Abstract
Aripiprazole lauroxil (Aristada) for the treatment of schizophrenia
INTRODUCTION
Schizophrenia, a chronic brain disorder that affects about 1% of the adult population in the United States and approximately 26 million people worldwide, is considered among the most disabling and economically catastrophic medical disorders as ranked by the World Health Organization.1,2 In 2013, the economic burden of schizophrenia in the U.S. was estimated at $155.7 billion, including excess direct health care costs of $37.7 billion, direct non-health care costs of $9.3 billion, and indirect costs of $117.3 billion (compared to individuals without schizophrenia).3 Only about 10% to 15% of people who suffer from schizophrenia maintain full-time employment of any type.3
Characteristics of the illness involve positive symptoms, including delusions, hallucinations, trouble with thinking and concentration, and lack of motivation, as well as negative symptoms, such as having a flat affect or poverty of speech, and impairments in cognition and executive functions.4 When their symptoms are treated, most people with schizophrenia, which affects more men than women, will greatly improve over a period of time. However, the mortality rate among patients with the disease is approximately 50% higher than that of the general population, which results from high incidence of suicides and violent deaths, and a wide range of health problems.5
There is no cure for schizophrenia, and researchers are working to unravel its genetics, conduct behavioral studies, and analyze advanced imaging of the brain, all in anticipation of novel approaches and more effective treatments. One recent therapy to have reached the market is aripiprazole lauroxil (Aristada, Alkermes, Inc.), an extended-release, long-acting intramuscular (IM) injection indicated for the treatment of adults 18 to 65 years of age with schizophrenia.
CHEMISTRY AND PHARMACOLOGY
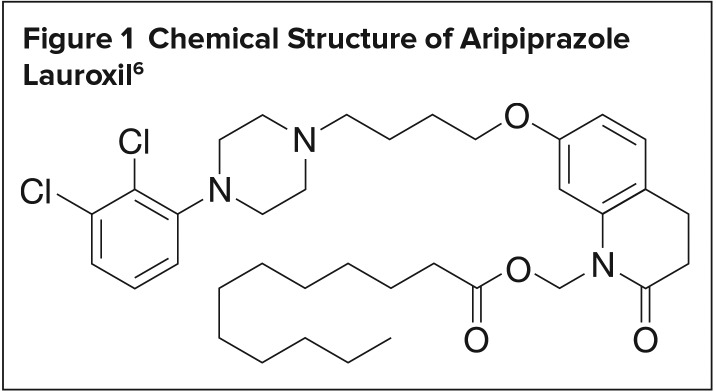
Aripiprazole lauroxil is an atypical antipsychotic available as a white to off-white sterile aqueous extended-release suspension for IM injection with the chemical name 7-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl] butoxy}-2-oxo-3,4-dihydro-2H-quinolin-1-yl) methyl dodecanoate, empirical formula of C36H51Cl2N3O4, and molecular weight of 660.7 g/mol (Figure 1).6
Figure 1.
