Abstract
Patterning of C. elegans vulval cell fates relies on inductive signaling. In this induction event, a single cell, the gonadal anchor cell, secretes LIN-3/EGF and induces three out of six competent precursor cells to acquire a vulval fate. We previously showed that this developmental system is robust to a four-fold variation in lin-3/EGF genetic dose. Here using single-molecule FISH, we find that the mean level of expression of lin-3 in the anchor cell is remarkably conserved. No change in lin-3 expression level could be detected among C. elegans wild isolates and only a low level of change—less than 30%—in the Caenorhabditis genus and in Oscheius tipulae. In C. elegans, lin-3 expression in the anchor cell is known to require three transcription factor binding sites, specifically two E-boxes and a nuclear-hormone-receptor (NHR) binding site. Mutation of any of these three elements in C. elegans results in a dramatic decrease in lin-3 expression. Yet only a single E-box is found in the Drosophilae supergroup of Caenorhabditis species, including C. angaria, while the NHR-binding site likely only evolved at the base of the Elegans group. We find that a transgene from C. angaria bearing a single E-box is sufficient for normal expression in C. elegans. Even a short 58 bp cis-regulatory fragment from C. angaria with this single E-box is able to replace the three transcription factor binding sites at the endogenous C. elegans lin-3 locus, resulting in the wild-type expression level. Thus, regulatory evolution occurring in cis within a 58 bp lin-3 fragment, results in a strict requirement for the NHR binding site and a second E-box in C. elegans. This single-cell, single-molecule, quantitative and functional evo-devo study demonstrates that conserved expression levels can hide extensive change in cis-regulatory site requirements and highlights the evolution of new cis-regulatory elements required for cell-specific gene expression.
Author Summary
Diversification of mechanisms regulating gene expression of key developmental factors is a major force in the evolution of development. However, in the past, comparisons of gene expression across different species have often been qualitative (i.e. ‘expression is on versus off’ in a certain cell) without precise quantification. New experimental methods now allow us to quantitatively compare the expression of gene homologs across species, with single cell resolution. Moreover, the development of genome editing tools enables the dissection of regulatory DNA sequences that drive gene expression. We use here a well-established “textbook” example of animal organogenesis in the microscopic nematode, Caenorhabditis elegans, focusing on the expression of lin-3, coding for the main inducer of the vulva, in a single cell called the anchor cell. We find that the lin-3 expression level is remarkably conserved, with 20–25 messenger RNAs per anchor cell, in species that are molecularly as distant as fish and mammals. This conservation occurs despite substantial changes and compensation in the regulatory elements required for cell-specific gene expression.
Introduction
Developmental systems operate in the presence of stochastic, environmental and genetic perturbations. While the output of a developmental system may be under stabilizing selection and remain mostly invariant, many internal variables such as the expression of a key gene or the activity of signalling pathways can be sensitive to perturbations. To reach a quantitative understanding of developmental systems, a key approach is to measure the sensitivity of the developmental system output to induced variation in an intermediate developmental phenotype. Whether and how this intermediate developmental phenotype varies within and among species then becomes a relevant evolutionary question [1]. The present work addresses the evolution of the expression level of the inducer of vulval development, lin-3, on which we previously performed a sensitivity analysis by manipulating its genetic dosage and addressing the phenotypic consequences for the developmental system [2].
The site and level of transcription of a gene can be modulated both in cis to the gene through cis-regulatory DNA sites directly influencing its transcription, or in trans due to evolution of trans-factors modifying the cellular context in which the gene is acting [3]. cis-regulatory sites containing binding sites for transcription factors often occur upstream of the coding region or in introns. These binding sites are often organized in modules, hence the designation as cis-regulatory modules (CRMs), acting in concert to enhance or repress gene expression in a given tissue at a given time. Changes in the number, relative order, orientation and spacing of transcription factor binding sites can affect transcription, often in a tissue-specific manner [4–6]. Tissue-specificity of CRMs is important for organismal evolution as it is thought to contribute to evolutionary novelty by minimizing pleiotropy [7–12]. Comparative studies in closely related species have revealed that transcriptional regulation can evolve through either extensive rewiring, or quantitative variation in the molecular components of a conserved network [11,13–17]. In particular, changes in cis-regulatory elements directly influencing the expression of critical developmental regulators have been shown to be a driving force for evolutionary innovation and phenotypic novelty in a variety of organisms. One example in Caenorhabditis concerns evolution between C. elegans and C. briggsae in the expression pattern of the transcription factor lin-48 in the excretory system, resulting in a morphological change in excretory cell position. In this case, lin-48 expression was gained in the excretory duct cell of C. elegans due to the acquisition of upstream binding sites for the transcription factor CES-2 [18,19].
Several features now make nematodes excellent experimental systems to understand gene expression evolution. First, rhabditid nematode species present a great advantage because homologous cells are easy to identify [20] so gene expression can be measured in a given cell. Second, the model organism Caenorhabditis elegans and other congeneric nematodes are amenable to functional genetics, transgenesis and now genome editing [21–26]. While transgenesis in C. elegans has long relied on formation of extra-chromosomal arrays containing many copies of the injected DNA that rearrange in an uncontrolled manner [27], the integration of a single copy at a defined locus is now possible, either at the endogenous locus using CRISPR/Cas9-mediated replacements [24–26,28] or at a controlled insertion locus using Mos1-mediated single-copy insertions (MosSCI) [29]. Third, Caenorhabditis species are highly divergent at the molecular level [30,31]. For example, C. elegans is as molecularly distant to C. briggsae as human is to mouse, and C. angaria as far as zebrafish to mouse [31], providing an opportunity to study the turnover of regulatory sequences at a large evolutionary scale where the nucleotide turnover is many times saturated yet the cellular context unchanged [32]. Many new Caenorhabditis species have recently been found and fully sequenced genomes are now available [33,34] (M. Blaxter, pers. comm.). Finally, the recent advent of quantitative methods, such as single-molecule fluorescent in situ hybridisation (smFISH) [35,36], allows to compare gene expression across species. The intensity of the conventional in situ hybridization signal cannot be meaningfully compared among species (regardless of whether the same probes or different probes targeting orthologs are used), while in the smFISH method the number of spots reflecting individual mRNA molecules can be counted, allowing a quantitative study of gene expression evolution.
Here, we take advantage of these recent developments to study the expression and requirement of lin-3, a model developmental gene involved in C. elegans vulval induction. The vulva is the egg-laying and copulatory organ of nematodes, and the C. elegans vulva is now a ‘textbook’ example of animal organogenesis [37]. C. elegans vulval development involves induction of three ventral epidermal cells (P5.p-P7.p) in response to the secretion of the LIN-3 signal from the anchor cell of the somatic gonad. LIN-3 activates the EGF receptor in the vulval precursor cells closest to the anchor cell and thereby acts as the upstream major inducer of vulval fates, in three precursor cells out of the six competent cells (Fig 1A). Induction of vulval fates involves interactions between EGF-Ras-MAPK, Notch and Wnt signalling, including some established pathway crosstalks [38]. We previously showed by modulating lin-3 expression via single-copy transgenesis that the genomic level of lin-3 expression is limited within a four-fold range for the vulva to develop normally in the C. elegans N2 background [2].
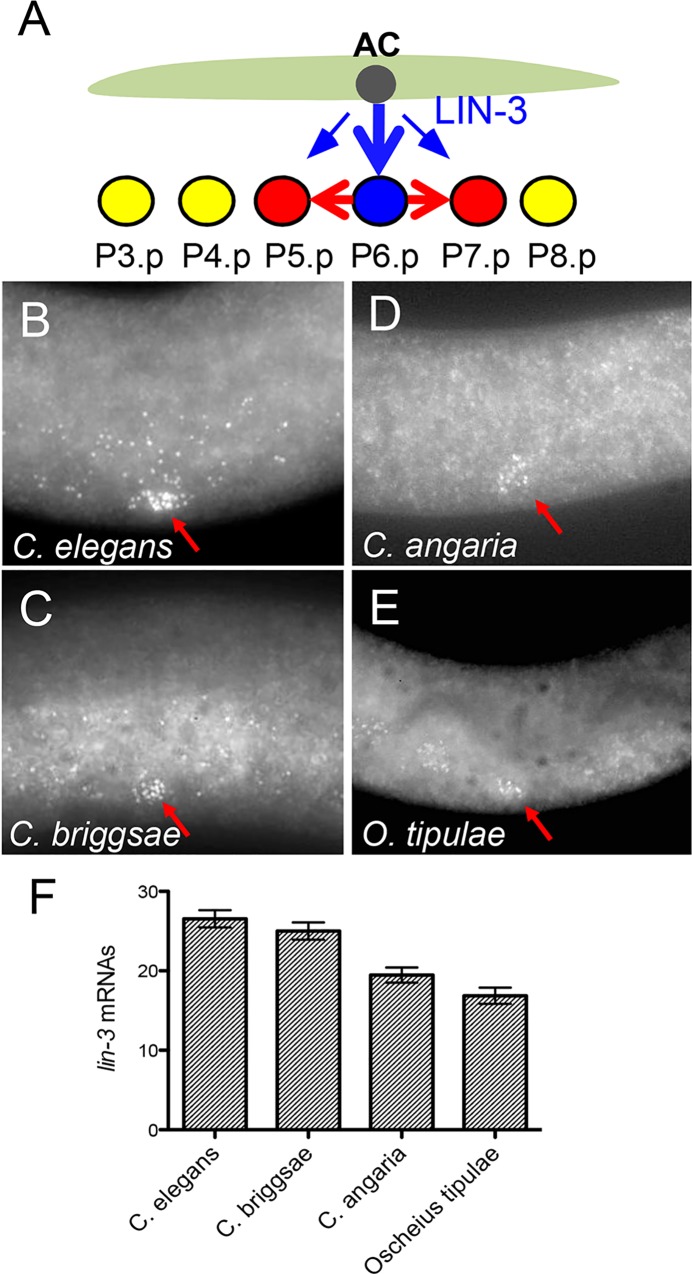
Fig 1. lin-3 expression level in the anchor cell is overall conserved in different Caenorhabditis species.
