Abstract
Objectives:
The aim of the study was to estimate the implications of androgen receptor (AR) expression in estrogen receptor (ER)-positive subset of invasive breast carcinoma patients.
Patients and Methods:
We assessed the AR expression in a subset of 96 predominantly ER-positive invasive breast carcinomas and correlated this expression pattern with several clinical and pathologic parameters: histologic type and grade, tumor size, lymph node status, progesterone receptor (PgR) status, and human epidermal growth factor receptor type 2 (HER2) overexpression and evaluated the association of these parameters with 10-year survival using univariate and multivariate analyses. Data used for analysis were derived from medical records. Immunohistochemical analysis for AR, ER, PgR, and HER2 were carried out and semiquantitative evaluation of stainings was performed.
Results:
AR expression was demonstrated in 43.7% of patients. AR was significantly related to well-differentiated tumors and positive PgR/HER2 status. No statistical difference was demonstrated in AR expression in relation to tumor size, lymph node status, menopausal status, and tumor histologic type. AR expression was not an independent prognostic factor related to 10-year survival in ER-positive cancers. In multivariate analyses, older age at diagnosis, larger tumor size, and positive lymph node status were significantly associated with poorer 10-year survival.
Conclusions:
AR expression is significantly associated with ER/PgR/HER2 status and positively related to well-differentiated tumors. Although AR status in ER-positive cancers is not an independent prognostic factor, it might provide important additional information on prognosis and become a promising object for targeted therapy.
Key Words: androgen receptor, estrogen receptor, progesterone receptor, epidermal growth factor receptor type 2, breast cancer, prognostic factor, predictive factor
Breast carcinoma is the most common cancer in females globally, and the second most common cancer overall. Approximately, 1.67 million new cases were diagnosed in 2012 (25% of female cases and 12% of the total).1 Breast carcinoma is, furthermore, the first cause of cancer death in females worldwide, with nearly 460,000 deaths estimated to have occurred in 2008 (14% of the total), slightly outrivaling the lung cancer (427,000 deaths in females). About 50% of all cases are reported in the economically high developed countries.2 In the European Union, both incidence and death rate of breast cancer in women are significantly higher than the global average. In 2006, there were 320,000 incident cases of breast cancer (30.9% of all female cases) and over 85,000 deaths (16.7% of all cancer deaths in women) reported in the EU.3
Consequently, an early and precise diagnosis and intensive, accurate treatment of breast cancer is one of the most important challenges of modern medicine.
Breast carcinoma is a heterogenous entity, varying in clinical, histologic, immunohistochemical, and genetic features. Tumor biology, natural history, therapeutic algorithm, and prognosis depend on the classic factors such as histopathologic type and grading, clinical stage of disease (primary tumor size, lymph node involvement, and presence of distant metastases), and hormonal receptors expression [progesterone receptor (PgR) and estrogen receptor (ER)]. Recently, immunohistochemical variables such as HER2 overexpression (human epidermal growth factor receptor type 2), Ki-67 index (nonhistone nuclear protein), E-Cadherin expression level (transmembrane glycoprotein),4–6 mutations in the TP53 gene, and Cathepsin D expression level (product of estrogen-inducible gene) have been considered valuable prognostic factors.7
Similar to a healthy breast tissue, breast carcinoma is highly hormone dependent. The family of steroid receptors includes the following nuclear receptors: estrogen (ER), progesterone (PgR), androgen (AR), and vitamin D receptor. Steroid hormones induce proliferation of breast cells by binding to their respective receptor. Numerous studies prove essential regulatory role of ER and PgR in the pathogenesis and growth of breast cancer, affecting cell proliferation and differentiation. Progesterone and estrogen hormone receptors are universally accepted, valuable prognostic and predictive factors. Moreover, targeted antiestrogen therapy has become a standard tool used to effectively treat a special subpopulation of breast cancer patients.8–10
AR presents functional and structural similarity to the above-discussed nuclear receptors.11–13 The crucial role of AR in pathogenesis, growth, and therapy of prostate cancer is profoundly documented.14 Many publications document the presence of AR on the breast cancer cells, but exact percentage of AR-positive cases differs significantly. Most authors report AR expression in 60% to 80% of breast cancer cases,14–18 but both lower (34%19 and 56%20) and higher (91.1%21) percentage has been described in certain studies. These differences can be explained by disparity between examined populations as well as by a diverse cutoff point of AR expression intensity.