Chemical Structure of Aripiprazole Lauroxil6
Aripiprazole lauroxil is available in the following strengths and deliverable volumes from single-use, prefilled syringes: 441 mg (1.6 mL), 662 mg (2.4 mL), and 882 mg (3.2 mL). Sorbitan monolaurate, polysorbate 20, sodium chloride, sodium phosphate dibasic anhydrous, sodium phosphate monobasic, and water for injection are the inactive ingredients.6
Aripiprazole lauroxil, a prodrug of aripiprazole, acts as a partial agonist at the D2 and 5-HT1A receptors, and as an antagonist at the 5-HT2A receptors. Enzyme-mediated hydrolysis likely converts the injected aripiprazole lauroxil to N-hydroxymethyl aripiprazole, which is then hydrolyzed to aripiprazole. Adverse reactions reported during clinical studies may have been due to the actions of the drug at other receptors, such as antagonist activity at the alpha1 receptors resulting in orthostatic hypotension.6
PHARMACOKINETICS AND PHARMACODYNAMICS
Aripiprazole lauroxil works primarily as a result of the activity and affinities for the D2 receptors of the parent drug, aripiprazole, and to a lesser extent, its major metabolite, dehydroaripiprazole, which also represents 30% to 40% of the aripiprazole exposure in plasma. Systemic absorption of aripiprazole following single IM administration occurs within five to six days and continues to be released for an additional 36 days. Concentrations of the drug continue to build and reach steady state following the fourth monthly administration. A therapeutic concentration of aripiprazole is achieved in four days with oral aripiprazole co administration for 21 days at the start of therapy. Aripiprazole exhibits extensive extravascular distribution following absorption with an apparent volume of distribution of 268 L.6
Aripiprazole lauroxil 441 mg yields similar concentrations when administered in either the deltoid or gluteal muscles. Administration of 882 mg every six weeks results in plasma aripiprazole concentrations that are within the established therapeutic range of 441–882 mg monthly.6
Aripiprazole lauroxil is metabolized extensively in the liver via cytochrome P450 (CYP) isoenzymes 3A4 and 2D6. It undergoes an enzyme-mediated hydrolysis to form N-hydroxymethyl-aripiprazole, which is then converted by water-meditated hydrolysis to aripiprazole.6
The mean elimination half-life of aripiprazole lauroxil after administration of 441 mg, 662 mg, or 882 mg ranges from 29.2 to 34.9 days.6
Aripiprazole exhibits high affinity for dopamine D2 and D3, and serotonin 5-HT1A and 5-HT2A receptors (Ki values, 0.34 nM, 0.8 nM, 1.7 nM, and 3.4 nM, respectively); moderate affinity for dopamine D4, serotonin 5-HT2C and 5-HT7, alpha1-adrenergic, and histamine H1 receptors (Ki values, 44 nM, 15 nM, 39 nM, 57 nM, and 61 nM, respectively); and moderate affinity for the serotonin reuptake site (Ki value, 98 nM). Aripiprazole has no appreciable affinity for cholinergic muscarinic receptors. Aripiprazole functions as a partial agonist at the dopamine D2 and the serotonin 5-HT1A receptors, and as an antagonist at the serotonin 5-HT2A receptor.6
CLINICAL TRIALS
A 12-week, randomized, double-blind, placebo-controlled, fixed-dose study that enrolled 622 adult patients 18–70 years of age demonstrated the efficacy of aripiprazole lauroxil. Eligible patients met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (text revision), criteria for schizophrenia. Patients also had to have a score of 70 to 120 on the Positive and Negative Syndrome Scale (PANSS), including a score of 4 or more for at least two positive scale items, and a score of 4 or more on the Clinical Global Impression Improvement Scale (CGI-I).6
Once tolerance was shown for oral aripiprazole, study participants received either oral aripiprazole or placebo once daily for the first three weeks of treatment. The IM injections were administered on days 1, 29, and 57. Two hundred and seven patients received aripiprazole lauroxil 441 mg, 208 patients received aripiprazole lauroxil 882 mg, and 207 patients received placebo.6
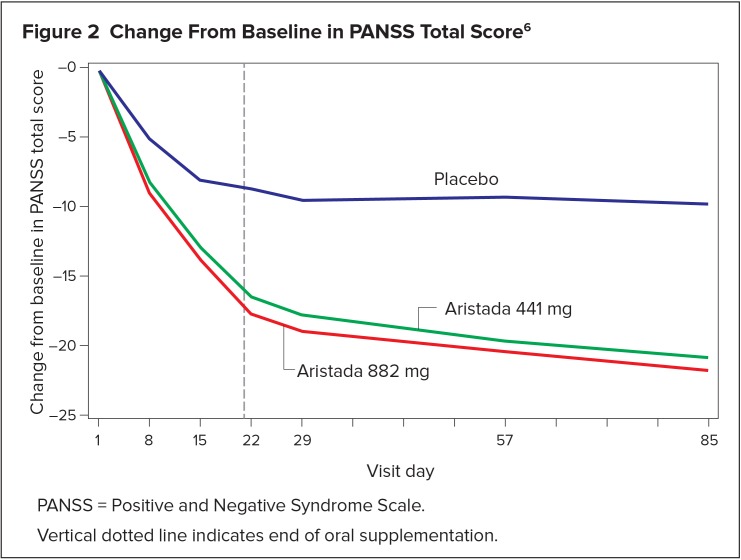
The primary outcome variable was the change from baseline to day 85 in PANSS total score. Statistically significant separation from placebo on PANSS total score change was observed in each aripiprazole lauroxil dose group (Table 1, Figure 2). No statistical difference in treatment outcome was observed with regard to age, gender, race, or weight.6
Table 1.