(A) Cartoon depicting the position of the anchor cell (AC) and Pn.p cells at the time of induction. Three Pn.p cells (P5.p –P7.p) are induced upon LIN-3 secretion. (B-E) smFISH using a lin-3 probe in C. elegans N2 (data from [2]) (B), C. briggsae AF16 (C), C. angaria RGD1 (D) and O. tipulae CEW1 (E). Red arrow marks the position of the anchor cell. (F) Quantification of the number of spots detected in the anchor cell of these species at the time of induction (n = 32* animals for N2, n = 24 for AF16, n = 26 for RGD1 and n = 22 for CEW1). *: these include 20 animals from [2] (see Fig 6 for an independent dataset with a similar result). The difference between C. elegans and C. briggsae is not statistically significant with a Tukey’s multiple comparison test (P value = 0.99), whereas the difference between C. elegans and C. angaria, or C. elegans and O. tipulae is significant (P values < 0.0002).
The C. elegans lin-3 gene has two alternative promoter regions, each including transcriptional and translational start sites. The lin-3 anchor cell isoform is driven by a specific cis-regulatory module lying immediately 5' of the second promoter, which is located in the first intron of the mRNA driven by the upstream promoter. Within this region, a 59 bp element was shown to be sufficient to drive expression in the anchor cell, acting as a transcriptional enhancer if placed upstream of a minimal promoter [39]. Anchor cell expression was shown to rely on two types of transcription factor binding sites in this 59 bp element, conserved in C. briggsae [39] (Fig 2): an NHR-binding site and two E-boxes. The lin-3(e1417) mutation substitutes a single nucleotide within the NHR-binding site and results in a strong reduction of lin-3 expression in the anchor cell [2,39]. This site can be bound in vitro by nuclear hormone receptors such as C. elegans NHR-25. The two E-boxes surround the NHR-binding site (E-boxL for left to the NHR and E-boxR for right), each consisting of the conserved sequence “CACCTG” but on opposite DNA strands to each other. When either of them is mutated in a lin-3::GFP transgene context, GFP expression in the anchor cell is strongly reduced [39]. We refer for simplicity to the ensemble of these three regulatory elements as the “regulatory triplet”.
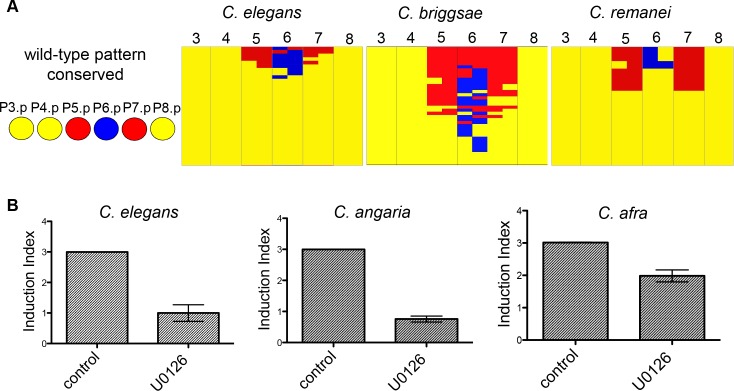
Fig 2. lin-3 activity in vulval induction is conserved in Caenorhabditis species.
(A) Comparative lin-3 RNAi effect on vulva induction in C. elegans, C. briggsae and C. remanei. Tables show graphically the observed defects in vulval cell fate pattern after scoring at least 100 nematodes. Every column is a distinct Pn.p cell (3 to 8) and 1° fate is depicted in blue, 2° fate is depicted in red and 3° fate in yellow. Half fates represent cases where the Pn.p daughter cells adopt different cell fates after the first cell division. The defects are ordered based on their consequence on vulval induction index, from high index to low. (B) Treatment with the MEK inhibitor U0126 decreases vulval induction in C. elegans (n = 15 for DMSO control and 10 μM U0126 treatment), C. angaria (n = 32 for control, n = 27 for 150 μM U0126 treatment) and C. afra (n = 100 for control, n = 30 for 150 μM treatment), as measured by the vulval induction index (average number of induced Pn.p cells at the population, wild-type index = 3). In all cases P<0.0001 with a Mann Whitney test.
We show here that a relative stability in lin-3 mRNA expression in the anchor cell and conservation of LIN-3 vulval induction activity contrasts with the turnover of cis-regulatory binding sites at the lin-3 locus. We show that the difference in requirement of regulatory elements for anchor cell expression is due to evolution in cis to the lin-3 locus without a need to infer evolution in trans. This evolution in cis occurs in a very short 58bp region upstream of the lin-3 vulval specific isoform. This study uncovers the evolution of new cis-regulatory motifs required for cell-specific gene expression.
Results
Evolutionary conservation of lin-3 mRNA expression in the anchor cell of Caenorhabditis and Oscheius
To determine the level of intraspecific variation in lin-3 expression, we quantified lin-3 expression in different C. elegans wild isolates. In the reference strain N2, a mean level of 25.4 lin-3 mRNA spots was detected using smFISH [2,40] (Fig 1B; S1A Table). We found that the mean and range of lin-3 expression in the anchor cell at the time of vulval induction are comparable between the C. elegans reference strain N2 and the most genetically divergent C. elegans isolates such as DL238 and QX1211 (S1A Fig; S1A Table).
We further explored lin-3 expression in different rhabditid species. First, we searched for the lin-3 ortholog in other available genomes (S2 Fig). The LIN-3 proteins can be aligned along their whole length, with a conserved signal peptide, EGF and trans-membrane domains. Interestingly, the most conserved parts of the proteins are the N-terminal part following the signal peptide and the intracellular domain [41].
We designed smFISH probes for the lin-3 gene of C. briggsae, C. afra, C. angaria and Oscheius tipulae and found that lin-3 is expressed in a single cell within the somatic gonad, immediately dorsal to P6.p, which we identified by DAPI staining as the anchor cell (Fig 1C–1E; S1B Fig; S1B Table). Similar to C. elegans, we also detected lin-3 expression at a lower level in the gonad outside the anchor cell and in the pharynx. We quantified fluorescent spots in the anchor cell and found no significant difference between C. elegans and C. briggsae (mean of 26.5±1 standard error in C. elegans vs. 25±1 in C. briggsae) (Fig 1F). In C. angaria and O. tipulae, we only found a small decrease compared to C. elegans (Fig 1F). Although lin-3 was clearly detected in the anchor cell of C. afra (S1B Fig), the inferior quality of the hybridisation signal compared to the background did not allow us to quantify fluorescent spots in this species. We conclude that despite the great genetic distance between these nematodes [31], the mean number of lin-3 mRNAs is remarkably conserved at least in C. briggsae and may only vary within a narrow range in C. angaria and O. tipulae.
Conserved role of LIN-3 in inducing vulval cell fates
The vulval cell fate pattern is conserved throughout the Rhabditidae family, to which the Caenorhabditis and Oscheius genera belong [42], nevertheless molecular underpinnings of vulval induction in species other than C. elegans remain mostly unknown. lin-3 RNAi experiments in C. briggsae so far produced a weak effect [43]. In Pristionchus pacificus, an outgroup and the only nematode species for which we currently have substantial molecular information related to vulval induction, vulval formation relies on Wnt signalling and is thought to be independent of the EGF pathway [44,45].
To address whether the lin-3 homolog plays a functional role in vulval induction in different Caenorhabditis species, we used a combination of RNAi and pharmacological inhibition. First, we used recently established strains of C. remanei and C. briggsae that are rendered sensitve to RNAi administered by feeding due to the expression of the C. elegans intestinal transporter sid-2 [21,46]. lin-3 RNAi treatment in these C. briggsae and C. remanei strains resulted in substantial reduction in vulval induction (Fig 2A; S3A–S3D Fig). We observed vulval cell fate phenotypes upon lin-3 RNAi that are not found in C. elegans, but are in keeping with published results revealing cryptic variation in vulval fate patterning following anchor cell laser ablations. Specifically, we found that P(5–7).p adopted a 2°-3°-2° cell fate pattern in C. remanei and a 2°-2°-2° pattern in C. briggsae [17,43]. Second, we used the MAP kinase (MEK) inhibitor U0126 that inhibits the downstream signalling events following EGF receptor activation. Application of this inhibitor has been previously shown to decrease vulval induction in O. tipulae [47]. Consistent with this result, we also obtained evidence for loss of overall vulval induction both in C. angaria and C. afra (Fig 1B; S3D Fig). Thus, we conclude that lin-3 is expressed in the anchor cell and plays a conserved role in inducing vulval fates in the Caenorhabditis genus.