The objectives of this study were to assess the AR expression in an ER-positive subset of breast carcinomas and to correlate this expression pattern with several clinical and pathologic parameters: histologic type and grade, tumor size, lymph node status, PgR/HER2 status and to correlate these parameters with 10-year survival.
PATIENTS AND METHODS
Patients
Patients with primary breast carcinoma treated surgically at the 2nd Department of General and Oncological Surgery, Wroclaw Medical University from January 1995 to April 2002 were enrolled in the study. A total of 96 ER-positive breast cancer cases were analyzed. Data used for analysis were derived from medical records. The following information was obtained from all patients’ medical records: age, menopausal status, lymph node status, tumor size, grade and histologic type of tumor, and PgR/HER2 status. Survival was measured from the date of definitive surgery to the date of patient’s death or last follow-up.
Methods
All patients underwent Patey’s conservative radical mastectomy. Postoperative adjuvant therapy was performed based on the recommendations of the Polish Union of Oncology. Immunohistochemistry analysis was performed on formalin-fixed and paraffin-embedded breast cancer tissues. Blocks were cut into 4 μm sections, deparaffinized in 2 changes of xylene, rehydrated in alcohols (96%, 80%, and 70% for 1 min each), washed in distilled water, stained in hematoxylin (Sigma-Aldrich, Steinheim, Germany), washed in tap water for 5 minutes, and then counterstained with eosin (Sigma-Aldrich). Sections were then washed in distilled water, dehydrated through alcohols, and mounted in mounting medium (Dako, Glostrup, Denmark). Sections stained with hematoxylin and eosin were evaluated with regard to the histopathologic diagnosis. Following (the) histopathologic analysis of the hematoxylin and eosin–stained sections, the most representative area of the tumor was marked, paraffin blocks were cut again into 4-μm-thick slices, and were stained using immunohistochemistry. Immunohistochemical staining was performed using the labeled streptavidin biotin (LSAB) method [LSAB+System horseradish peroxidase (HRP); Dako] with the following reagents: peroxidase blocking reagent, protein block reagent, antibody diluent with background-reducing components, biotinylated-conjugated antibody and streptavidin-HRP, and chromogen solution. Slides were deparaffinized in 2 changes of xylene for 10 minutes, then rehydrated in a series of graded alcohols (96%, 80%, and 70%) for 3 minutes each. Next, the specimens were washed twice for 4 minutes in distilled water, and were microwaved in a citric buffer [0.1 M citric acid, 0.05% Tween 20 (pH 6.0); Sigma-Aldrich] for 8 minutes for heat-induced epitope retrieval. Following 2 washes in distilled water for 4 minutes, the specimens were incubated for 10 minutes with peroxidase blocking reagent and rinsed twice for 5 minutes with phosphate-buffered saline. Next, incubation with protein block reagent was performed for 10 minutes, after which specimens were incubated with primary antibodies and stored overnight at 4°C [Monoclonal Mouse Anti Human Androgen Receptor Clone, AR441 (DAKO, cat. No. M3562)]. Following overnight incubation, the slides were incubated for 15 minutes with biotinylated-conjugated antibodies and streptavidin-HRP, rinsing twice with phosphate-buffered saline between and following the incubation. The reaction was detected and visualized using 3,3′-diaminobenzidine (DAB) in chromogen solution (Sigma-Aldrich). Finally, the samples were counterstained with hematoxylin, dehydrated using the aforementioned alcohols for 3 minutes each, cleared in 2 changes of xylene for 5 minutes, and mounted with xylene-based mounting medium (Dako).
For evaluation of histologic slides, a system previously described by Allred et al22 was used and a semiquantitative evaluation of stainings was carried out. The criteria for AR positivity were based on the intensity and percentage of tumor cells showing expression. The intensity was graded as negative, weak, intermediate, or strong. Tumors that had >10% of cells presenting intermediate or strong intensity of expression were considered positive. Evaluation of histologic slides was performed by 2 independent pathologists by means of light microscopes (Olympus BX51) and the results were agreed thereafter.
Statistics
Statistical analyses were performed using the Statistica 10 software (StatSoft). Comparison of continuous variables was performed by independent t tests. Categorical variables were tested by the χ2 test. Data were expressed as mean and SD for continuous variables. The Cox proportional hazard analysis was used to determine the risk of recurrence or mortality relative to the prognostic factors in breast cancer cases. The Kaplan-Meier method was used to assess the cumulative survival rates of breast cancer patients.
