Abstract
Evidence supporting rechallenge in patients responding to first exposure to trabectedin is limited. We report on a 39-year-old woman with advanced high-grade undifferentiated sarcoma (US) retreated twice with trabectedin after first response. The patient presented in June 2006 with an abdominal mass originating from the rear fascia of the rectus abdominis. Staging examinations did not indicate metastases and she underwent surgery; pathology showed a high-grade (FNCLCC G3) US. Subsequently, the patient received five cycles of adjuvant chemotherapy with epirubicin and ifosfamide. In February 2009 a computed tomography (CT) scan showed an abdominal mass involving the transverse mesocolon. R0 surgery was performed. In September 2009, peritoneal lesions appeared. Trabectedin was initiated at a dose of 1.5 mg/m2 by a 24 h intravenous infusion every 3 weeks, without relevant toxicity. After six cycles (March 2010), CT and PET-CT scans showed complete disappearance of metastases. In February 2012, new secondary lesions in the subdiaphragmatic region and a peritoneal lesion appeared. We rechallenged the patient with the same schedule of trabectedin; a complete response was achieved after two cycles. In October 2013, new secondary lesions in the subdiaphragmatic region and a retroperitoneal lesion were found. We rechallenged with the same schedule of trabectedin; PET-CT scans after two cycles showed complete response on the subdiaphragmatic lesion. Radiotherapy on the retroperitoneal lesion was performed. The patient underwent a total of 18 cycles and remains free from radiologically detectable disease. We report complete radiological remission after two rechallenges with trabectedin in a patient with previously responding high-grade US.
Keywords: chemotherapy, maintenance, rechallenge, soft-tissue sarcoma, trabectedin
Introduction
Trabectedin (yondelis) is a marine-derived antineoplastic agent. It is indicated in Europe for the treatment of adult patients with advanced soft-tissue sarcoma (STS), after failure of anthracyclines-based chemotherapy (±ifosfamide), or for those who are unsuited to receive these agents 1. Recently, the drug was also approved in undifferentiated sarcoma (US) in advanced pretreated liposarcoma and leiomyosarcoma on the basis of a phase III study versus dacarbazine in these two histotypes 2. Although patients with liposarcoma, especially the myxoid subtype, and leiomyosarcoma have benefitted the most from the drug, responses have consistently been observed among many other histotypes of STS 3–9.
Single-agent trabectedin has been reasonably well tolerated in clinical trials. A pooled analysis of trabectedin safety in 1132 patients treated in phase II trials showed a very favorable safety profile 10. Nausea, fatigue, and vomiting were the most common trabectedin-related adverse events, reported in greater than or equal to 20% of patients. Reversible myelosuppression (mainly neutropenia) and transient reversible transaminase increases were the most common laboratory abnormalities found with trabectedin, with a very low incidence of clinically relevant consequences.
For this reason, trabectedin can be administered for prolonged periods to patients with sustained clinical benefit without cumulative toxicities and strategies of maintenance after six cycles and rechallenge in previously responding patients can be considered useful options in advanced STS 11–14.
Here, we report a radiological complete response (CR) after two rechallenges with trabectedin in a 39-year-old woman with advanced high-grade US arising from the abdominal wall.
Case report
The patient presented in June 2006 with an abdominal mass originated from the rear fascia of the rectus abdominis muscle (12 cm in maximum diameter); a core-biopsy was positive for a high-grade mesenchymal neoplasm. She underwent a brain, chest, and abdominal computed tomography (CT) scan to complete the staging and no secondary lesions were found. She then underwent a surgical wide excision of this lesion; pathology indicated a high-grade sarcoma [G3 according to the French Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system], without any specific differentiation on immunohistochemical analysis. We performed a pathological review within the Italian Rare Cancer Network that confirmed the diagnosis of high-grade US (Fig. 1). MDM2 is a distinctive mutation in well-differentiated/dedifferentiated liposarcoma, the most likely differential diagnosis in this case. However, an examination by the reference pathologist for MDM2 mutation both in immunohistochemistry and by fluorescence in-situ hybridization analysis did not detect any mutation of this gene, which also served to confirm the initial diagnosis.
Fig. 1.