The lin-3 regulatory triplet evolved at the base of the Elegans species group
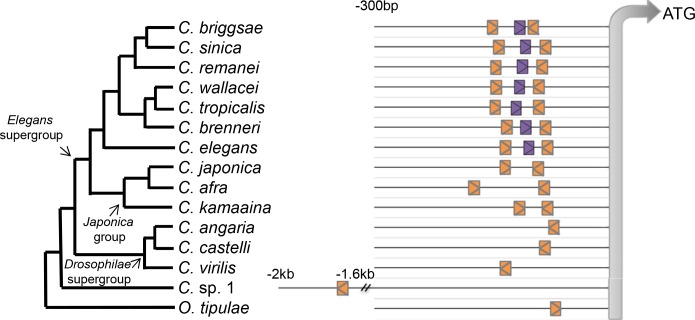
Three transcription-factor binding sites, an NHR-binding site and two E-boxes, are required for lin-3 expression in the anchor cell of C. elegans [39]. In light of the conserved expression pattern and level, we wondered whether these regulatory elements required for AC expression of lin-3 are also conserved. The regulatory triplet was found to be present in different species of the Elegans group of Caenorhabditis including C. briggsae (Figs 3, S4 and S5). However, in the sister clade, called the Japonica group, we were able to find the two E-boxes, but no putative NHR-binding site within a window of 2.5 kb upstream of the translational start site of the vulval isoform of lin-3. In further outgroup species, such as C. angaria, we only found a single E-box, and no NHR-binding site in this region. One E-box within the lin-3 CRM was also detected in the outgroup Oscheius tipulae (Fig 3). In C. sp. 1, we were able to detect a single ATG and the first E-box was only found 2 kb upstream. Overall, these observations suggest that the NHR-binding site was acquired in the branch leading to the Elegans group of the Caenorhabditis genus. The evolution of the second E-box at the base of the Caenorhabditis genus remains unclear: the second E-box may have been acquired in the branch leading to the Elegans supergroup or else be lost in the Drosophilae supergroup. No other sequence similarity could be found in the region upstream of the ATG of the vulva-expressed isoform of lin-3 (S4 Fig).
Fig 3. Evolution in the cis-regulatory elements necessary for lin-3 expression in C. elegans.
Distribution of cis-regulatory elements (the regulatory triplet) in different species. 300 bp upstream of the ATG of the vulval isoform of lin-3 are shown (and up to 2 kb for C. sp. 1, where no upstream ATG is found). Orange depicts the E-box and purple the NHR site. “>” or “<” show orientation of the regulatory site.
The above results raised an interesting conundrum. How is it possible that some elements that are required for lin-3 anchor cell expression in C. elegans are completely missing in related species, without any significant consequence for lin-3 spatial and quantitative expression?
A single C. elegans E-box cannot drive lin-3 expression in the anchor cell
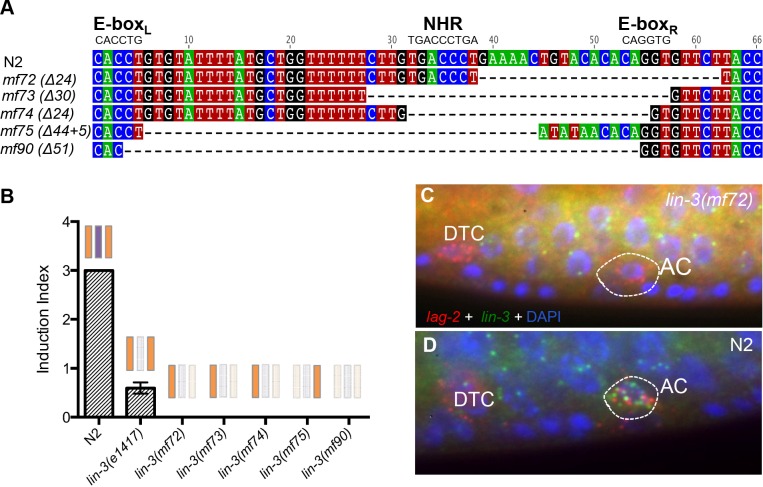
We first aimed to confirm that one E-box is not sufficient for lin-3 expression in the anchor cell in C. elegans. We used CRISPR-mediated genome editing [48] to select deletions of cis-regulatory elements of the C. elegans lin-3 gene. We generated a variety of alleles, in which either all three elements are deleted (mf90), or NHR and E-boxR are deleted leaving E-boxL intact (mf72-mf74) or only E-boxR is left intact (mf75), the latter recapitulating the cis-regulatory context of the C. angaria lin-3 upstream module (Fig 4A). All these alleles result in fully penetrant vulvaless phenotypes with no cell induced to a vulval fate, thus a stronger phenotype than the lin-3(e1417) allele with one-nucleotide substitution in the NHR binding-site (Fig 4B). We used smFISH to detect lin-3 transcripts and found no lin-3 expression in the anchor cell, which was visualised by the unperturbed expression of lag-2. Interestingly, we still detected lin-3 expression in the gonad of these mutant animals (Fig 4C and 4D). We conclude that these new lin-3 alleles are anchor cell-specific null alleles.
Fig 4. A single E-box in the Cel-lin-3 CRM is not sufficient for lin-3 expression in the anchor cell of C. elegans.
(A) New cis-regulatory lin-3 alleles with deleted E-boxL and NHR or NHR and E-boxR. (B) Quantification of vulval induction in these new mutants. Note the complete absence of any induction in the recovered lin-3 alleles (n>30). Scorings of lin-3(1417) animals are the same as those reported in Fig 5 and are used here to indicate that this mutation leads to vulval hypo-induction rather than no induction at all. (C-D) smFISH in lin-3(mf72) (C) and N2 (D) animals. Green spots correspond to lin-3 transcripts and red spots to lag-2 that is used as an anchor cell marker. Blue is DAPI staining of nuclei. Note the absence of lin-3 expression in the anchor cell in the lin-3(mf72) mutant animal. Absence of lin-3 signal in the anchor cell was also confirmed for the other lin-3 alleles.
These results confirmed that one E-box in the upstream cis-regulatory module of lin-3 is not sufficient for lin-3 expression in the anchor cell of C. elegans—whereas it appears sufficient in species of the Drosophilae group such as C. angaria.
Compensatory evolution occurs in cis to the lin-3 locus
The evolution in the requirement of transcription-factor binding sites for lin-3 expression in the anchor cell could be due to changes in cis or in trans to the lin-3 locus or both. We reasoned that if differences in trans were important, we would expect lin-3 genomic fragments derived from species missing one or two cis-regulatory elements from the regulatory triplet to be unable to be expressed in the anchor cell of C. elegans. We tested this hypothesis and obtained multiple lines of evidence suggesting no role for changes in trans to the lin-3 locus in explaining the differential binding site requirement.
First, we overexpressed in C. elegans a C. angaria lin-3 genomic fragment containing 200 bp of upstream sequence, the coding region and the 3’ UTR. This fragment drove anchor cell expression of Can-lin-3 and triggered vulval hyperinduction in C. elegans, further showing that the Can-LIN-3 protein could activate the C. elegans LET-23/EGF receptor (S6A and S6B Fig). Vulval hyperinduction was also observed when an equivalent genomic fragment from C. elegans was expressed in C. angaria or a fragment from C. afra was expressed in C. elegans (S6C and S6D Fig). These results indicate that the injected lin-3 fragments from different Caenorhabditis species contain the necessary information for anchor cell-specific expression, despite the fact that a superficially equivalent C. elegans fragment with only one E-box, as in the new lin-3 alleles described above, cannot be expressed in this cell.
Since the regulatory triplet for C. elegans anchor cell expression is missing in these transgenes, we tested whether sequences in the introns, exons or 3'UTR sequences were required for expression of the C. angaria transgene in the anchor cell. To this end, we fused the Can-lin-3 upstream sequences to a fragment containing the C. briggsae lin-3 coding sequence and 3’ UTR. We expressed this fragment in C. elegans N2 and again observed clear expression in the anchor cell. As expected, in control injections containing only the promoterless C. briggsae fragment, the transgene was not expressed anywhere in the body (S7 Fig). To further strengthen these results, we fused the lin-3 cis-regulatory modules amplified from C. elegans, C. briggsae, C. afra and C. angaria to sequences encoding an unrelated protein, the fluorescent protein Cherry, and the unrelated unc-54 3'UTR. In all cases, we observed clear expression in the anchor cell (Fig 5A), indicating again that these short cis-regulatory modules alone contain the necessary information for anchor cell-specific expression in C. elegans. We conclude that evolution within the 200 bp upstream cis-regulatory module of lin-3 is sufficient to explain the difference in requirement of regulatory elements for anchor cell expression within Caenorhabditis.
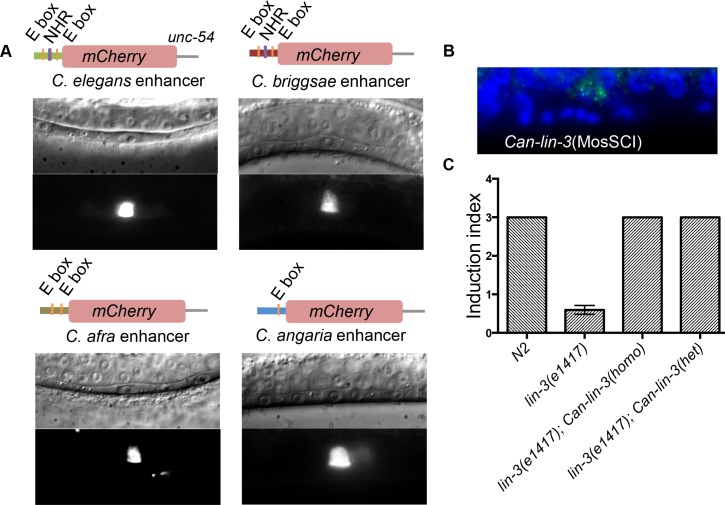
Fig 5. Evolution in lin-3 cis-element requirement does not result from a modified trans environment.
(A) Transcriptional lin-3-CRM::Cherry fusions from different Caenorhabditis species are all expressed in the anchor cell of C. elegans. (B) A Can-lin-3 single-copy transgene is expressed in the C. elegans anchor cell. smFISH detection of Can-lin-3 in C. elegans strain harbouring a single extra-copy of Can-lin-3. Green spots correspond to lin-3 expression, while blue is DAPI staining. (C) A single extra-copy of Can-lin-3 fully rescues vulval induction of the Cel-lin-3(e1417) mutant to wild-type levels, both when homozygous (two copies, n = 100) or when hemizygous (one copy, n = 30). See the corresponding brood size rescue results in S8 Fig.