Primary Efficacy Results as Determined by PANSS Total Score6
| Study Number | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-Subtracted Differencea (95% CI) |
|---|---|---|---|---|
| Study 1 | Aripiprazole lauroxil 441 mgb | 92.6 (10.2) | −20.9 (1.4) | −10.9 (−14.5 to −7.3) |
| Aripiprazole lauroxil 882 mgb | 92.0 (10.8) | −21.8 (1.4) | −11.9 (−15.4 to −8.3) | |
| Placebo | 93.9 (11.3) | −9.8 (1.4) | – |
CI = confidence interval, not adjusted for multiple comparisons; LS Mean = least-squares mean; PANSS = Positive and Negative Syndrome Scale; SD = standard deviation; SE = standard error.
Difference (drug minus placebo) in least-squares mean change from baseline
Doses that are demonstrated to be effective
Figure 2.
Change From Baseline in PANSS Total Score6
PANSS = Positive and Negative Syndrome Scale.
Vertical dotted line indicates end of oral supplementation.
The secondary outcome variable was CGI-I score at day 85, with the drug treatment groups showing statistically significant superior CGI-I scores when compared with the placebo group.6
In addition to this clinical study, the efficacy of aripiprazole lauroxil was established, in part, on the efficacy data from trials of the oral formulation of aripiprazole.6
SAFETY PROFILE
Warnings and Precautions
A boxed warning is included in the prescribing information to reflect an increased mortality rate in elderly patients with dementia-related psychosis treated with antipsychotic drugs, such as aripiprazole lauroxil.6
As with other atypical antipsychotics, serious adverse effects may occur. Antipsychotic drugs have been associated with cerebrovascular adverse reactions, including stroke, in elderly patients with dementia-related psychosis; neuroleptic malignant syndrome; tardive dyskinesia; metabolic abnormalities, such as hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain; orthostatic hypotension; leukopenia, neutropenia, and agranulocytosis; seizures; potential for cognitive and motor impairment; disruption of body temperature regulation; and dysphagia. In addition, post-marketing case reports suggest that some patients can develop pathological gambling and impulse-control problems while taking aripiprazole.6
Adverse Reactions
During clinical trials, the most commonly observed adverse reaction with aripiprazole lauroxil (incidence of 5% or greater and at least twice the rate of placebo) was akathisia. The most common injection site reaction was pain (4% and 5% in the 441-mg and 882-mg treatment groups, respectively; and 2% in the placebo group), which occurred mostly during the first injection and decreased with sub sequent administration. Induration, swelling, and redness at the injection site were reported in less than 1% of patients receiving aripiprazole lauroxil. Adverse reactions occurring in 2% or more of aripiprazole lauroxil-treated patients (and that occurred at greater incidence than in the placebo-treated patients) are listed in Table 2.6
Table 2.
