Abstract
Aims/Objectives/Background
Individuals with current or previous infection with the hepatitis B virus (HBV) can experience viral reactivation when treated with immunosuppression. Rituximab, an anti-CD20 antibody used to treat many diseases, has potent immunosuppressant effects with a high risk of causing HBV reactivation. Reactivation can range from elevated liver enzymes to acute severe hepatitis with liver failure and a significant mortality risk. HBV screening and appropriate use of prophylactic antiviral therapy can prevent reactivation. This work describes the introduction of a local policy for HBV testing in patients before rituximab treatment and assesses its impact.
Methods and Results
A baseline review (before policy introduction) of 90 patients showed that only 21 (23%) had hepatitis B surface antigen (HBsAg) and 17 (19%) had hepatitis B core antibody (anti-HBcAb) tested before receiving rituximab. Following introduction of the policy (on the basis of international guidelines), improved laboratory reporting protocols and targeted education sessions, two further reviews of HBV testing rates among patients being initiated onto rituximab were performed. There was a marked increase in pre-rituximab testing for HBsAg from 23 to 79% and for anti-HBcAb from 19 to 78%. Throughout the study period, a total of one (0.8%) HBsAg-positive and six (4.7%) anti-HBcAb-positive patients were identified.
Conclusions
This work clearly indicates that simple strategies can markedly improve appropriate HBV screening. In our cohort, 6% (of whom only 43% had recognized HBV risk factors) required antiviral prophylaxis, which emphasizes the importance of universal screening before rituximab. Reinforcement of the guidelines and ongoing education is needed to further increase testing rates.
Keywords: hepatitis B, immunosuppression, reactivation, rituximab, screening
Introduction
It is estimated that globally, 240 million individuals are chronically infected with the hepatitis B virus (HBV) and two billion individuals have evidence of previous infection 1,2. Chronic infection with HBV (cHBV) causes hepatitis, which can progress to cirrhosis, liver failure and hepatocellular carcinoma. The prevalence of cHBV varies widely across the world, with areas such as Asia and Africa being highly endemic [>8% hepatitis B surface antigen (HBsAg) seropositive] 3. Although, overall, the UK is a low-endemicity area, rates of cHBV are higher in individuals who have migrated from endemic countries 4. A previous community screening study from the North-East of England found that 8.7% of the British-Chinese population and 3.1% of individuals born in Pakistan were chronically infected with HBV, and the majority did not know that they were infected 5.
It is well known that individuals with cHBV or past infection with HBV can experience a reactivation of the virus when treated with immunosuppressive therapy 6,7. As a result, screening for HBV [HBsAg and hepatitis B core antibody (anti-HBcAb) testing] has been recommended for all patients receiving immunosuppression or chemotherapy by the Centers for Disease Control and Prevention (CDC) since 2008 8, but this has not been adopted widely 9,10. It is well recognized that screening for HBV remains variable, with a large US series showing that only 16% of patients were screened before receiving chemotherapy 11. Interestingly, the latest guidance from the American Gastroenterological Association (AGA) does not recommend testing for HBV in all patients receiving chemotherapy, although they do specifically recommend testing in patients before treatment with rituximab 12.
Rituximab is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of B cells. It has potent immunosuppressant effects and is now used to treat many diseases including haematological malignancies, rheumatological conditions and other autoimmune disorders. Because of its particularly potent immunosuppressive effects, 67–85% of HBsAg-positive patients not on antiviral therapy who are treated with rituximab will experience a flare of hepatitis 13,14 and up to 25% of patients with past infection (anti-HBcAb positive, HBsAg negative) will have reactivation of HBV 15,16. HBV reactivation can range from a subclinical increase in HBV DNA levels, to elevated liver enzymes, to acute severe hepatitis with liver failure and a significant risk of death (ranging from 4 to 60%) 14,17,18. Because of its particularly high risk of causing reactivation of HBV, the Food and Drug Administration (FDA) issued specific guidance in September 2013 specifically recommending that patients treated with rituximab are screened for HBsAg and anti-HBcAb before the initiation of treatment 19. Importantly, reactivation of HBV can be prevented by the use of prophylactic antiviral therapy, which has been shown to almost eliminate the risk of HBV reactivation 20–22. In the UK, the National Institute for Health and Care Clinical Excellence (NICE) advises that all HBsAg-positive or anti-HBcAb-positive patients receive prophylactic antiviral therapy to prevent reactivation 6.