The C. angaria lin-3 transgene quantitatively mimics a C. elegans lin-3 transgene
Above, we used multicopy transgenesis, which may cause sufficient expression and hyperinduction due to summing of weak transcriptional activity of many copies. We thus next asked whether the C. angaria lin-3 fragment had quantitatively a similar activity to that of its C. elegans counterpart when introduced in single copy at a targeted genomic location outside the lin-3 locus (using MosSCI transgenesis, see Methods). We found that a single-copy Can-lin-3 insertion in C. elegans N2 is expressed in the anchor cell (Fig 5B) and does not cause hyperinduction, like an equivalent Cel-lin-3 transgene copy [2]. Most interestingly, this single copy transgene could completely rescue the induction and brood size of lin-3(e1417) mutants, both in homozygous and hemizygous states (Figs 5C, S8). This quantitative behavior of the Can-lin-3 transgene (rescue in the hemizygous and homozygous state, no effect when added to the endogenous locus) recapitulates the activity of a C. elegans copy inserted at the same genomic location [2]. This experiment shows that the C. angaria lin-3 gene driven by its cis-regulatory element acts in a similar quantitative manner to the C. elegans fragment, even in the absence of the regulatory triplet.
A 58 bp cis-regulatory fragment from C. angaria with a single E-box can replace the entire C. elegans regulatory triplet
To pin down the regulatory elements in the C. angaria transgene that are required for anchor cell expression, we mutated the E-box, which is the only distinguishable regulatory element in this short upstream region. We found that Can-lin-3 genomic fragments with a mutated E-box lose their ability to be expressed in the anchor cell of C. elegans and to trigger vulval hyperinduction when expressed as multi-copy transgenes (Fig 6B, 6D and 6E). This shows that the single E-boxR of C. angaria is necessary for lin-3 expression in the anchor cell of C. elegans.
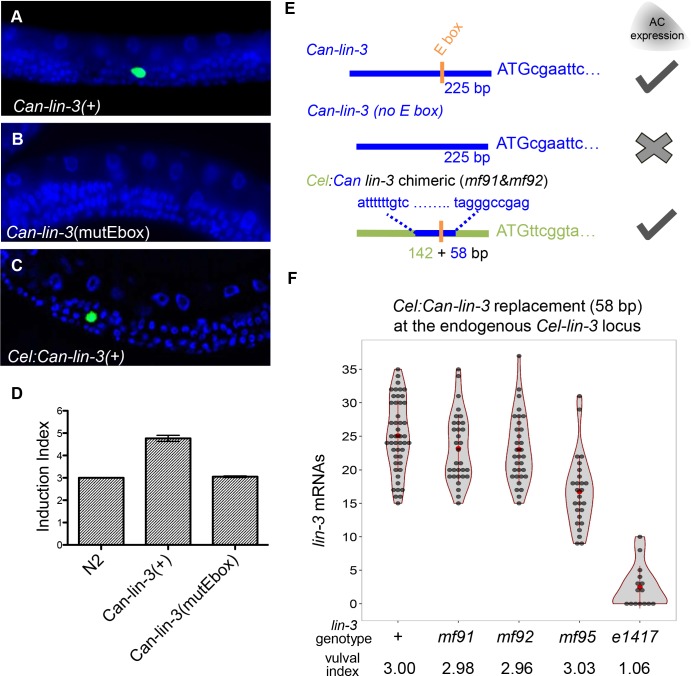
Fig 6. A 58 bp C. angaria fragment with a single E-box is able to replace the C. elegans regulatory triplet.
(A) Expression of a Can-lin-3 fragment in C. elegans containing the Can-lin-3 CRM, coding sequences and 3’ UTR leads to Can-lin-3 expression in the anchor cell, as detected by FISH. (B) Expression of the same fragment with a mutated E-box in the CRM results in loss of anchor cell expression. (C) A chimeric CRM that is mostly C. elegans apart from a 58 bp region around the regulatory triplet that is taken from C. angaria is also expressed in the anchor cell of C. elegans. (A) and (C) are using classical transgenesis in multicopy arrays experiments. (D) Over-expression of the Can-lin-3(+) fragment in C. elegans causes an increase in vulval induction, but not if the Can-lin-3 fragment with a mutated E-box is used. (E) Summary of the compensatory changes in cis to the C. angaria lin-3 locus allowing lin-3 expression in the anchor cell. C. elegans sequences are depicted in green and C. angaria sequences in blue. Orange box corresponds to the E-box. (F) Modification at the Cel-lin-3 endogenous locus, replacing the regulatory triplet with 58 bp from C. angaria containing a single canonical E-box with its genomic context (mf91 and mf92) or with 7 bp modified to its right (mf95 and mf112). The violin plots show the number of lin-3 mRNAs spots quantified in the anchor cell in the CRISPR replacements compared to N2 and lin-3(e1417) which are used as control strains. The whisker plot is superimposed in red. The average number of induced VPCs is shown below with the number of scored animals being 130, 159, 111, 42, and 35 from left to right.
Changes in the flanking sequences to core binding sites have been shown to contribute to binding efficiency of transcription factors, so we reasoned that perhaps the difference in requirement of regulatory elements for lin-3 expression in the anchor cell may rely on nucleotides adjacent to the single E-box. To this end, we synthesised a chimeric CRM, where a 58 bp central portion harbouring the regulatory triplet in C. elegans was replaced with 58 bp from C. angaria containing E-boxR (Fig 6E). We first showed that this chimeric fragment can be expressed in the C. elegans anchor cell when used in multiple-copy extra-chromosomal array transgenesis (Fig 6C). Furthermore, we used genome editing at the Cel-lin-3 locus to replace the endogenous lin-3 CRM with this chimeric CRM. We found that the genome-edited animals expressed lin-3 in the anchor cell at a normal level and produced a phenotypically wild-type vulva (Fig 6F; S2 Table).
These results demonstrate that the difference in requirement of cis-regulatory elements between C. elegans and C. angaria is explained by compensatory evolution within a very short cis-regulatory fragment (58 bp), rendering the presence of a second E-box and the NHR binding site unnecessary in C. angaria. Despite this loss of transcription factor binding sites, the activity of the cis-regulatory module in driving transcription in the anchor cell remains at the same quantitative level.
The compensation could be explained by the gain of new transcription factor binding sites in the C. angaria 58 bp regulatory region. To identify putative transcription factor binding sites, we performed a motif discovery approach in the anchor cell cis-regulatory lin-3 regions of Caenorhabditis species close to C. angaria and an exhaustive search of transcription factors that could bind the 58 bp sequence (see Methods). We found the GTTTATG sequence, a possible Forkhead-binding site, to be significantly over-represented. This sequence is only one bp to the right of the C. angaria E-box. We tested whether modifying this sequence in the 58 bp C. angaria replacement would change the lin-3 expression level. Indeed, when scrambling these 7 bp (see Methods; S2 Fig), lin-3 expression was reduced significantly to about 60% of the wild-type level (mf95 allele in Fig 6F; t-test, p-value < 6 10−8). However, as expected from a less than two-fold decrease [2], this new replacement, like the intact C. angaria CRM, produced phenotypically wild-type vulva cell fate induction (Fig 6F). Thus, we could affect the expression of the C. angaria CRM by modifying a motif adjacent to the E-box. This motif contributes to the compensation in cis in the 58 bp, but does not explain all of it, as lin-3 expression in the mf95 mutated replacement allele was still much higher than with a single C. elegans E-box.
Discussion
A quantitative account of gene expression evolution
This study addressed the level of expression of a critical developmental regulator in a single cell. We showed that both lin-3 expression level in the anchor cell and its requirement for the induction of vulval cell fates are conserved in Caenorhabditis and Oscheius nematode species. We found that the mean lin-3 mRNA level in the anchor cell only varies within 30%, despite the vast genetic divergence in this group—corresponding to that found among the most diverged vertebrates. We previously showed using quantitative perturbations that the mean level of lin-3 expression in C. elegans needs to stay within a four-fold range for a correct vulva pattern to arise and that the mean C. elegans N2 level is in the very middle (on a log scale) of this permissible zone. Therefore, it is likely that stabilizing selection acting on vulva formation [49] leads to stasis both in lin-3 expression level and in its effect on vulval induction.
By contrast with this evolutionary stasis in vulval pattern and in the lin-3 mRNA level, we showed that this cell-specific level of lin-3 expression involves substantial turnover of key cis-regulatory elements, namely the appearance of a novel binding site (NHR) and the turnover of a second copy of an existing binding site (E-box). Each of these elements is required for anchor cell expression in C. elegans yet is absent in some Caenorhabditis species. We further focused on the difference in requirement of cis-regulatory elements for lin-3 expression between C. elegans and C. angaria. A 58 bp fragment from C. angaria with a single E-box can replace the three C. elegans binding sites, demonstrating that compensatory evolution within this short cis-regulatory fragment at the lin-3 locus is sufficient to explain this difference in transcriptional regulation
Among evo-devo studies that center on comparisons of gene expression patterns and the evolution of cis-regulatory sequences, this is to our knowledge the first study taking advantage of the latest available capabilities to edit genomes and to quantify the level of mRNA expression at the single-cell level in a multicellular eukaryote.
Turnover of transcription-factor binding sites
Gene expression may evolve due to changes in cis or in trans to a given locus, two possibilities that are not mutually exclusive. Cis-regulation may occur from sites quite distant to the transcriptional unit due to long-range chromatin interactions. Our data provided strong support for compensatory cis-changes, and this in a DNA fragment directly upstream of the translational start site of the vulva specific isoform of lin-3. We cannot exclude that some further trans-changes facilitate the difference in requirement of regulatory elements between the two species. However, the cis-regulatory changes that we uncovered in this work are at least sufficient to explain the difference in requirement of regulatory elements for anchor-cell-specific gene expression in Caenorhabditis.