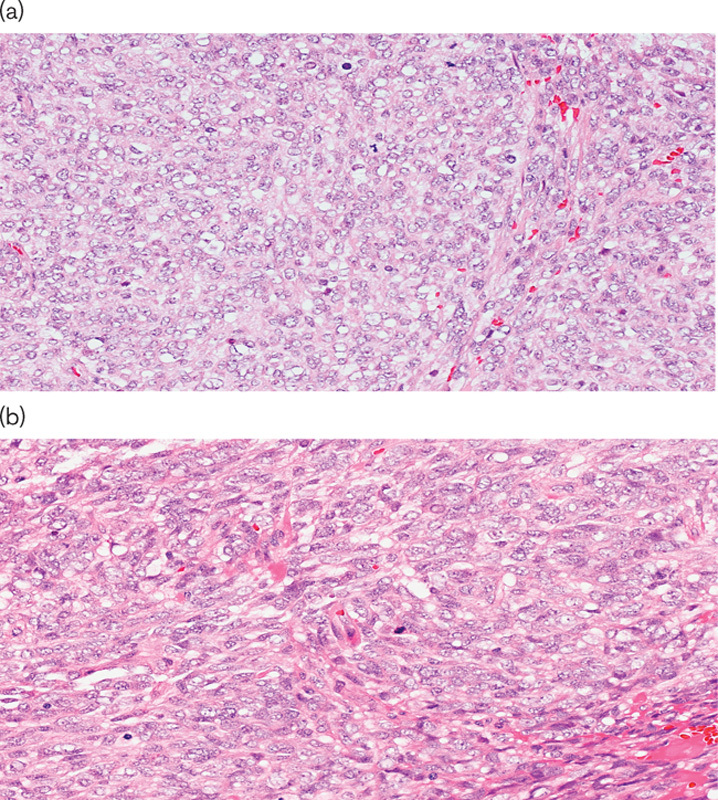
Pathological evaluation. Hematoxylin and eosin-stained section showing a high-grade neoplasm composed of large epithelioid cells harboring vesicular nuclei with prominent nucleoli (a); a spindle cell component is associated (b). Mitotic figures are frequently identified.
We shared with the patient the option of an adjuvant treatment and she was treated with five cycles of adjuvant chemotherapy with epirubicin (60 mg/m2 intravenous days 1–2 every 3 weeks) and ifosfamide (3000 mg/m2 intravenous days 1–3 every 3 weeks). She remained free of disease until February 2009, when a CT scan showed an abdominal mass (7 cm in diameter) that involved the transverse mesocolon. Margin-negative (R0) surgery was performed without any additional treatment; the pathological examination confirmed a relapse of previous high-grade US.
The patient remained free of disease until September 2009, when a CT scan showed multifocal peritoneal lesions, localized in the pelvis (5 cm in maximum diameter). Considering the previous treatment with anthracycline-based chemotherapy, the second relapse, the progression-free interval, and the multifocality of pelvic disease, we decided to propose a chemotherapy treatment with trabectedin.
The patient began trabectedin (at the dose of 1.5 mg/m2 as a 24 h intravenous infusion every 3 weeks) on October 2009; no relevant hepatic or hematologic toxicity was reported. After six cycles (March 2010), CT and fluorine-18 fluorodeoxyglucose (18F-FDG) PET-CT scans showed complete disappearance of metastases and we decided to stop the treatment.
In February 2012, CT and PET-CT scans showed new secondary lesions in the subdiaphragmatic region, near to the fifth liver segment, and a peritoneal lesion near the right common iliac artery. We shared with the patient the option of a new chemotherapy treatment (e.g. high-dose continuous-infusion ifosfamide), but she refused a treatment that could possibly cause alopecia; we then decided to rechallenge the patient with the same schedule of trabectedin. After two cycles, CT and PET-CT scans showed again a CR (Fig. 2). She continued the treatment for up to six cycles, without any relevant toxicity.
Fig. 2.