RESULTS
A total of 96 adult females diagnosed with estrogen-positive primary invasive breast carcinomas were enrolled and an average age of patients was 58.19 years (SD: 9.52). AR expression was demonstrated in 43.7% (42 of 96) of patients. Compared with AR-positive patients, those with AR negative tended to have higher grade II (62% in AR+ vs. 74% in AR−) and grade III tumors (0% in AR+ vs. 11% in AR−) (P=0.0058). The ratio of PgR expression was higher in AR+ subgroup than in AR− (52% vs. 30%, P=0.0237). A significant number of AR-positive tumors was associated with positive HER2 status (95% in AR+ vs. 67% in AR−, P=0.0012). No statistical difference was demonstrated in AR expression with relation to tumor size, lymph node status, menopausal status, and tumor type (Table 1). In univariate Cox regression analysis, AR expression subgroup (AR+ vs. AR−) was not an independent prognostic factor related to 10-year survival in addition to menopausal status, PgR, and HER2 statuses. Age, tumor size, lymph node status, and grade were factors independently related to 10-year survival (Table 2). In multivariate analyses, only age, tumor size, and lymph node status were associated with poor 10-year survival (Table 2). In Kaplan-Meier log-rank analysis, AR expression did not display statistical significance in cumulative 10-year survival (Figs. 1A, B).
TABLE 1.
Descriptive Statistics of Women With Androgen Receptor Positive (AR+) and Androgen Receptor Negative (AR−) Tumor
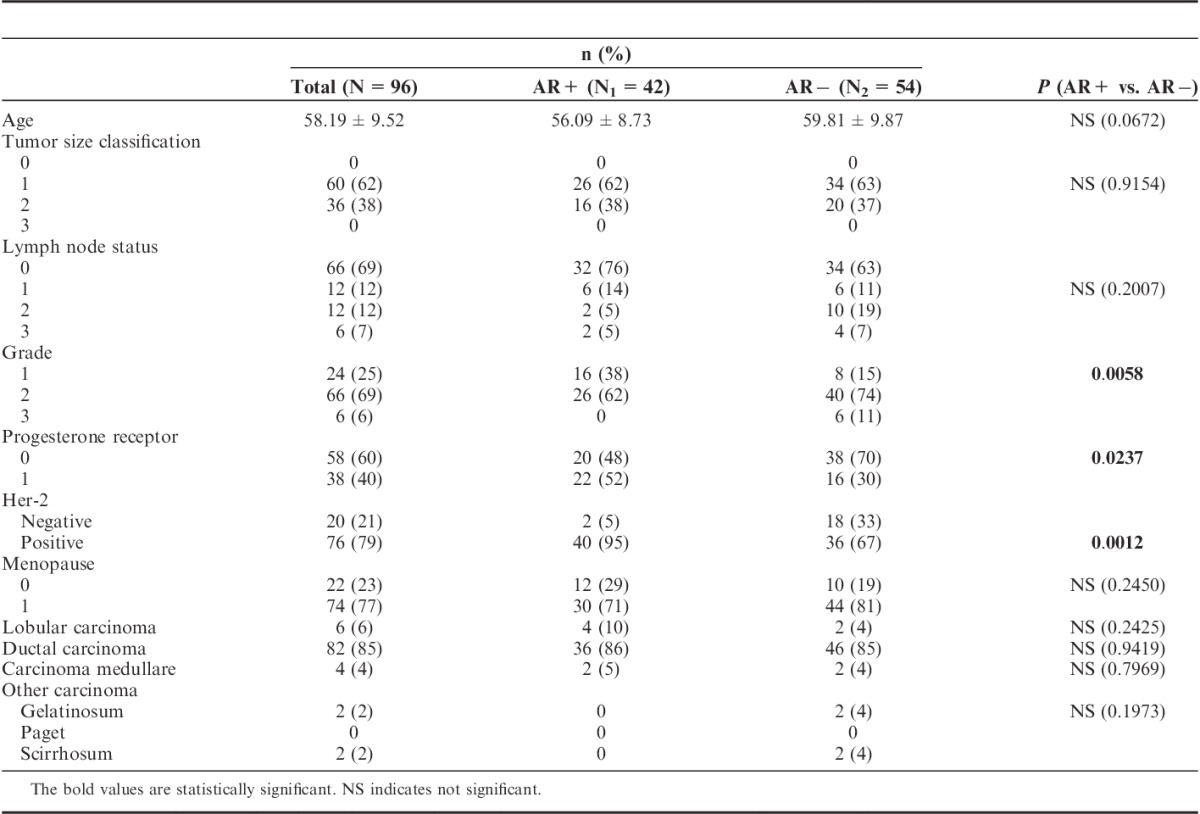
TABLE 2.