We have narrowed down the compensatory changes that allow the C. angaria lin-3 to be expressed in the anchor cell in a very short region of 58 bp. To explain the compensatory changes, we performed an exhaustive search of transcription factor binding sites and found a putative Forkhead binding site immediately adjacent to the E-box in C. angaria and absent from the replaced 58 bp region of C. elegans. Mutation of this site significantly lowered lin-3 expression, but insufficiently to affect the vulval induction level and it thus only partially explained the compensatory evolution in cis (Fig 6E). We further note that, because this putative Forkhead binding site is immediately adjacent to the E-box, we cannot distinguish between two scenarios: a role for another specific transcription factor binding site versus an alteration of the affinity of the E-box itself. An alternative model would indeed be that compensation occurs through a stronger affinity of the E-box in the C. angaria regulatory region, while the C. elegans E-box is insufficient to drive expression. Such differences in affinity may arise from changes in the sequences flanking the core binding sites as it has been shown for bHLH factors binding to E-boxes [50,51]. Variation in the flanking sequences next to core transcription factor binding sites has also recently been shown to influence both the levels and sites of gene expression for another developmentally important gene [52]. We conclude that the GTTTATG sequence contributes to the compensation, but does not explain it entirely.
Evolution of transcriptional regulation without change in gene expression
Here we described some evolution in cis-regulatory elements that occurs without consequences at the level of gene expression, as observed in many other genes and various groups of organisms [53–56]. This cis-regulatory element turnover in the absence of phenotypic consequence can be viewed as an extension to the notion of developmental systems drift, which posits that distinct molecular mechanisms may underlie the emergence of similar developmental phenotypes [57]. In a similar way, the conservation of gene expression pattern and level may depend on distinct molecular mechanisms due to the loss and gain of binding sites. Indeed, if the invariant output phenotype that we consider is lin-3 expression level in the anchor cell, the molecular events leading to it, such as transcription factor binding, do vary in evolution.
The best-studied example for conservation of gene expression pattern despite turnover of cis-regulatory elements is the stripe 2 enhancer of the Drosophila pair-rule gene even-skipped. The minimal stripe 2 enhancer (eve2) in D. melanogaster is a DNA region of approximately 500 bp that consists of multiple binding sites for activators such as Bicoid and Hunchback and for repressors such as Giant and Krüppel: their combination allows a confined expression in the second stripe along the antero-posterior axis of the early Drosophila embryo [58]. Compared to the described lin-3 cis-regulatory module, the eve2 stripe element involves more transcription-factor binding sites and results in expression in a group of cells (nuclei) rather than in a single cell. Similar to the lin-3 CRM, the transcription-factor binding sites change in Drosophila species in a way that binding sites required for correct expression in D. melanogaster are absent in the stripe 2 element of other species, though without leading to alteration in the expression domain, due to compensatory cis-changes [53,59]. Here we went further in replacing the endogenous cis-regulatory sequences at the locus by those of a distant species, and show a quantitative rescue of gene expression and vulval induction.
One previous example in C. elegans of turnover of binding sites involves lin-48 expression in hindgut cells, which is conserved between C. elegans and C. briggsae despite turnover of EGL-38 upstream response elements [60]. This turnover shows both similarities and differences to the described evolution of lin-3 cis-regulatory elements. The similarity is that there is an increase in the number of EGL-38 response elements in C. elegans. However, in the lin-48 case, there is evolution towards redundancy because the gain in one EGL-38 response element decreases the reliance on the existing element for correct gene expression.
More recently, evolution of cis-regulatory elements between C. elegans and C. briggsae has been studied by placing exogenous cis-regulatory elements from C. briggsae into C. elegans. A main result over several genes whose expression is conserved between the two species is the appearance of ectopic gene expression domains in these transgenic experiments, implying evolution both in cis and in trans [61,62]. In one case, the ability of the unc-47 proximal promoter from C. briggsae to drive ectopic expression in some C. elegans neurons was mapped next to a conserved cis-regulatory motif [61].
We note that the C. angaria fragment conveys the same level of transcriptional activity yet that a few vulval cell fate patterning "errors" occur in the replacement lines (Fig 6F). We observed both hypoinduced and hyperinduced variants in each of the two replacement lines (S2 Table), but the very low frequency of these variants make them difficult to study quantitatively. In the case of the eve2 enhancer, the minimal stripe element is embedded within a larger region of approximately 800 bp, and these flanking sites contribute to robustness to some genetic and environmental perturbations [63]. In Caenorhabditis, the distal promoter of unc-47, although largely not conserved, is also important for robust gene expression, acting perhaps in a sequence-independent manner [64]. It remains unclear whether any regions within and/or outside the lin-3 CRM can play a similar role in stabilizing expression of lin-3 in Caenorhabditis to different perturbations.
An evolutionary gain in binding site requirement
The distribution of lin-3 cis-regulatory elements in different Caenorhabditis nematodes and the mapping of changes on the phylogeny suggests as the most likely evolutionary scenario a gain of regulatory sites: the likely acquisition of an E-box before the common ancestor between the Elegans and Japonica groups and a gain of an NHR-binding site before the origin of the Elegans group. In addition, these sites not only appeared, but also became indispensable for lin-3 anchor cell expression at least in C. elegans.
The acquisition of such new short regulatory motifs (6 bp) is easy and gains of regulatory motifs have been observed in other systems as well [65]. Given the high robustness of vulval development to several perturbations, the evolution towards a dependence on a higher number of sites for anchor cell expression is counter-intuitive and suggestive of evolution towards fragility. It is currently unclear what drove the evolution of these novel motifs with a conserved gene expression, whether selection or drift. Gains in interconnectedness between components of transcriptional networks may often occur non-adaptively, for example if they do not disrupt the underlying regulation [66]. Such gains can also be reshaped in equivalent network configurations and eventually become necessary depending on the evolution of the transcriptional network [67].
Materials and Methods
Nematode culture, genetics and pharmacology
A complete list of strains used in this study is presented in the supplement (S3 Table). All strains were maintained at 20°C and handled according to standard procedures [68]. We used the Bristol N2 strain as a reference C. elegans strain on standard NGM plates with OP50 as a food source. The U0126 treatments were performed by supplying the DMSO-dissolved inhibitor to NGM plates at a concentration between 10–150 μM and letting synchronised L2 stage nematodes develop into L4 larvae. Control treatments in this case were performed by growing nematodes on plates supplemented with DMSO only.
For the Can-lin-3 rescue of the C. elegans lin-3(e1417) mutant, JU2495 hermaphrodites were crossed to JU2498 males and the F1 or F2 progeny were analysed for hemizygous or homozygous insertion phenotypic rescue, respectively.
Identification of lin-3 orthologs in Caenorhabditis genomes
The lin-3 genomic sequences of the different species were accessed in WormBase (www.wormbase.org; version WS252) or from the Caenorhabditis Genomes Project by Mark Blaxter's laboratory (http://bang.bio.ed.ac.uk:4567) or from Matt Rockman’s laboratory. The Oscheius tipulae genome was sequenced and assembled as a collaborative effort between M. Blaxter's and our lab (Besnard, Kotsouvolos et al., in preparation) and is available (http://oscheius.bio.ed.ac.uk/). We first used the TBLASTN algorithm conditioning only to the most identical hits, favouring those with high similarity in the N-terminal part and signal peptide, and lower e-value. Afterwards, we proceeded to predict gene bodies in these contigs using FGNESH (http://www.softberry.com) with a hidden Markov model specific to C. elegans. Finally, manual curation and annotation of the lin-3 sequences were performed using as a reference the amino-acid sequence of the closest available lin-3 ortholog.
Transcription-factor motif recognition in lin-3 promoter sequences
To study the evolution of the regulatory triplet in the Caenorhabditis clade, we analysed the promoter regions upstream of the downstream ATG corresponding to the N-terminal exon homologous to that known to be expressed in the AC of C. elegans (S2 Fig). First, to address whether the cis-regulatory C. elegans NHR-binding sites and E-boxes were present in the other species, we performed a scan in the promoters with the position weight matrices of HLH-2 and NHR-proteins available in JASPAR [69] using matrix-scan [70] and a n = 2 Hidden Markov Model specific to C. elegans (Fig 3). Similarly, we looked in these regions for DNA patterns known to be binding sites of bHLH proteins [51] using the dna-pattern tool present in the RSAT suite [71]. Once we had the position of these sites across the promoter regions, we proceeded to plot their location using RSAT feature-map tool (S5 Fig).
Additionally, we looked for DNA motifs different from the cis-regulatory C. elegans NHR and E-boxes binding sites by performing a motif-discovery approach in Caenorhabditis lin-3 promoters using the RSAT tool oligo-analysis [71]. The top over-represented words of length 6, 7 and 8 base pairs were compared to known motifs available in JASPAR. We thus identify the GTTTATG to the right of the E-box. Finally, to identify possible transcription factors acting on the AC lin-3 expression in the 58 bp C. angaria fragment, we performed an exhaustive search of the full JASPAR motif repertoire in the 58 bp replaced sequence using RSAT matrix-scan. This search found the putative Forkhead-binding motif and a putative overlapping bZIP-binding motif (Fos/Jun repressors). The 7 bp modification in the mf95 replacement also affected this predicted binding site of bZip transcription factors.
Cloning
All lin-3 CRMs reside directly upstream of the ATG of the vulval isoform of lin-3. To create the lin-3 CRM::Cherry::unc-54 constructs, we used a three-fragment Gateway approach merging the lin-3 CRMs cloned in pDONOR P4-P1R, the Cherry ORF cloned in pDONOR 221 and the unc-54 3’UTR cloned in pDONOR P2R-P3. All primer sequences containing attB4 forward and attB1 reverse recombination sites used to amplify the CRMs from gDNA from different species are shown in S4 Table.
unc-54 3’ UTR was amplified from N2 genomic DNA using primers unc-54attB2 and unc-54attB3. Worm-optimised Cherry was amplified from pAA64 using primers containing the attB1and attB2 sites. All constructs were injected at 10 ng/μl with myo-2::GFP as co-injection marker and pBluescript as carrier DNA.