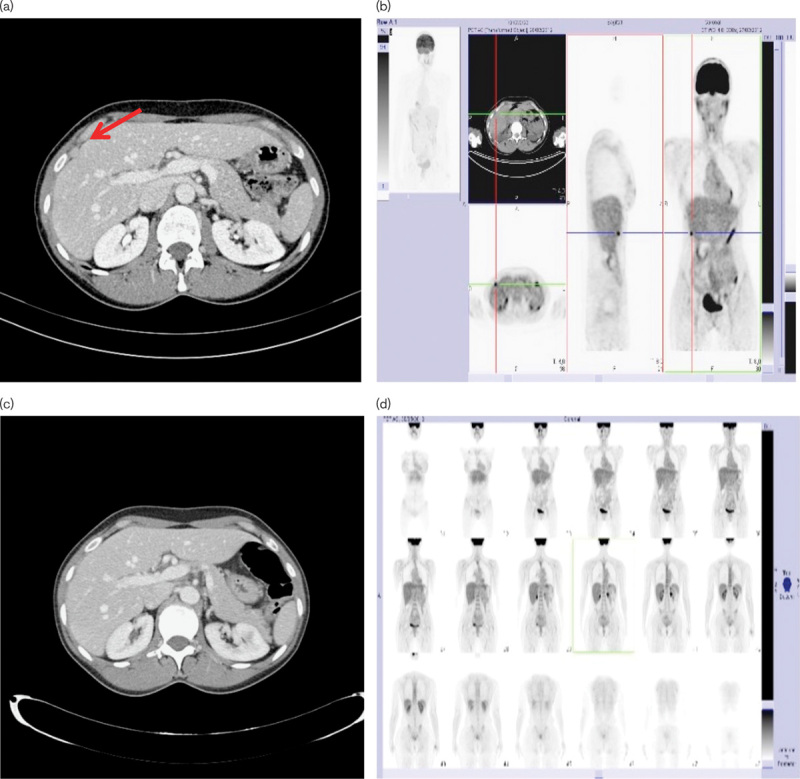
18F-FDG-PET/CT and CT scan tumor assessment before the first rechallenge (a, b); 18F-FDG-PET/CT and CT scan tumor assessment showing CR after the first rechallenge (c, d). The arrow points to the lesion near the fifth liver segment. CR, complete response; CT, computed tomography; 18F-FDG, fluorine-18 fluorodeoxyglucose.
In October 2013, CT and PET-CT scans showed new secondary lesions in the subdiaphragmatic region and a retroperitoneal lesion near L5. We decided to rechallenge the patient for the third time with the same schedule of trabectedin, considering the previous good response, the progression-free interval, and the good safety profile. A PET-CT scan performed after two cycles showed a CR of the subdiaphragmatic lesion, whereas the retroperitoneal lesion showed a lower 18F-FDG uptake, but it did not respond completely; we decided to perform radiotherapy on this lesion. The dose was 25 Gy in five daily fraction (accelerated hypofractionated radiotherapy) using intensity-modulated arc therapy in helical tomotherapy; we obtained a CR at the subsequent PET-CT scan evaluation. This time, the patient underwent a total of 18 cycles before the treatment was interrupted in November 2014 at the patient’s request, at which point in time she was free of radiologically detectable disease (Fig. 3).
Fig. 3.
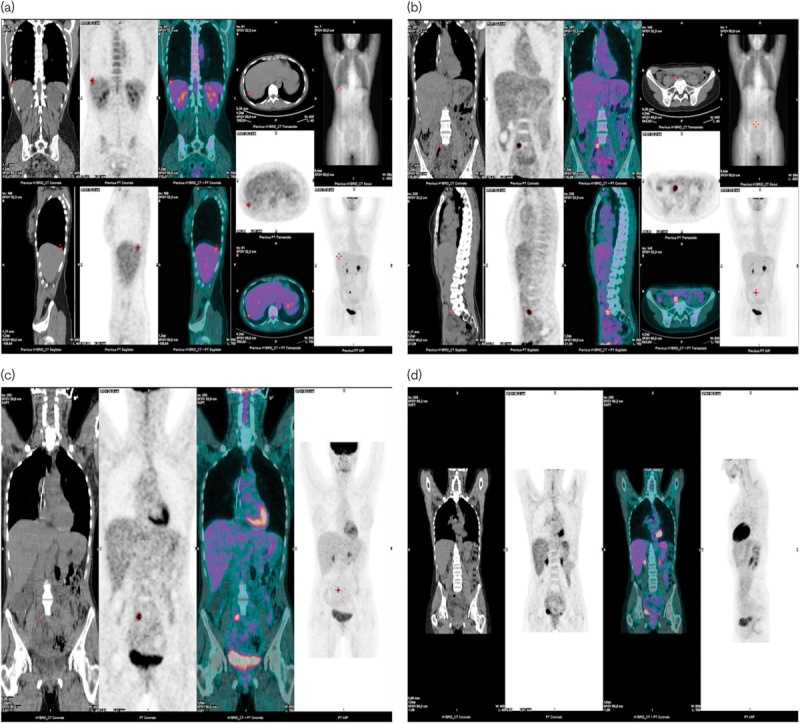
18F-FDG-PET/CT tumor assessment before the second rechallenge (a, b); 18F-FDG-PET/CT tumor assessment showing CR in the subdiaphragmatic lesion after the second rechallenge (c); 18F-FDG-PET/CT tumor assessment showing CR in the retroperitoneal lesion after the second rechallenge and radiotherapy (d). CR, complete response; CT, computed tomography; 18F-FDG, fluorine-18 fluorodeoxyglucose.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. This analysis was approved by the appropriate Institutional Ethics Committee.