Adverse Reactions That Occurred in 2% or More of Aripiprazole Lauroxil-Treated Patients and at Greater Incidence Than in Placebo-Treated Patients6
| Adverse Reaction System Organ Class Preferred Term | Placebo n = 207 (%) | Aripiprazole Lauroxil | |
|---|---|---|---|
| 441 mg n = 207 (%) | 882 mg n = 208 (%) | ||
| General disorders and administration site conditions | |||
| Injection site pain | 2 | 3 | 4 |
| Investigations | |||
| Increased weight | 1 | 2 | 2 |
| Increased blood creatine phosphokinase | 0 | 2 | 1 |
| Nervous system disorders | |||
| Akathisia | 4 | 11 | 11 |
| Headache | 3 | 3 | 5 |
| Psychiatric disorders | |||
| Insomnia | 2 | 3 | 4 |
| Restlessness | 1 | 3 | 1 |
Reactions observed during the post-marketing period of oral aripiprazole include allergic reaction (anaphylactic reaction, angioedema, laryngospasm, pruritus/urticaria, or oropharyngeal spasm), pathological gambling, hiccups, and blood glucose fluctuation.6
Contraindications
Aripiprazole lauroxil is contraindicated in patients with a known hypersensitivity to aripiprazole. Hypersensitivity reactions have ranged from pruritus/urticaria to anaphylaxis.6
Monitoring Requirements
Patients on aripiprazole lauroxil should be monitored for any symptoms of metabolic change and should be educated about the risks associated with the drug’s use. Routine blood glucose, lipids, and weight assessments should be conducted frequently. Patients with a pre-existing low white blood cell count or a history of drug-induced leukopenia/neutropenia should have their complete blood count monitored while undergoing treatment.6
Use in Specific Populations
Pediatric and Geriatric Use
The safety and effectiveness of aripiprazole lauroxil in patients younger than 18 years or older than 65 years of age have not been evaluated.6
Pregnancy and Lactation
Women exposed to aripiprazole lauroxil should register during pregnancy with the National Pregnancy Registry for Atypical Antipsychotics at (866) 961-2388, or visit http://tinyurl.com/pregnancy-registry for more information.6
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms after delivery. The limited published data on aripiprazole use in pregnant women are not sufficient to inform any drug-associated risks for birth defects or miscarriage. Advise pregnant women of the potential risk to a fetus.6
Aripiprazole is present in human breast milk. Because there are insufficient data at this time, the amount in human milk, the effects on the breastfed infant, or the effects on milk production are unknown. The manufacturer recommends evaluation of the need for the drug versus any potential adverse effects of the drug on the infant and the mother.6
Drug Interactions
When given in combination with strong CYP3A4 or CYP2D6 inhibitors for more than two weeks, aripiprazole lauroxil dosage must be reduced due to the increased exposure of aripiprazole. In contrast, dosage must be increased when the drug is given in combination with strong CYP3A4 inducers for more than two weeks due to decreased aripiprazole concentration (Table 3).6
Table 3.
Dose Adjustments for CYP450 Considerations6
| Concomitant Drug | Dose Change for Aripiprazole Lauroxila |
|---|---|
| Strong CYP3A4 inhibitor | Reduce the dose of aripiprazole lauroxil to the next lower strength. No dosage adjustment is necessary in patients taking 441 mg aripiprazole lauroxil, if tolerated. For patients known to be poor metabolizers of CYP2D6: Reduce dose to 441 mg from 662 mg or 882 mg. No dosage adjustment is necessary in patients taking 441 mg aripiprazole lauroxil, if tolerated. |
| Strong CYP2D6 inhibitor | Reduce the dose of aripiprazole lauroxil to the next lower strength. No dosage adjustment is necessary in patients taking 441 mg aripiprazole lauroxil, if tolerated. For patients known to be poor metabolizers of CYP2D6: No dose adjustment required. |
| Both strong CYP3A4 inhibitor and strong CYP2D6 inhibitor | Avoid use for patients at 662-mg or 882-mg dose. No dosage adjustment is necessary in patients taking 441 mg aripiprazole lauroxil, if tolerated. |
| CYP3A4 inducer | No dose adjustment for 662-mg and 882-mg dose, but increase the 441-mg dose to 662 mg. |
For the 882-mg dose administered every six weeks, the next lower strength should be 441 mg administered every four weeks.
Because aripiprazole lauroxil may potentially enhance the effects of some antihypertensive drugs through alpha-adrenergic antagonism, monitor patient blood pressure when the medications are coadministered.6
Monitor patients for sedation and orthostatic hypotension when aripiprazole lauroxil and lorazepam are given concomitantly.6
DOSAGE AND ADMINISTRATION
Aripiprazole-naïve patients should be assessed for tolerability by establishing them on the oral formulation for up to 14 days before initiating the IM aripiprazole lauroxil injections. Depending on condition and response, some patients may require treatment with aripiprazole lauroxil 441 mg (deltoid or gluteal), 662 mg (gluteal only), or 882 mg (gluteal only) every month, which corresponds to 300 mg, 450 mg, and 600 mg of aripiprazole, respectively, while others may require a starting dose of 882 mg every six weeks. Oral aripiprazole should be taken daily for 21 days in addition to the first IM injections.6
Adjustments may be necessary for doses that are missed, but administer the missed injection as soon as possible, and give oral aripiprazole supplementation with the next scheduled injection (Table 4). Dosage adjustments are also required when aripiprazole lauroxil is administered to CYP2D6 poor metabolizers, or to patients taking CYP3A4 inhibitors, CYP2D6 inhibitors, or CYP3A4 inducers for more than two weeks (Table 3).