To create the Can-lin-3 insertion by MosSCI, we amplified a 2.9 kb lin-3 fragment from C. angaria genomic DNA using primers Canlin-3AvrII and Canlin-3XhoI. The amplicon was cloned into pCFJ151 (chromosome II targeting vector) [29] as an AvrII/XhoI fragment. Injections and recovery of insertions were performed using the direct insertion protocol, as previously described.
To overexpress lin-3 fragments in C. elegans or C. angaria, we amplified genomic fragments amplified from C. elegans (5.2 kb), C. angaria (3.2 kb) and C. afra (5.1 kb) using primer pairs RH9for/RH9rev, Canlin-3F2/Canlin-3R1 and Caflin-3oxF2/Caflin-3oxR1, respectively. The PCR products were injected directly (30 ng/μl) together with pBluescript as carrier and myo-2::GFP as co-injection marker.
To mutagenize the E-box in the C. angaria lin-3 CRM, the above 3.2 kb fragment was cloned into pGEM-Teasy and the 5’-CAGGTG-3’ sequence was modified to 5’-CAGGAA-3’ using primers t211a_g212a/ t211a_g212a_anti and standard in vitro site directed mutagenesis.
The chimeric construct replacing a 58 bp region containing the C. elegans regulatory triplet (5’-cacctgtgtattttatgctggttttttcttgtgaccctgaaaactgtacacacaggtg-3’) with a similar in length sequence from C. angaria containing only one E-box (5’-attttttgtcaaagatttttcggcgccaggtgtgtttatgactcatgttagggccgag-3’) was synthesised by Genewiz. This construct was used as PCR template to permute 7 bp to the right of the C. angaria E-box (5’-CAGGTGtGTTTATG-3’ to 5’-CAGGTGtTTGGATT-3’).
The chimeric construct to drive Cbr-lin-3 under the C. angaria CRM was built using fusion PCR. Briefly, the Can-lin-3 CRM was amplified from C. angaria genomic DNA with primers Canlin-3 F2 and CaACFusion and the Cbr-lin-3 region coding region and 3’ UTR from C. briggsae genomic DNA with primers Cbrlin-3F1 and Cbrlin-3R1. The two amplicons were then fused together using a third PCR reaction with primers Canlin-3F2 and Cbrlin-3R1. The final product was injected as a PCR fragment at 20 ng/μl concentration.
Single molecule fluorescence in situ hybridization
smFISH was performed in synchronized populations of L3 stage animals using short fluorescently labelled oligos as probes, as previously described [2]. The animals were age-synchronized by bleaching, followed by hatching of embryos in M9 buffer. The L1 larvae were then placed onto culture plates with food until the L3 stage, as determined by Nomarski microscopy, and then fixed.
The C. elegans lin-3 and lag-2 probes have been previously described [2]. The low level of genetic divergence within C. elegans allowed us to detect fluorescent spots while using the same FISH probe as in the N2 strain.
For all other species we followed the same protocol as with C. elegans with the following two modifications to decrease the more pronounced background fluorescence. We used 20% formamide in the hybridisation and wash solutions and performed three washes post-hybridisation instead of two in C. elegans. Given that we are using different probes consisting of fewer oligos for the detection of lin-3 transcripts in these species together with slightly more stringent hybridisation conditions, the observed difference in the number of fluorescence spots may thus even be due to technical rather than biological reasons. The sequences of the new lin-3 probes can be found in S5 Table. The probes were labelled with Quasar 670 (Biosearch Technologies) and diluted to 100–200 nM for the overnight hybridisation.
RNAi
RNAi was performed by feeding the animals with dsRNA-expressing bacteria, as previously described [2]. The C. elegans lin-3 RNAi feeding clone used in this study is from the Ahringer RNAi library (Source Bioscience). A Cre-lin-3 fragment was amplified using oligos Crelin-3RNAiF1 and Crelin-3RNAiR1 that contain an XhoI restriction site. The PCR product was cloned into L4440 as an XhoI fragment. To create the C. briggsae lin-3 RNAi clone, a fragment was amplified using primers Cbrlin-3RNAiF1 and Cbrlin-3RNAiR1 and then cloned into pDONR 221 (Invitrogen) using attB1F and attB2R universal oligonucleotides. The lin-3 fragment was sequence verified and transferred to a Gateway compatible L4440 plasmid. Both constructs were transformed into E. coli HT115 for use in C. elegans feeding.
Phenotypic characterisation
To score the vulval cell fate pattern, nematodes were mounted with M9 on 3% agar pads containing 10 mM sodium azide and analysed under Nomarski optics. Standard criteria were used to infer cell fates based on the topology and number of cells at the L4 stage [43,72]. Half fates were assigned when two daughters of the Pn.p cells acquired distinct fates after the first cell division.
Genome editing
We followed the CRISPR/Cas9 target design and used reagents as previously described [48]. We targeted the following region at the C. elegans lin-3 CRM 5’-accctgaaaactgtacacacAGG-3’ with AGG representing the PAM motif. We replaced the unc-119 target site under the pU6 promoter [48] with the lin-3 target site using fusion PCR first with primers E-box2A gRNA-F/ U6prom HindIII and E-box2A gRNA-R/ oligos U6prom EcoRI F followed by amplification of the full sgRNA fragment with U6prom EcoRI F/ U6prom HindIII R. The only modification was that we did not clone the lin-3 sgRNA in a vector but injected it directly as a PCR product (40 ng/μl, together with 40 ng/μl eft-3::Cas-9 and myo-2::GFP as co-injection marker).
To replace the endogenous lin-3 cis-regulatory element of C. elegans by a 58 bp lin-3 element from C. angaria, we first obtained a chimeric double-stranded DNA as homologous recombination template, using Gibson assembly of C. elegans lin-3 promoter extremities with 58 bp of the C. angaria lin-3 upstream sequence. In a similar fashion, we obtained a homologous recombination template identical to the previous but with modified bases next to the C. angaria E-box. Oligonucleotide sequences are found in S4 Table. C. elegans N2 animals were injected with a DNA mix containing the Peft-3::Cas9 plasmid, the pU6::dpy-10 sgRNA plasmid (co-CRISPR marker), the Ebox-2A sgRNA containing plasmid and the double-stranded DNA repair templates (independently), with final concentrations of 50, 40, 100, and 30 ng/μl, respectively. On plates with a high number of animals displaying the Dpy phenotype, the F1 progeny were singled, and their progeny screened by PCR. Broods from independent P0 animals were found positive and rendered homozygous (two independent lines for each replacement). Both replacements were confirmed by Sanger sequencing. The resulting lines were given allele names mf91 and mf92 for the first replacement, and mf95 and mf112 for the second one.
Supporting Information
(A) smFISH quantification of Cel-lin-3. The level of lin-3 expression in other C. elegans isolates is similar to that in the N2 reference strain (n≥14 animals; S1A Table. (B) smFISH localising lin-3 transcripts in the anchor cell of C. afra. Serial optical sectioning through the anchor cell of a single animal showing lin-3 fluorescent spots.
(TIF)
The sequences of the enhancers used in Fig 5A are in bold. The endogenous 3’ UTR used for the overexpression experiments in Figs 5B, 6A–6D, S6 and S7 is underlined.
(DOC)
(A-D) Nomarski images of L4 stage animals upon lin-3 RNAi (A-C) or MEK inhibitor (U0126) treatment (D). (A-C) lin-3 RNAi by feeding in C. remanei strain JU1184 results in 2°-3°-2° (B) or 3°-3°-3° (C) vulval cell fates for P(5–7).p compared to the 2°-1°-2° of the wild-type (A). (D) Treatment with U0126 decreases vulval induction in C. angaria and C. afra. Note uninduced cells in both cases. The vulva in control L4 animals shows the typical “Christmas tree” morphology in all species.
(TIF)
(A-C) Alignment of the 300 bp region upstream of the lin-3 ATG shows no other similarity in different species outside the E-box (B) and NHR (C) binding sites. Cbr = C. briggsae, Csi = C. sinica, Cre = C. remanei, Cwa = C. wallacei, Ctr = C. tropicalis, Cbn = C. brenneri, Cel = C. elegans, Cja = C. japonica, Caf = C. afra, Can = C. angaria. (D) Comparison of the Drosophila FushiTarazu/F1 (FTZ-F1) binding site, the NHR-binding site in wild-type C. elegans and lin-3(e1417) mutant. At least two nucleotide changes are required to align putative NHR binding sites from the Japonica group of the Caenorhabditis genus to the sequence in C. elegans and multiple changes are required for C. angaria.
(TIF)
Location of transcription factor binding sites belonging to the bHLH protein family (as described in [51]) across DNA sequences upstream the TSS of the vulval form of lin-3 mRNA. The location of the NHR-binding site belonging to the lin-3 regulatory triplet is also depicted. Only the first 500 bp before the ATG are displayed for most of the species, except for C. virilis (only 300 bp) and C. sp. 1 (up to 1.9 Kb).
(TIF)
(A) Wild-type C. elegans vulval invagination in the L4 stage as seen by Nomarski optics. (B) Over-expression of Can-lin-3(+) in C. elegans via transgenesis with repeated extra-chromosomal arrays results in vulval hyper-induction, with several additional invaginations in the L4 stage (arrowheads). (C) Injection of a Cel-lin-3 fragment in C. angaria leads to vulval hyperinduction. (D) Over-expression of a Caf-lin-3 fragment in C. elegans with repeated extra-chromosomal arrays leads to vulval hyperinduction.