Discussion
The favorable safety profile and the lack of cumulative toxicity of trabectedin presents a clinical opportunity with respect to maintenance strategies in patients achieving at least stable disease (SD) and for rechallenge strategies in previously responding patients after a ‘drug-holiday’.
We know that trabectedin has a high level of activity in myxoid liposarcoma 3–15. Retrospective data on the efficacy of rechallenge with trabectedin in patients with myxoid liposarcoma who were responding at the time of discontinuation were presented at the American Society of Clinical Oncology meeting in 2009 by Sanfilippo et al. 13; they analyzed eight responding patients who resumed the treatment at the time of progression. Following rechallenge with trabectedin, no progressive disease was observed at the first assessment and none of the patients had to discontinue or reduce the dose because of toxicity. The response rate according to RECIST was 50%. At a median follow-up of 7 months (range 2.7–12 months), the median time to progression had not yet been reached.
At the American Society of Clinical Oncology meeting in 2012, the French Sarcoma Group reported a retrospective analysis of 49 patients who had one or more trabectedin rechallenges 14. After the first reintroduction of trabectedin, 33 out of 49 patients obtained further clinical benefit (partial response or SD) and three out of 33 showed a response following a second rechallenge. Interestingly, the median overall survival was 5.0 years (2.7–7.3 years) from trabectedin introduction and 1.5 years (0.1–4.8 years), 0.8 years (0.5–1.3 years), and 0.6 years, respectively, following second, third, and fourth trabectedin reintroduction. The authors observed that patients with tumors of low or intermediate grade of differentiation were more often rechallenged than patients with more aggressive histologies; this observation is not in agreement with our case report, whereas our patient had a very aggressive histology.
The effect of this approach in the sarcoma community still remains an open question as there is no evidence to show the absence of a deleterious effect of ‘trabectedin holidays’ in responding patients. There is instead retrospective as well as recent prospective evidence that a maintenance strategy with trabectedin provides a benefit in the outcome of patients with advanced STS treated beyond the sixth cycle.
In a retrospective analysis of the French compassionate expanded-access program in 181 heavily pretreated patients, those who were in partial response or SD after six cycles and who continued treatment had a better progression-free survival (median 5.3 vs. 10.5 months, P=0.001) and overall survival (median 13.9 vs. 33.4 months, P=0.009) compared with those who stopped after six cycles 11. The results of the worldwide expanded-access program (1803 patients) 16 reinforced these findings, reporting that 29.6% of patients received six or more cycles of treatment and had a median overall survival of 11.9 months.
Recently, the French Sarcoma Group published the results of a phase II noncomparative, randomized study investigating the clinical benefit of continuation of trabectedin treatment until progression versus interruption of therapy after six treatment cycles in patients with advanced STS (the T-DIS trial) 12. In 178 evaluable patients, 91 (51%) had not progressed after six cycles. Of these patients, 53 patients were assigned randomly to the two treatment groups: 27 to the continuation group and 26 to the interruption group. After randomization, progression-free survival at 6 months was 51.9% (95% confidence interval 31.9–68.6) in the continuation group versus 23.1% (9.4–40.3) in the interruption group (P=0.0200). The authors do not recommend trabectedin discontinuation in patients who have not progressed after six cycles of treatment.
In recognition of these emerging data, at the second rechallenge with trabectedin in our patient, we decided to continue the treatment up to 18 cycles, without any relevant cumulative toxicity. At the time of the second CR, it could be considered that concomitant radiotherapy may have helped in obtaining a CR in one of the two lesions. However, as we obtained an early metabolic response at the PET-CT scan after only two cycles of trabectedin and have often experienced a late response with this drug, it is possible that by continuing treatment, we could have achieved a CR without radiotherapy.
Trabectedin has a unique mechanism of action in that it shares the mechanisms of action of a cytotoxic agent and of a targeted therapy. This mechanism may be different among STS histotypes and this could be the reason why trabectedin has shown activity in multiple histological subtypes, even in this case of high-grade US, a disease with complex genetic alterations in the increasing group of molecular subtypes of STS. To our knowledge, this is the first report of complete radiological remission with trabectedin in this sarcoma histotype.
Conclusion
We report a complete radiological remission after two rechallenges with trabectedin in a patient with multifocal abdominal lesions from previously responding US. This case report supports the favorable safety profile of trabectedin, which can be administered for prolonged periods with no evidence of cumulative toxicity, which is consistent with previous reports 1,10. We also confirm that strategies of maintenance after six cycles of trabectedin and/or rechallenge in previously responding patients can be reasonable options during the course of treatment of advanced STS. Prospective studies are needed in this setting to confirm these observations from our ‘real-world’ experience.