Prognostic Factors Related to 10-year Survival (Cox Univariate and Multivariate Regression Analysis)
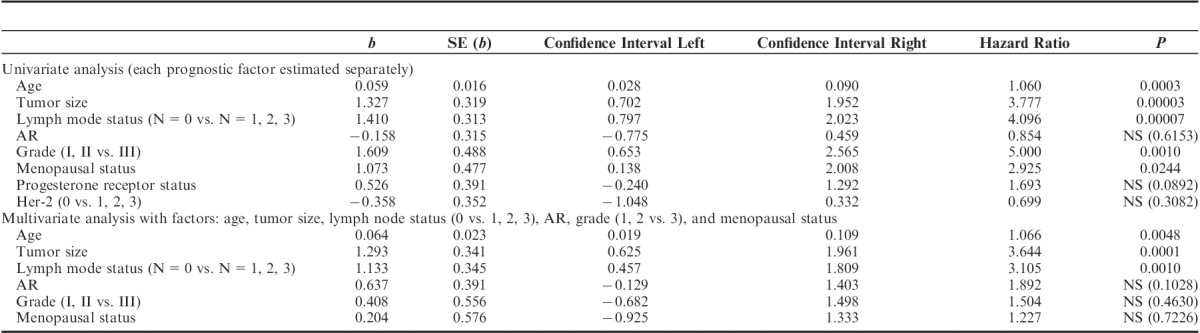
FIGURE 1.
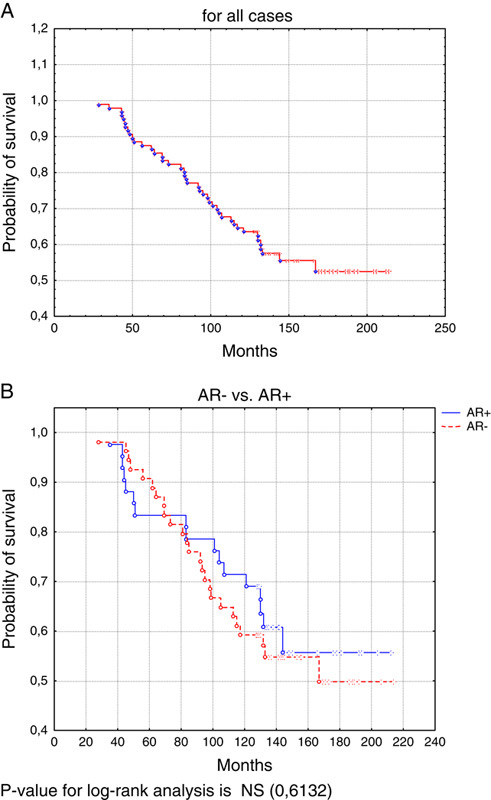
Kaplan-Meier log-rank analysis for survival rate in time (10 y). (A) For all cases. (B) AR− versus AR+. P-value for log-rank analysis is NS (0.6132). AR indicates androgen receptor.