(TIF)
(A) A promoterless C. briggsae fragment introduced into C. elegans is not expressed in N2. (B) The same fragment under the Can-lin-3 CRM drives expression in the anchor cell of N2, as monitored using Cbr-lin-3 FISH. Green corresponds to lin-3 expression and blue is DAPI staining of nuclei.
(TIF)
A single-copy insertion of Can-lin-3(+) rescues brood size defects of lin-3(e1417) mutants (n>15). Note that the presence of a myo-2::GFP transgene linked to lin-3(e1417) in the background enhances the lin-3(e1417) brood size defects and does not allow rescue to wild-type brood size. Vulval induction in this experiment is presented in Fig 5C.
(TIF)
lin-3 mRNA single-molecule FISH quantification in the anchor cell in different C. elegans wild isolates (sheet A) and different Caenorhabditis species (sheet B). Each entry is an individual animal. Mean and standard deviation (SD) are indicated at the bottom.
(XLSX)
The genotype is indicated in the name of the sheet. Gonad size of each individual is given in pixels. Experimental batch is indicated in the "Batch" column.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Lewis Stevens, Mark Blaxter and the Caenorhabditis Genomes Project, as well as Matt Rockman and Luke Noble for early access to unpublished genome assemblies. We are grateful to Jean-Louis Bessereau and Aurélien Richaud for advice and technical help on CRISPR replacements. We gratefully acknowledge John Reece-Hoyes and Marian Walhout for one-hybrid screens with lin-3 fragments from different species. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We also thank WormBase.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by a grant from the Agence Nationale de la Recherche (ANR12-BSV2-0004-01). MB was supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale (FRM) and a Research Project Grant by the Leverhulme Trust. AMVV is supported by the French government through ANR10-LABX-54 MEMOLIFE. We also acknowledge the support of the Bettencourt Schueller Foundation (Coup d’Elan 2011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Félix MA (2012) Evolution in developmental phenotype space. Curr Opin Genet Dev 22: 593–599. 10.1016/j.gde.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Barkoulas M, van Zon JS, Milloz J, van Oudenaarden A, Félix MA (2013) Robustness and epistasis in the C. elegans vulval signaling network revealed by pathway dosage modulation. Dev Cell 24: 64–75. 10.1016/j.devcel.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Carroll SB (2005) Evolution at two levels: on genes and form. PLoS Biol 3: e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson CI, Evans NC, Barolo S (2010) Structural rules and complex regulatory circuitry constrain expression of a Notch- and EGFR-regulated eye enhancer. Dev Cell 18: 359–370. 10.1016/j.devcel.2009.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson CI, Schwimmer DB, Barolo S (2011) Rapid evolutionary rewiring of a structurally constrained eye enhancer. Curr Biol 21: 1186–1196. 10.1016/j.cub.2011.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erceg J, Saunders TE, Girardot C, Devos DP, Hufnagel L, Furlong EE (2014) Subtle changes in motif positioning cause tissue-specific effects on robustness of an enhancer's activity. PLoS Genet 10: e1004060 10.1371/journal.pgen.1004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern DL (2000) Evolutionary developmental biology and the problem of variation. Evolution 54: 1079–1091. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro MD, Marks ME, Peichel CL, Blackman BK, Nereng KS, Jonsson B, Schluter D, Kingsley DM (2004) Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature 428: 717–723. [DOI] [PubMed] [Google Scholar]
- 9.Gompel N, Prud'homme B, Wittkopp PJ, Kassner VA, Carroll SB (2005) Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature 433: 481–487. [DOI] [PubMed] [Google Scholar]
- 10.McGregor AP, Orgogozo V, Delon I, Zanet J, Srinivasan DG, Payre F, Stern DL (2007) Morphological evolution through multiple cis-regulatory mutations at a single gene. Nature 448: 587–590. [DOI] [PubMed] [Google Scholar]
- 11.Tuch BB, Galgoczy DJ, Hernday AD, Li H, Johnson AD (2008) The evolution of combinatorial gene regulation in fungi. PLoS Biol 6: e38 10.1371/journal.pbio.0060038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung H, Loehlin DW, Dufour HD, Vaccarro K, Millar JG, Carroll SB (2014) A single gene affects both ecological divergence and mate choice in Drosophila. Science 343: 1148–1151. 10.1126/science.1249998 [DOI] [PubMed] [Google Scholar]
- 13.Gasch AP, Moses AM, Chiang DY, Fraser HB, Berardini M, Eisen MB (2004) Conservation and evolution of cis-regulatory systems in ascomycete fungi. PLoS Biol 2: e398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanay A, Regev A, Shamir R (2005) Conservation and evolvability in regulatory networks: the evolution of ribosomal regulation in yeast. Proc Natl Acad Sci U S A 102: 7203–7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnoult L, Su KF, Manoel D, Minervino C, Magrina J, Gompel N, Prud'homme B (2013) Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science 339: 1423–1426. 10.1126/science.1233749 [DOI] [PubMed] [Google Scholar]
- 16.Wittkopp PJ (2007) Variable gene expression in eukaryotes: a network perspective. J Exp Biol 210: 1567–1575. [DOI] [PubMed] [Google Scholar]
- 17.Hoyos E, Kim K, Milloz J, Barkoulas M, Pénigault JB, Munro E, Félix MA (2011) Quantitative variation in autocrine signaling and pathway crosstalk in the Caenorhabditis vulval network. Curr Biol 21: 527–538. 10.1016/j.cub.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Chamberlin H (2002) Multiple regulatory changes contribute to the evolution of the Caenorhabditis lin-28 ovo gene. Genes Dev 16: 2345–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Chamberlin HM (2004) Evolutionary innovation of the excretory system in Caenorhabditis elegans. Nat Genet 36: 231–232. [DOI] [PubMed] [Google Scholar]
- 20.Félix M-A (1999) Evolution of developmental mechanisms in nematodes. J Exp Zool (Mol Dev Evol) 28: 3–18. [DOI] [PubMed] [Google Scholar]
- 21.Nuez I, Félix M-A (2012) Evolution of susceptibility to ingested double-stranded RNAs in Caenorhabditis nematodes. PLoS One 7: e29811 10.1371/journal.pone.0029811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Q, Shen Y, Chen X, Shifman Y, Ellis RE (2014) Rapid creation of forward-genetics tools for C. briggsae using TALENs: lessons for nonmodel organisms. Mol Biol Evol 31: 468–473. 10.1093/molbev/mst213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frokjaer-Jensen C (2013) Exciting prospects for precise engineering of Caenorhabditis elegans genomes with CRISPR/Cas9. Genetics 195: 635–642. 10.1534/genetics.113.156521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B (2013) Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat Methods 10: 1028–1034. 10.1038/nmeth.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Fenk LA, de Bono M (2013) Efficient genome editing in Caenorhabditis elegans by CRISPR-targeted homologous recombination. Nucleic Acids Res 41: e193 10.1093/nar/gkt805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arribere JA, Bell RT, Fu BX, Artiles KL, Hartman PS, Fire AZ (2014) Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198: 837–846. 10.1534/genetics.114.169730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mello C, Fire A (1995) DNA transformation In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans Modern Biological Analysis of an Organism. San Diego: Academic Press; pp. 451–482. [Google Scholar]
- 28.Paix A, Wang Y, Smith HE, Lee CY, Calidas D, Lu T, Smith J, Schmidt H, Krause MW, Seydoux G (2014) Scalable and versatile genome editing using linear DNAs with microhomology to Cas9 Sites in Caenorhabditis elegans. Genetics 198: 1347–1356. 10.1534/genetics.114.170423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM (2008) Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40: 1375–1383. 10.1038/ng.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent MR, et al. (2003) The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol 1: 166–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DH (2004) Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci USA 101: 9003–9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Félix M-A (2004) Genomes: a helpful cousin for our favourite worm. Curr Biol 14: R75–R77. [PubMed] [Google Scholar]
- 33.Kiontke K, Félix M-A, Ailion M, Rockman MV, Braendle C, Pénigault J-B, Fitch DH (2011) A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol Biol 11: 339 10.1186/1471-2148-11-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortazavi A, Schwarz EM, Williams B, Schaeffer L, Antoshechkin I, Wold BJ, Sternberg PW (2010) Scaffolding a Caenorhabditis nematode genome with RNA-seq. Genome Res 20: 1740–1747. 10.1101/gr.111021.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raj A, Rifkin SA, Andersen E, van Oudenaarden A (2010) Variability in gene expression underlies incomplete penetrance. Nature 463: 913–918. 10.1038/nature08781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregor T, Garcia HG, Little SC (2014) The embryo as a laboratory: quantifying transcription in Drosophila. Trends Genet 30: 364–375. 10.1016/j.tig.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sternberg PW (2005) Vulval development. In WormBook, The C elegans Research Community, ed.,http://www.wormbook.org, 10.1895/wormbook.1.6.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Félix M-A, Barkoulas M (2012) Robustness and flexibility in nematode vulva development. TIG 28: 185–195. 10.1016/j.tig.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 39.Hwang BJ, Sternberg PW (2004) A cell-specific enhancer that specifies lin-3 expression in the C. elegans anchor cell for vulval development. Development 131: 143–151. [DOI] [PubMed] [Google Scholar]