The risks associated with reactivation of HBV with rituximab treatment were highlighted in our own unit recently when an anti-HBcAb-positive, HBsAg-negative patient with diffuse large B-cell lymphoma developed severe fibrosing cholestatic hepatitis secondary to HBV reactivation with rituximab (as part of the R-CHOP regimen) that required a prolonged hospital admission despite initiation of antiviral therapy 23. The aim of this work is to describe the introduction of a hospital-wide policy on HBV testing in patients before treatment with rituximab and to assess its impact.
Materials and methods
Collection of baseline rates of testing for hepatitis B virus before initiation of the policy
An initial retrospective review was performed of HBV testing in adult patients receiving rituximab for the first time in the Newcastle upon Tyne Hospitals NHS Foundation Trust between September and December 2012 (4 months). The review was registered and approved by the Hospital Clinical Governance Department. The patients were identified from Pharmacy records as all rituximab was dispensed by the hospital pharmacy. Paediatric patients, patients with a bone marrow or solid organ transplant and those receiving rituximab as part of a clinical trial were excluded as they have HBV testing as part of their treatment protocol. Clinical details were collected from electronic health records including the following:
Demographic information.
Indication for rituximab.
Details of coprescribed immunosuppressants/chemotherapy.
Whether HBsAg and anti-HBcAb tests were performed before rituximab administration (within previous 1 year).
Results of HBV testing.
Risk factors for infection if HBsAg or anti-HBcAb positive.
Use of prophylactic antivirals.
Patient outcomes.
Development and introduction of a hospital-wide policy on hepatitis B virus testing before immunosuppression/chemotherapy
In January 2013, a hospital policy was introduced on the basis of the European Association of the Study of the Liver (EASL) guidelines 7 that recommended that all patients who were commencing chemotherapy or long-term immunosuppression should be screened for evidence of current or past HBV infection. To help ensure that the pathway was adhered to, additional laboratory reporting protocols were put in place.
Laboratory testing and reporting
All samples submitted for preimmunosuppression HBV testing were screened for HBsAg and total HBcAb using Elecsys HBsAg II and Elecsys anti-HBc Assay (Roche Diagnostics Ltd, Burgess Hill, United Kingdom). Laboratory protocols were designed for additional testing, interpretation and reporting of preimmunosuppression HBV screening samples yielding a reactive result.
Hepatitis B surface antigen-reactive samples
Other HBV markers including HB core IgM, HB e antigen and anti-HB e antibody were tested and results were interpreted and reported with appropriate comments according to the Laboratory Standard Operating Procedure in line with the recommended Standard for Microbiology Investigation methods 24. An additional report comment for urgent referral to Hepatology was added. Results were telephoned to the requesting clinician and a repeat sample was requested to confirm HBV status and check the HBV viral load level.
Antihepatitis B virus core-positive (hepatitis B surface antigen-negative) samples
Additional testing for anti-HBsAb was performed, and where anti-HBs was not detected at a level greater than 10 IU/l, the presence of anti-HBV core antibodies was confirmed using a second assay 24. Results were communicated to the requesting clinician either by telephone or by a secure e-mail, and a report was issued with the comment: ‘Evidence of past, resolved hepatitis B virus infection. Potential for HBV reactivation associated with immunosuppressive therapy. Recommend referral to viral hepatology services’.
Where the presence of anti-HBc could not be confirmed, including testing of a repeat sample, the result was reported as indeterminate and where the index of suspicion of past HBV infection was very low, results were considered to be nonspecific and clinicians were advised to check for HBV markers if liver function tests became elevated during immunosuppression.
In some cases of positive and indeterminate anti-HBc results, it is deemed likely that the result was because of passively acquired antibodies from recently received blood products. Where possible, this is confirmed by testing of earlier and later samples. The pathway for managing patients with positive HBV serology is shown in Fig. 1.
Fig. 1.
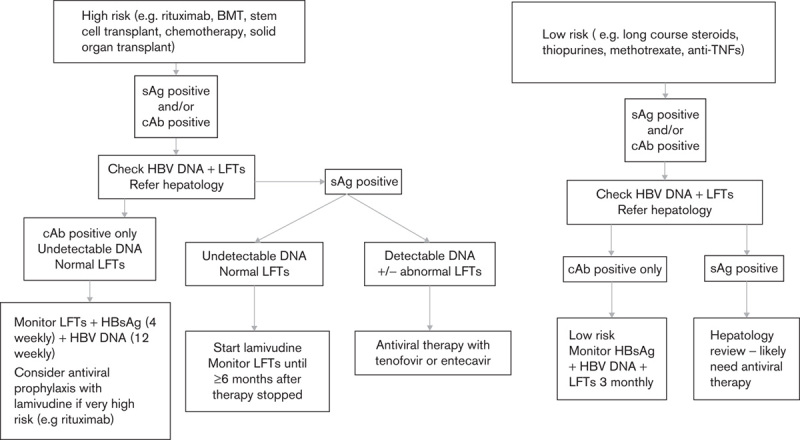
Algorithm for prevention of HBV reactivation in patients undergoing immunosuppression. BMT, bone marrow transplant; cAb, core antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IBD, inflammatory bowel disease; LFTs, liver function tests; sAg, surface antigen; TNF, tumour necrosis factor.