DISCUSSION
The role of androgen signaling in neoplastic cells remains controversial. It has been reported to be involved in differentiation and growth of normal breast cells.23,24 Szelei et al25 have distinguished 3 mechanisms of androgen control of cellular balance: proliferation stimulation, proliferation inhibition, and apoptosis inhibition. Yu et al8 have described important role of AR in homeostasis of healthy breast tissue as a counterbalance for the proliferative effects of ER. Nevertheless, androgens could possibly influence risk of breast carcinoma and tumor growth through several (often contradictory) mechanisms: by binding to AR (directly stimulating malignant cell proliferation), binding directly to ER (competitive inhibition of 17β-estradiol stimulatory effect on neoplastic cells), or by conversion to estradiol.20 Clinical effect of AR stimulation can also be influenced by hormonal (interactions between AR and ER, modulating transcriptive efficiency) and extrahormonal factors (eg, intracellular MAP-kinase activity). Moreover, different ligands of AR (eg, Dihydrotesteron-DHT, R188, Medroxyprogesteron-MPA) present diverse specificity to AR and other steroid receptors and differently undergo a conversion to estrogens.26
Similarly, published data concerning association of AR presence with breast cancer survival, acclaimed prognostic factor. Peters et al20 have reported significant inverse correlation between AR expression on breast cancer cells and 10-year survival. Gonzalez et al18 and Bryan et al27 have published opposite dependence, observing a significantly longer overall survival in group of AR-positive patients. In studies by Hu et al14 and Agoff et al,28 association of AR presence and patient’s survival differs depending on ER status. In subpopulation of ER-negative patients, expression of AR was considered a good prognostic factor. Inversely, in population of ER-positive patients, AR expression was associated with shorter overall survival. Castellano et al29 have presented opposite results: in population of ER-positive patients, AR expression was considered a good prognostic factor. In publication of Soreide et al,21 no correlation between AR− expression and overall survival was observed (Figs. 2–4).
FIGURE 2.
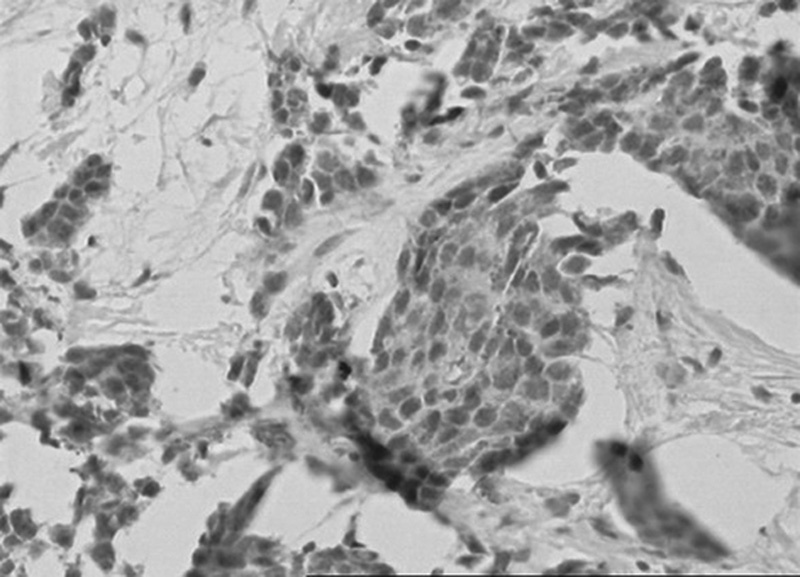
Positive nuclear immunohistochemical staining for androgen receptor, AR in cells of invasive ductal breast carcinoma; DAB was used as a reaction substrate and hematoxylin for counterstaining ×150 magnification.
FIGURE 4.
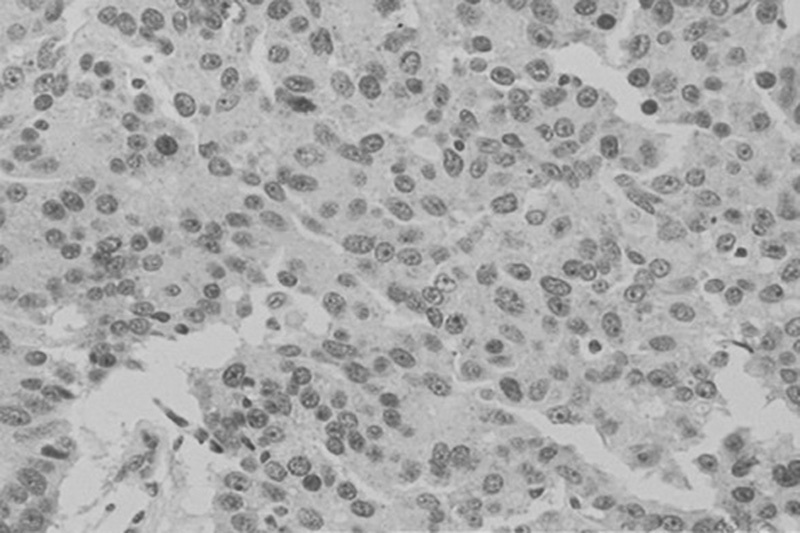
Positive nuclear immunohistochemical staining for estrogen receptor, ER in cells of invasive ductal breast carcinoma; DAB was used as a reaction substrate and hematoxylin for counterstaining ×150 magnification.