Acknowledgements
The authors thank Ray Hill, an independent medical writer, who provided English-language and copy editing and journal styling before submission on behalf of Health Publishing & Services Srl.
Authors’ contributions: G.G.B. and M.P. contributed case material, and contributed to the conception and design, to the analysis and interpretation of data, and to manuscript drafting; R.F., M.D., E.P., and L.F. carried out the radiological evaluation and contributed to the analysis and interpretation of data and manuscript drafting; M.S. contributed to pathological evaluation and pathological images; R.B. shared the clinical decisions in the Italian Rare Cancer Network; G.G.B. and M.P. contributed to the analysis and interpretation of data, and manuscript drafting. All the authors have read and approved the final version of manuscript.
Conflicts of interest
G.G. Baldi has received honoraria and travel coverage from Pharmamar. For the remaining authors there are no conflicts of interest.
References
- 1.Demetri GD, Chawla SP, von Mehren M, Ritch P, Baker LH, Blay JY, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 2009; 27:4188–4196. [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, von Mehren M, Jones RL, Hensley ML, Shuetze SM, Staddon A, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016; 34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosso F, Jones RL, Demetri GD, Judson IR, Blay JY, Le Cesne A, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007; 8:595–602. [DOI] [PubMed] [Google Scholar]
- 4.Le Cesne A, Cresta S, Maki RG, Blay JY, Verweij J, Poveda A, et al. A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer 2012; 48:3036–3044. [DOI] [PubMed] [Google Scholar]
- 5.Sanfilippo R, Grosso F, Jones RL, Banerjee S, Pilotti S, D’Incalci M, et al. Trabectedin in advanced uterine leiomyosarcomas: a retrospective case series analysis from two reference centers. Gynecol Oncol 2011; 123:553–556. [DOI] [PubMed] [Google Scholar]
- 6.López-González A, Cantos B, Tejerina E, Provencio M. Activity of trabectidin in desmoplastic small round cell tumor. Med Oncol 2011; 28 (Suppl 1):S644–S646. [DOI] [PubMed] [Google Scholar]
- 7.Frezza AM, Whelan JS, Dileo P. Trabectedin for desmoplastic small round cell tumours: a possible treatment option? Clin Sarcoma Res 2014; 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaigneau L, Kalbacher E, Thiery-Vuillemin A, Fagnoni-Legat C, Isambert N, Aherfi L, et al. Efficacy of trabectedin in metastatic solitary fibrous tumor. Rare Tumors 2011; 3:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanfilippo R, Dileo P, Blay JY, Constantinidou A, Le Cesne A, Benson C, et al. Trabectedin in advanced synovial sarcomas: a multicentre retrospective study from four European institutions and the Italian Rare Cancer Network. Anticancer Drugs 2015; 26:678–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Cesne A, Yovine A, Blay JY, Delaloge S, Maki RG, Misset JL, et al. A retrospective pooled analysis of trabectedin safety in 1132 patients with solid tumors treated in phase II clinical trials. Invest New Drugs 2012; 30:1193–1202. [DOI] [PubMed] [Google Scholar]
- 11.Blay JY, Italiano A, Ray-Coquard I, Le Cesne A, Duffaud F, Rios M, et al. Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: an analysis of 181 patients of the French ATU compassionate use program. BMC Cancer 2013; 13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Cesne A, Blay JY, Domont J, Tresch-Bruneel E, Chevreau C, Bertucci F, et al. Interruption versus continuation of trabectedin in patients with soft-tissue sarcoma (T-DIS): a randomised phase 2 trial. Lancet Oncol 2015; 16:312–319. [DOI] [PubMed] [Google Scholar]
- 13.Sanfilippo R, Grosso F, Virdis E, Morosi C, Tercero JC, Gronchi A, et al. Rechallenge with trabectedin in patients with responding myxoid liposarcoma [abstract]. J Clin Oncol 2009; 27 (Suppl):10575. [Google Scholar]
- 14.Saada E, Rahal C, Coquard IR, Italiano A, Chevreau C, Isambert N, et al. Rechallenge with trabectedin in patients with locally advanced or metastatic soft tissue sarcoma following drug holiday: the experience of the French Sarcoma Group (FSG) [abstract]. J Clin Oncol 2012; 30 (Suppl):10062. [Google Scholar]
- 15.Gronchi A, Bui BN, Bonvalot S, Pilotti S, Ferrari S, Hohenberger P, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 2012; 23:771–776. [DOI] [PubMed] [Google Scholar]
- 16.Samuels BL, Chawla S, Patel S, von Mehren M, Hamm J, Kaiser PE, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013; 24:1703–1709. [DOI] [PubMed] [Google Scholar]