The guideline was published on the hospital guidelines website and all hospital clinicians were advised of the policy by e-mail. In addition, six 30-min education sessions were conducted for clinical teams (consultants, junior doctors, nurse specialists and pharmacists) who regularly prescribe chemotherapy or immunosuppression, which specifically included all the major rituximab prescribers. Education sessions were a PowerPoint presentation delivered by S.M. or J.D. describing a case of HBV reactivation following rituximab therapy, explaining the clinical implications of HBV and summarizing the evidence on reactivation and rituximab use. A simple referral proforma for HBsAg-positive and anti-HBcAb-positive patients was developed to ease the process of referral to the viral hepatitis service.
Review of hepatitis B virus testing rates following the introduction of the policy
Two further retrospective reviews of HBV testing rates among patients being initiated onto rituximab were performed after the introduction of the HBV testing policy to assess its impact. These were performed between February and April 2014 (3 months) and September and December 2014 (4 months), and followed the same methodology as the original audit. No further specific education sessions were conducted between the two postpolicy assessment periods. However, the policy was reinforced with feedback from subsequent audit results and contacts between the virology and the clinical teams with positive results.
Definition of hepatitis B virus reactivation
HBV reactivation was defined as any of the following:
Reappearance of HBV DNA and/or HBsAg in previously anti-HBcAb-positive individuals.
Increase in HBV DNA of more than 1 log IU/ml with or without increase in alanine aminotransferase levels in an HBsAg-positive patient.
Results
Baseline demographics and hepatitis B virus testing rates in the original cohort
A total of 90 patients were initiated on rituximab between September and December 2012 (4 months). The median age of the patients was 62 years (range: 18–88 years) and 60 (67%) were women. The main rituximab prescribing specialties were rheumatology (56%) and haematology (32%). Rituximab was used with other immunosuppression in 45%, alone in 29% and with other chemotherapy in 26% of patients (Fig. 2).
Fig. 2.
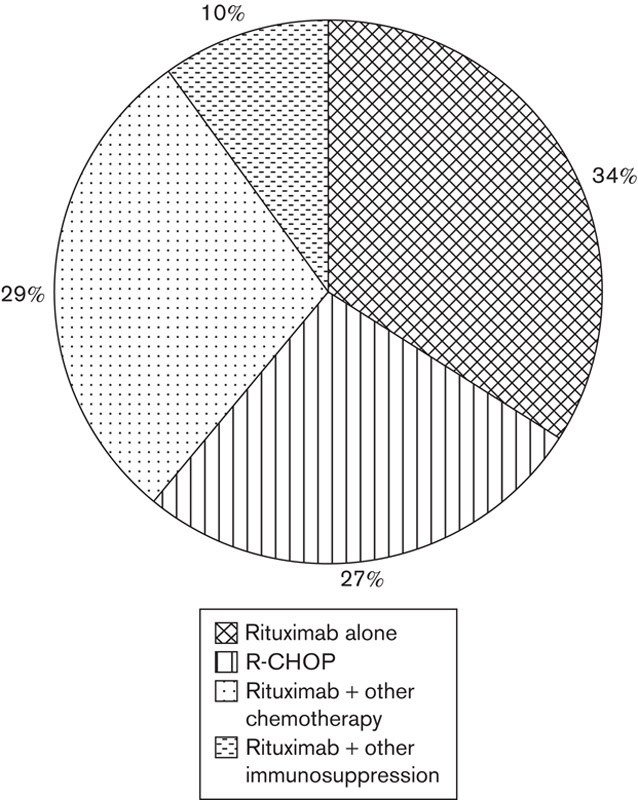
Prescription of rituximab.