FIGURE 3.
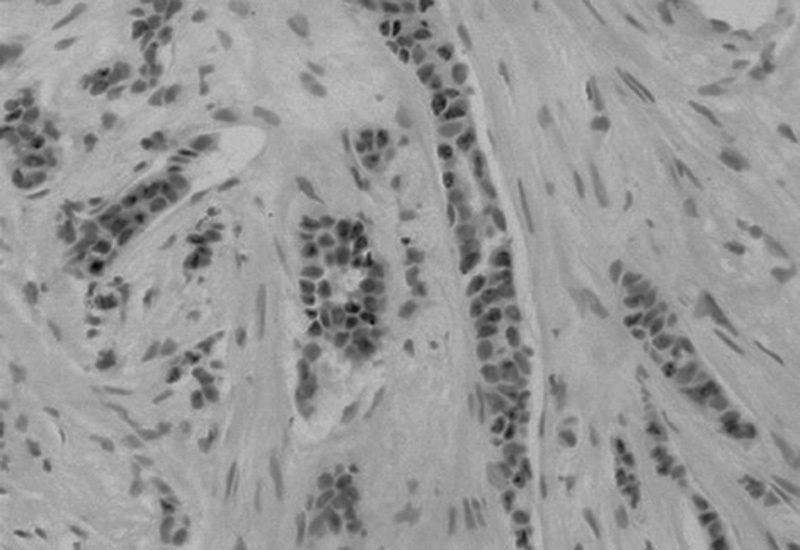
Positive nuclear immunohistochemical staining for progesterone receptor, PgR in cells of invasive ductal breast carcinoma; DAB was used as a reaction substrate and hematoxylin for counterstaining ×150 magnification.
Published data regarding clinicopathologic parameters of tumors in relation to AR status are also incoherent. ER−8,11,17 and PgR−8,11,17,29–31 expression has been reported significantly positively associated with AR− expression, but some authors deny this correlation.18 No correlation between HER2 overexpression and AR status has been found.8,11 AR-positive tumors tend to have a lower histologic grade,11,16,29–31 but opposite observations are also documented17 and some authors have reported these parameters as independent.8,18 Ogawa et al11 have reported AR expression as a favorable biomarker related to a lower tumor size, lower incidence of distant metastases, and lymph node involvement. In other publications, no significant correlation with tumor size,8 lymph node status,8,18,19 and distant metastases18 has been found. AR expression reveals no association with menopausal status.11,19
The steroid hormones govern the growth and differentiation of breast normal and cancer cells. The role of ER and PgR as predictive and prognostic factors in breast carcinoma is well documented, but the significance of AR is still uncertain.
Our study showed that AR expression in ER-positive breast cancer is related to a lower histologic grade and a higher rate of PgR expression, which is consistent with previous reports.29–31 Of note, however, is that there was a significant interaction between AR and HER2 in this study. In contrast to other studies, our results show that AR was not associated with age, tumor size, lymph node involvement, menopausal status, and tumor type.32–34
It has been indicated that in patients with ER-positive tumor, AR expression was associated with a better outcome. Our results contrasted with those from other studies with respect to prognostic factors crucial for survival.29,30,34 Our univariate Cox regression analysis demonstrated that age, tumor size, grade, lymph node status, and menopausal status were prognostic factors for 10-year survival, whereas AR expression did not show statistical significance. In addition, the prognostic significance of AR was not demonstrated in multivariate analyses for 10-year survival. Park and colleagues suggested that AR might provide a predictive role for endocrine treatment. In our study, all patients of ER-positive subgroup received endocrine therapy.