- 40.Saffer AM, Kim DH, van Oudenaarden A, Horvitz HR (2011) The Caenorhabditis elegans synthetic multivulva genes prevent ras pathway activation by tightly repressing global ectopic expression of lin-3 EGF. PLoS Genet 7: e1002418 10.1371/journal.pgen.1002418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Tzou P, Hill RJ, Sternberg PW (1999) Structural requirements for the tissue-specific and tissue-general functions of the C. elegans epidermal growth factor LIN-3. Genetics 153: 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiontke K, Barrière A, Kolotuev I, Podbilewicz B, Sommer RJ, Fitch DHA, Félix M-A (2007) Trends, stasis and drift in the evolution of nematode vulva development. Curr Biol 17: 1925–1937. [DOI] [PubMed] [Google Scholar]
- 43.Félix M-A (2007) Cryptic quantitative evolution of the vulva intercellular signaling network in Caenorhabditis. Curr Biol 17: 103–114. [DOI] [PubMed] [Google Scholar]
- 44.Tian H, Schlager B, Xiao H, Sommer RJ (2008) Wnt signaling induces vulva development in the nematode Pristionchus pacificus. Curr Biol 18: 142–146. 10.1016/j.cub.2007.12.048 [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Sommer RJ (2011) Antagonism of LIN-17/Frizzled and LIN-18/Ryk in nematode vulva induction reveals evolutionary alterations in core developmental pathways. PLoS Biol 9: e1001110 10.1371/journal.pbio.1001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winston WM, Molodowitch C, Hunter CP (2000) Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456–2459. [DOI] [PubMed] [Google Scholar]
- 47.Dichtel-Danjoy M-L, Félix M-A (2004) The two steps of vulval induction in Oscheius tipulae CEW1 recruit common regulators including a MEK kinase. Dev Biol 265: 113–126. [DOI] [PubMed] [Google Scholar]
- 48.Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM, Calarco JA (2013) Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods 10: 741–743. 10.1038/nmeth.2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braendle C, Baer CF, Félix MA (2010) Bias and evolution of the mutationally accessible phenotypic space in a developmental system. PLoS Genet 6: e1000877 10.1371/journal.pgen.1000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walhout AJ, van der Vliet PC, Timmers HT (1998) Sequences flanking the E-box contribute to cooperative binding by c-Myc/Max heterodimers to adjacent binding sites. Biochim Biophys Acta 1397: 189–201. [DOI] [PubMed] [Google Scholar]
- 51.Grove CA, De Masi F, Barrasa MI, Newburger DE, Alkema MJ, Bulyk ML, Walhout AJ (2009) A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell 138: 314–327. 10.1016/j.cell.2009.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farley EK, Olson KM, Zhang W, Brandt AJ, Rokhsar DS, Levine MS (2015) Suboptimization of developmental enhancers. Science 350: 325–328. 10.1126/science.aac6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludwig MZ, Bergmann C, Patel NH, Kreitman M (2000) Evidence for stabilizing selection in a eukaryotic enhancer element. Nature 403: 564–567. [DOI] [PubMed] [Google Scholar]
- 54.Fisher S, Grice EA, Vinton RM, Bessling SL, McCallion AS (2006) Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science 312: 276–279. [DOI] [PubMed] [Google Scholar]
- 55.Hare EE, Peterson BK, Iyer VN, Meier R, Eisen MB (2008) Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genet 4: e1000106 10.1371/journal.pgen.1000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paris M, Kaplan T, Li XY, Villalta JE, Lott SE, Eisen MB (2013) Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet 9: e1003748 10.1371/journal.pgen.1003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.True JR, Haag ES (2001) Developmental system drift and flexibility in evolutionary trajectories. Evol Dev 3: 109–119. [DOI] [PubMed] [Google Scholar]
- 58.Small S, Blair A, Levine M (1992) Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J 11: 4047–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludwig MZ, Palsson A, Alekseeva E, Bergman CM, Nathan J, Kreitman M (2005) Functional evolution of a cis-regulatory module. PLoS Biol 3: e93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Greenberg JF, Chamberlin HM (2004) Evolution of regulatory elements producing a conserved gene expression pattern in Caenorhabditis. Evol Dev 6: 237–245. [DOI] [PubMed] [Google Scholar]
- 61.Barrière A, Gordon KL, Ruvinsky I (2012) Coevolution within and between regulatory loci can preserve promoter function despite evolutionary rate acceleration. PLoS Genet 8: e1002961 10.1371/journal.pgen.1002961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrière A, Ruvinsky I (2014) Pervasive divergence of transcriptional gene regulation in Caenorhabditis nematodes. PLoS Genet 10: e1004435 10.1371/journal.pgen.1004435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ludwig MZ, Manu, Kittler R, White KP, Kreitman M (2011) Consequences of eukaryotic enhancer architecture for gene expression dynamics, development, and fitness. PLoS Genet 7: e1002364 10.1371/journal.pgen.1002364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrière A, Gordon KL, Ruvinsky I (2011) Distinct functional constraints partition sequence conservation in a cis-regulatory element. PLoS Genet 7: e1002095 10.1371/journal.pgen.1002095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold CD, Gerlach D, Spies D, Matts JA, Sytnikova YA, Pagani M, Lau NC, Stark A (2014) Quantitative genome-wide enhancer activity maps for five Drosophila species show functional enhancer conservation and turnover during cis-regulatory evolution. Nat Genet 46: 685–692. 10.1038/ng.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch M (2007) The evolution of genetic networks by non-adaptive processes. Nat Rev Genetics 8: 803–813. [DOI] [PubMed] [Google Scholar]
- 67.Sorrells TR, Johnson AD (2015) Making sense of transcription networks. Cell 161: 714–723. 10.1016/j.cell.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathelier A, Zhao X, Zhang AW, Parcy F, Worsley-Hunt R, et al. (2014) JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res 42: D142–147. 10.1093/nar/gkt997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turatsinze JV, Thomas-Chollier M, Defrance M, van Helden J (2008) Using RSAT to scan genome sequences for transcription factor binding sites and cis-regulatory modules. Nat Protoc 3: 1578–1588. 10.1038/nprot.2008.97 [DOI] [PubMed] [Google Scholar]
- 71.Medina-Rivera A, Defrance M, Sand O, Herrmann C, Castro-Mondragon JA, et al. (2015) RSAT 2015: Regulatory Sequence Analysis Tools. Nucleic Acids Res 43: W50–56. 10.1093/nar/gkv362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katz WS, Hill RJ, Clandinin TR, Sternberg PW (1995) Different levels of the C. elegans growth factor LIN-3 promote distinct vulval precursor fates. Cell 82: 297–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) smFISH quantification of Cel-lin-3. The level of lin-3 expression in other C. elegans isolates is similar to that in the N2 reference strain (n≥14 animals; S1A Table. (B) smFISH localising lin-3 transcripts in the anchor cell of C. afra. Serial optical sectioning through the anchor cell of a single animal showing lin-3 fluorescent spots.
(TIF)
The sequences of the enhancers used in Fig 5A are in bold. The endogenous 3’ UTR used for the overexpression experiments in Figs 5B, 6A–6D, S6 and S7 is underlined.
(DOC)
(A-D) Nomarski images of L4 stage animals upon lin-3 RNAi (A-C) or MEK inhibitor (U0126) treatment (D). (A-C) lin-3 RNAi by feeding in C. remanei strain JU1184 results in 2°-3°-2° (B) or 3°-3°-3° (C) vulval cell fates for P(5–7).p compared to the 2°-1°-2° of the wild-type (A). (D) Treatment with U0126 decreases vulval induction in C. angaria and C. afra. Note uninduced cells in both cases. The vulva in control L4 animals shows the typical “Christmas tree” morphology in all species.
(TIF)
(A-C) Alignment of the 300 bp region upstream of the lin-3 ATG shows no other similarity in different species outside the E-box (B) and NHR (C) binding sites. Cbr = C. briggsae, Csi = C. sinica, Cre = C. remanei, Cwa = C. wallacei, Ctr = C. tropicalis, Cbn = C. brenneri, Cel = C. elegans, Cja = C. japonica, Caf = C. afra, Can = C. angaria. (D) Comparison of the Drosophila FushiTarazu/F1 (FTZ-F1) binding site, the NHR-binding site in wild-type C. elegans and lin-3(e1417) mutant. At least two nucleotide changes are required to align putative NHR binding sites from the Japonica group of the Caenorhabditis genus to the sequence in C. elegans and multiple changes are required for C. angaria.
(TIF)
Location of transcription factor binding sites belonging to the bHLH protein family (as described in [51]) across DNA sequences upstream the TSS of the vulval form of lin-3 mRNA. The location of the NHR-binding site belonging to the lin-3 regulatory triplet is also depicted. Only the first 500 bp before the ATG are displayed for most of the species, except for C. virilis (only 300 bp) and C. sp. 1 (up to 1.9 Kb).
(TIF)
(A) Wild-type C. elegans vulval invagination in the L4 stage as seen by Nomarski optics. (B) Over-expression of Can-lin-3(+) in C. elegans via transgenesis with repeated extra-chromosomal arrays results in vulval hyper-induction, with several additional invaginations in the L4 stage (arrowheads). (C) Injection of a Cel-lin-3 fragment in C. angaria leads to vulval hyperinduction. (D) Over-expression of a Caf-lin-3 fragment in C. elegans with repeated extra-chromosomal arrays leads to vulval hyperinduction.
(TIF)
(A) A promoterless C. briggsae fragment introduced into C. elegans is not expressed in N2. (B) The same fragment under the Can-lin-3 CRM drives expression in the anchor cell of N2, as monitored using Cbr-lin-3 FISH. Green corresponds to lin-3 expression and blue is DAPI staining of nuclei.
(TIF)
A single-copy insertion of Can-lin-3(+) rescues brood size defects of lin-3(e1417) mutants (n>15). Note that the presence of a myo-2::GFP transgene linked to lin-3(e1417) in the background enhances the lin-3(e1417) brood size defects and does not allow rescue to wild-type brood size. Vulval induction in this experiment is presented in Fig 5C.
(TIF)
lin-3 mRNA single-molecule FISH quantification in the anchor cell in different C. elegans wild isolates (sheet A) and different Caenorhabditis species (sheet B). Each entry is an individual animal. Mean and standard deviation (SD) are indicated at the bottom.
(XLSX)
The genotype is indicated in the name of the sheet. Gonad size of each individual is given in pixels. Experimental batch is indicated in the "Batch" column.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.