Overall, only 21 (23%) patients had HBsAg and 17 (19%) patients had anti-HBcAb tested before treatment with rituximab. Two patients were anti-HBcAb positive, but none were HBsAg positive. Both anti-HBcAb patients were HBV DNA negative and neither were administered prophylactic antiviral therapy. One anti-HBcAb-positive patient (HBsAg and anti-HBsAb negative with an undetectable HBV DNA) developed a significant reactivation of HBV with jaundice. He required admission to hospital and was treated with tenofovir, and the jaundice resolved after 8 weeks.
This review highlighted poor compliance with international recommendations for HBV testing before initiation of immunosuppression. As a result, a hospital-wide guideline was developed (Fig. 1) and targeted education sessions were delivered to all the teams that treat patients with rituximab.
Hepatitis B virus testing rates after the introduction of a hepatitis B virus testing policy
Table 1 shows a comparison of the testing rates before and after the introduction of a policy on testing for HBV in all patients before initiation of rituximab. Overall, there was a marked increase in prerituximab testing for HBsAg from 23 to 79%. Testing rates for anti-HBcAb also increased considerably from 19 to 78%.
Table 1.
Hepatitis B virus testing practice across three time periods
Prescription of prophylactic antiviral therapy for hepatitis B surface antigen-positive and antihepatitis B core antibody-positive individuals
Throughout the entire study period, a total of one (0.8%) HBsAg-positive and six (4.7%) anti-HBcAb patients were identified. According to the NICE guidelines that were issued in 2013, it was recommended that all these patients should receive prophylactic antiviral therapy to prevent reactivation. Neither of the two anti-HBcAb-positive patients identified in the original cohort were treated with prophylactic antivirals as this was just before the publication of NICE guidelines. Of the five HBsAg-positive or anti-HBcAb-positive patients identified after the policy introduction, three received antiviral therapy before receiving rituximab. The HBsAg-positive patient was already on treatment with entecavir with an undetectable HBV DNA. Two of the anti-HBcAb-positive patients were commenced on lamivudine prophylactically. On further investigation, both patients who did not receive antiviral therapy were anti-HBcAb positive only with undetectable HBV DNA. One of these patients had received high-dose intravenous immunoglobulin for low-grade Waldenstrom’s macroglobulinaemia and was believed to have passively acquired the anti-HBcAb. Repeat testing 3 weeks later showed decreasing levels of anti-HBcAb consistent with this hypothesis. The other patient was noted to be anti-HBcAb positive after four cycles of rituximab and was commenced on lamivudine at that stage. His rituximab was suspended until it had been confirmed that the HBV DNA PCR was undetectable.
Of the seven patients in our total cohort who tested positive for HBsAg or anti-HBcAb, four (57%) had no obvious risk factors for hepatitis B infection. The other three patients were at risk because of their ethnicity; they were from Egypt, Pakistan and China.
Discussion
It is well recognized that treatment with immunosuppressive therapy can reactivate HBV in patients with current or previous infection. Reactivation of HBV can have significant clinical consequences including liver failure, which carries a high mortality rate 14,17,18,25,26. The risk of HBV reactivation associated with rituximab, a very potent immunosuppressant, is particularly high, occurring in 67–85% of untreated HBsAg-positive individuals and up to 25% of anti-HBcAb patients. Prophylactic treatment with oral antivirals, such as lamivudine and entecavir, has been shown to prevent viral reactivation 20–22,27,28. As a result, guidelines recommend screening for HBV with HBsAg and anti-HBcAb in all patients before the initiation of rituximab and, if either is detectable, then antiviral prophylaxis is recommended to reduce the risk of reactivation 6,7,12,19.
The initial aim of this project was to review practice in our hospital and determine the proportion of patients who had appropriate HBV testing before the initiation of rituximab, and if necessary, implement measures to improve testing and prophylaxis rates to improve patient safety. Disappointingly, our baseline assessment of HBV testing indicated low rates of HBV screening (23% HBsAg and 19% anti-HBcAb), suggesting the need to implement a change to improve practice. The results of our baseline assessment were in agreement with previous studies carried out elsewhere, where appropriate HBV testing rates ranged from 14 to 32% 9–11,27. Although we did not formally assess the reasons for the low rates of HBV testing, discussion with clinicians indicated that there was a general lack of awareness of the risks of HBV reactivation with rituximab and few clinicians had ever encountered a case; thus, they did not perceive it as a problem.