In conclusion, the problem of association between expression of AR and overall survival and clinicopathologic parameters remains unsolved. In this 10-year follow-up study, AR expression was not associated with better outcomes in ER-positive breast cancer subgroup. Therefore, further studies on larger cohorts of patients are required to assess the role of AR in breast cancer.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v10, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. [DOI] [PubMed] [Google Scholar]
- 4.Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3,655 invasive breast carcinomas. Am J Clin Pathol. 2005;123:541–546. [DOI] [PubMed] [Google Scholar]
- 5.Mallon E, Osin P, Nasiri N, et al. The basic pathology of human breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:139–163. [DOI] [PubMed] [Google Scholar]
- 6.Koo JS, Jung W, Jeong J. The predictive role of E-cadherin and androgen receptor on in vitro chemosensitivity in triple-negative breast cancer. Jpn J Clin Oncol. 2009;39:560–568. [DOI] [PubMed] [Google Scholar]
- 7.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Q, Niu Y, Liu N, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011;22:1288–1294. [DOI] [PubMed] [Google Scholar]
- 9.Mersin H, Yildirim E, Berberoglu U, et al. The prognostic importance of triple negative breast carcinoma. Breast. 2008;17:341–346. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura R, Arima N. Is triple negative a prognostic factor in breast cancer? Breast Cancer. 2008;15:303–308. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa Y, Hai E, Matsumoto K, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13:431–435. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal AK, Jelen M, Grzebieniak Z, et al. Androgen receptors as a prognostic and predictive factor in breast cancer. Folia Histochem Cytobiol. 2008;46:269–276. [DOI] [PubMed] [Google Scholar]
- 13.Moe RE, Anderson BO. Androgens and androgen receptors: a clinically neglected sector in breast cancer biology. J Surg Oncol. 2007;95:437–439. [DOI] [PubMed] [Google Scholar]
- 14.Hu R, Dawood S, Holmes MD, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Amicis F, Thirugnansampanthan J, Cui Y. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moinfar F, Okcu M, Tsybrovskyy O, et al. Androgen receptors frequently are expressed in breast carcinomas. Cancer. 2003;98:703–711. [DOI] [PubMed] [Google Scholar]
- 17.Bryś M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85–90. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez LO, Corte MD, Vazquez J, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer. 2008;8:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller WR, Telford J, Dixon JM. Androgen receptor activity in human breast cancer and its relationship with oestrogen and progestogen receptor activity. Eur J Cancer Clin Oncol. 1985;21:539–542. [DOI] [PubMed] [Google Scholar]
- 20.Peters KM, Edwards SL, Nair SS, et al. Androgen receptor expression predicts breast cancer survival: the role of genetic and epigenetic events. BMC Cancer. 2012;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soreide JA, Lea OA, Varhaug JE, et al. Androgen receptors in operable breast cancer: relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur J Surg Oncol. 1992;18:112–118. [PubMed] [Google Scholar]
- 22.Allred DC, Harvey JM, Berardo MD, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 23.Zhu X, Daffada AA, Chan CM, et al. Identification of an exon 3 deletion splice variant androgen receptor mRNA in human breast cancer. Int J Cancer. 1997;72:574–580. [DOI] [PubMed] [Google Scholar]
- 24.Birrell SN, Hall RE, Tilley WD. Role of the androgen receptor in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:95–103. [DOI] [PubMed] [Google Scholar]
- 25.Szelei J, Jimenez J, Soto AM, et al. Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology. 1997;138:1406–1412. [DOI] [PubMed] [Google Scholar]
- 26.Garay JP, Park BH. Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res. 2012;2:434–445. [PMC free article] [PubMed] [Google Scholar]
- 27.Bryan RM, Mercer RJ, Bennett RC, et al. Androgen receptors in breast cancer. Cancer. 1984;54:2436–2440. [DOI] [PubMed] [Google Scholar]
- 28.Agoff SA, Swanson PA, Linden H. Androgen receptor expression in estrogen receptor–negative breast cancer immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725–731. [DOI] [PubMed] [Google Scholar]
- 29.Castellano I, Allia E, Accortanzo V, et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124:607–617. [DOI] [PubMed] [Google Scholar]
- 30.Peters AA, Buchanan G, Ricciardelli C, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–6140. [DOI] [PubMed] [Google Scholar]
- 31.Macedo LF, Guo Z, Tilghman SL, et al. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006;66:7775–7782. [DOI] [PubMed] [Google Scholar]
- 32.Shim HS, Jung WH, Kim H, et al. Expression of androgen receptors and inhibin/activin alpha and betaA subunits in breast apocrine lesions. APMIS. 2006;114:352–358. [DOI] [PubMed] [Google Scholar]
- 33.Gatalica Z. Immunohistochemical analysis of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract. 1997;193:753–758. [DOI] [PubMed] [Google Scholar]
- 34.Riva C, Dainese E, Caprara G, et al. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch. 2005;447:695–700. [DOI] [PubMed] [Google Scholar]