Table 4.
| Dose of Patient’s Last Aripiprazole Injection | Length of Time Since Last Injection | ||
|---|---|---|---|
| No Oral Supplementation Required | Supplement with 7 Days Oral Aripiprazole | Supplement with 21 Days Oral Aripiprazole | |
| 441 mg monthly | ≤ 6 weeks | > 6 and ≤ 7 weeks | > 7 weeks |
| 662 mg monthly | ≤ 8 weeks | > 8 and ≤ 12 weeks | > 12 weeks |
| 882 mg monthly | ≤ 8 weeks | > 8 and ≤ 12 weeks | > 12 weeks |
| 882 mg every 6 weeks | ≤ 8 weeks | > 8 and ≤ 12 weeks | > 12 weeks |
The patient should supplement with the same dose of oral aripiprazole as when the patient began aripiprazole injection.
Patients with mild-to-severe hepatic impairment (Child–Pugh score, 5–15) or mild-to-severe renal impairment (glomerular filtration rate, 15–90 mL/min) do not require dosage adjustment for aripiprazole lauroxil injection.6
COST
Aripiprazole lauroxil is supplied as an extended-release injectable suspension in prefilled, single-use syringes. The cost of the drug is dose-dependent. The average wholesale prices for the 441-mg, 662-mg, and 882-mg injections are $1,304, $1,956, and $2,606, respectively.7
P&T COMMITTEE CONSIDERATIONS
Schizophrenia is a lifelong mental disorder that is a major public health issue and a burden on families and the community. Current therapies for the illness focus on eliminating symptoms with antipsychotic medications and psychosocial treatment.
Aripiprazole lauroxil is a novel long-acting atypical IM agent approved in October 2015 by the Food and Drug Administration for the treatment of schizophrenia. The efficacy data have been evaluated for doses of 441 mg and 882 mg and have shown statistically significant separation of the treatment groups from the placebo group using PANSS and CGI-I criteria.
Patients with no prior use of aripiprazole should be established on the oral formulation to determine tolerability before starting on the long-acting injection. Based on the patient’s current condition, the starting dose can be 441 mg, 662 mg, or 882 mg every month, or 882 mg every six weeks. Oral aripiprazole supplementation should be provided in conjunction with the first injection and continue for 21 days. Because the injection is administered only every four to six weeks, patient adherence to therapy may be improved.
Akathisia stands out as the most common adverse effect reported in clinical trials. As with other atypical antipsychotics, adverse effects such as movement disorders, metabolic abnormalities, and cardiac problems may occur; however, the drug poses a minimal potential risk for nausea, vomiting, sedation, and dizziness.
In comparison to other long-acting injectable atypical antipsychotics, aripiprazole lauroxil’s favorable metabolic profile, low incidence of extrapyramidal symptoms, low risk of QTc prolongation, and longer half-life make it a valuable agent to be considered when making a formulary decision.
REFERENCES
- 1.Murray CJL, Lopez AD, editors. The Global Burden of Disease. Cambridge, Massachusetts: Harvard University Press; 1996. p. 21. [Google Scholar]
- 2.National Institute of Mental Health . Schizophrenia. Available at: www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtml. Accessed January 16, 2015. [Google Scholar]
- 3.Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. doi: 10.4088/JCP.15m10278. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, Virginia: American Psychiatric Association; 2013. [Google Scholar]
- 5.National Collaborating Centre for Mental Health . Schizophrenia: The NICE Guideline on Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. London, United Kingdom: Royal College of Psychiatrists; 2010. [Google Scholar]
- 6.Aristada (aripiprazole lauroxil) prescribing information. Waltham, Massachusetts: Alkermes, Inc.; 2016. Available at: www.aristada.com/downloadables/ARIS-TADA-PI.pdf. Accessed July 20, 2016. [Google Scholar]
- 7.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Accessed July 20, 2016. [Google Scholar]