stone composition in phantom and patients using single-source dual-energy computed tomography. J Comput Assist Tomogr. 2013;37:37–45. doi: 10.1097/RCT.0b013e3182720f66. [DOI] [PubMed] [Google Scholar]
- 7.Eiber M, Holzapfel K, Frimberger M, et al. Targeted dual-energy single-source CT for characterisation of urinary calculi: experimental and clinical experience. Eur Radiol. 2012;22:251–258. doi: 10.1007/s00330-011-2231-2. [DOI] [PubMed] [Google Scholar]
- 8.Leng S, Shiung M, Ai S, et al. Feasibility of discriminating uric acid from non-uric acid renal stones using consecutive spatially registered low- and high-energy scans obtained on a conventional CT scanner. AJR Am J Roentgenol. 2015;204:92–97. doi: 10.2214/AJR.13.11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Zhao R, Liu B, Yu Y. Gemstone spectral imaging dual-energy computed tomography: a novel technique to determine urinary stone composition. Urology. 2013;81:727–730. doi: 10.1016/j.urology.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Eliahou R, Hidas G, Duvdevani M, Sosna J. Determination of renal stone composition with dual-energy computed tomography: an emerging application. Semin Ultrasound CT MR. 2010;31:315–320. doi: 10.1053/j.sult.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Hidas G, Eliahou R, Duvdevani M, et al. Determination of renal stone composition with dual-energy CT: in vivo analysis and comparison with x-ray diffraction. Radiology. 2010;257:394–401. doi: 10.1148/radiol.10100249. [DOI] [PubMed] [Google Scholar]
- 12.Xu D, Langan D, Wu X, et al. Dual energy CT via fast kVp switching spectrum estimation. Proc SPIE. 2009;7258:72583T. [Google Scholar]
- 13.Matlaga BR, Kawamoto S, Fishman E. Dual source computed tomography: a novel technique to determine stone composition. Urology. 2008;72:1164–1168. doi: 10.1016/j.urology.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Thomas C, Heuschmid M, Schilling D, et al. Urinary calculi composed of uric acid, cystine, and mineral salts: differentiation with dual-energy CT at a radiation dose comparable to that of intravenous pyelography. Radiology. 2010;257:402–409. doi: 10.1148/radiol.10100526. [DOI] [PubMed] [Google Scholar]
- 15.Qu M, Ramirez-Giraldo JC, Leng S, et al. Dual-Energy Dual-Source CT With Additional Spectral Filtration Can Improve the Differentiation of Non-Uric Acid Renal Stones: An Ex Vivo Phantom Study. AJR Am J Roentgenol. 2011;196:1279–1287. doi: 10.2214/AJR.10.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zilberman DE, Ferrandino MN, Preminger GM, Paulson EK, Lipkin ME, Boll DT. In vivo determination of urinary stone composition using dual energy computerized tomography with advanced post-acquisition processing. J Urol. 2010;184:2354–2359. doi: 10.1016/j.juro.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 18.Daudon M, Donsimoni R, Hennequin C, et al. Sex- and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urol Res. 1995;23:319–326. doi: 10.1007/BF00300021. [DOI] [PubMed] [Google Scholar]
- 19.Kasidas GP, Samuell CT, Weir TB. Renal stone analysis: why and how? Ann Clin Biochem. 2004;41:91–97. doi: 10.1258/000456304322879962. [DOI] [PubMed] [Google Scholar]
- 20.Qu M, Jaramillo-Alvarez G, Ramirez-Giraldo JC, et al. Urinary stone differentiation in patients with large body size using dual-energy dual-source computed tomography. Eur Radiol. 2013;23:1408–1414. doi: 10.1007/s00330-012-2727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krambeck AE, Khan NF, Jackson ME, Lingeman JE, McAteer JA, Williams JC., Jr Inaccurate reporting of mineral composition by commercial stone analysis laboratories: implications for infection and metabolic stones. J Urology. 2010;184:1543–1549. doi: 10.1016/j.juro.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarse CA, McAteer JA, Sommer AJ, et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urol. 2004;4:15. doi: 10.1186/1471-2490-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pramanik R, Asplin JR, Jackson ME, Williams JC., Jr Protein content of human apatite and brushite kidney stones: significant correlation with morphologic measures. Urol Res. 2008;36:251–258. doi: 10.1007/s00240-008-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JC, Jr, McAteer JA, Evan AP, Lingeman JE. Micro-computed tomography for analysis of urinary calculi. Urol Res. 2010;38:477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- 25.Primak A, Ramirez-Giraldo JC, Eusemann C, et al. Dual-source dual-energy CT with additional tin filtration: Dose and image quality evaluation in phantoms and in-vivo. AJR Am J Roentgenol. 2010;195:1–11. doi: 10.2214/AJR.09.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Primak AN, Ramirez Giraldo JC, Liu X, Yu L, McCollough CH. Improved dual-energy material discrimination for dual-source CT by means of additional spectral filtration. Med Phys. 2009;36:1359–1369. doi: 10.1118/1.3083567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolzmann P, Leschka S, Scheffel H, et al. Characterization of urinary stones with dual-energy CT: improved differentiation using a tin filter. Invest Radiol. 2010;45:1–6. doi: 10.1097/RLI.0b013e3181b9dbed. [DOI] [PubMed] [Google Scholar]
- 28.Thomas C, Krauss B, Ketelsen D, et al. Differentiation of urinary calculi with dual energy CT: effect of spectral shaping by high energy tin filtration. Invest Radiol. 2010;45:393–398. doi: 10.1097/RLI.0b013e3181df9d28. [DOI] [PubMed] [Google Scholar]
- 29.McCollough CH, Bruesewitz MR, Kofler JM., Jr CT dose reduction and dose management tools: overview of available options. Radiographics. 2006;26:503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 30.Duan X, Wang J, Qu M, et al. Kidney stone volume estimation from computerized tomography images using a model based method of correcting for the point spread function. J Urol. 2012;188:989–995. doi: 10.1016/j.juro.2012.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]