In an attempt to improve HBV testing rates and initiation of appropriate antiviral prophylaxis in patients undergoing immunosuppression or chemotherapy, a hospital guideline (Fig. 1) was developed on the basis of international recommendations 7, which was disseminated to all clinicians. In addition, six 30-min education sessions were delivered to staff involved in the management of patients on immunosuppression or chemotherapy. Each session included a case example of an HBsAg-negative/anti-HBcAb-positive patient with lymphoma who developed liver failure secondary to HBV reactivation on R-CHOP chemotherapy 23, followed by the rationale for HBV testing and prophylaxis. Following the introduction of this hospital guideline, there was a marked increase in appropriate HBV screening in patients before receiving rituximab from 19 to 78%. The improved screening rates in our Trust now exceed those reported by the American Society of Clinical Oncology (ASCO) Quality Oncology Practice Initiative (QOPI) 29, which successfully screened nearly 70% of patients with non-Hodgkin lymphoma before the initiation of rituximab. QOPI was created in 2002 to measure quality and enable quality improvement in cancer care. Ongoing work is needed to further improve testing rates in our hospital as one in five patients treated with rituximab remains untested for HBV. Alternative methods of improving HBV testing rates could include the use of a computer-assisted hospital-based screening reminder system, which was shown to achieve an HBsAg screening rate of 85.5% 29. However, that method was still associated with a disappointing rate of appropriate antiviral prophylaxis of only 45.5% 30.
Some clinicians cite the low prevalence of HBV in some areas as a reason not to offer universal HBV screening before immunosuppression/chemotherapy, and instead target individuals with associated risk factors (such as country of birth, injecting drug use, sexual contact, etc.) or do not screen at all 11. Importantly, in this study, even in a low HBV prevalence country (0.5% HBsAg seropositivity), there was a high rate of HBsAg and anti-HBcAb positivity (6%), which suggests that screening in patients receiving rituximab is worthwhile as all these patients would be offered prophylactic antiviral therapy. A previous study showed that only 60% of patients with HBV infection have obvious risk factors for the infection, which means that targeting risk factors will miss a significant proportion of cases 31. This was mirrored in the present study, where 57% of the patients who tested positive did not have any obvious risk factors for HBV, which reinforces the need for universal testing. Screening all patients for HBV who are receiving rituximab-based chemotherapy has also been shown to be more cost effective than screening ‘high-risk’ individuals or not screening at all 32.
It is increasingly being recognized that individuals with past infection (anti-HBcAb positive) have a significant risk of HBV reactivation with rituximab (up to 25%), which is higher than that for other immunosuppressants (5%) 33,34. HBV incorporates covalently closed circular HBV DNA (cccDNA) in hepatocyte nuclei, which remains present even after the immune control of the infection, with subsequent disappearance of circulating HBsAg from the peripheral blood 35. If patients are immunosuppressed with drugs, such as rituximab, HBV viral replication can be reinitiated, leading to reactivation. Patients who develop HBV reactivation following treatment with rituximab have a 20–50% risk of mortality 34,36–38, which appears to be higher than that found with other immunosuppressants. The mechanisms leading to HBV reactivation with rituximab remain to be elucidated, but may be more complex than B-cell depletion alone 39. Various hypotheses have been suggested including changes in T lymphocyte activity and number 40, and reduction of anti-HBV antibodies 41. It is interesting to note that HBV reactivation in patients receiving BCR-ABL tyrosine kinase inhibitors has recently been recognized. The European Medicines Agency (EMA) now advises testing for HBV before initiating treatment with these agents (e.g. imatinib, dasatinib, nilotinib) 42.
There is convincing evidence that preemptive antiviral prophylaxis is more effective than treating patients once their HBV DNA starts to increase, both in terms of preventing liver injury and reducing mortality 27,28. Huang et al. 20 compared the use of prophylactic entecavir before rituximab-based chemotherapy to therapeutic entecavir (at the time of HBV reactivation and HBsAg reverse seroconversion since chemotherapy). The cumulative HBV reactivation rates at months 6, 12 and 18 after chemotherapy were 8, 11.2 and 25.9%, respectively, in the control group and 0, 0 and 4.3% in the entecavir prophylactic group (P=0.019). A recent randomized, open-label, phase 3 study from China 22 compared entecavir with lamivudine for the treatment of HBsAg-positive patients with low viral loads receiving R-CHOP. The results showed significantly lower rates of HBV-related hepatitis, HBV reactivation and chemotherapy disruption in the entecavir group. There was no significant difference between treatment-related adverse events between the two groups. Both the EASL and NICE guidance provide clear advice on the use of prophylactic antivirals for all HBsAg-positive patients receiving immunosuppression of chemotherapy 6,7.
It is important that HBV screening be performed before starting any immunosuppression to avoid false-negative HB core results because of immunosuppression, ideally at the time of the diagnosis of conditions requiring immunosuppression. Although the HB core-positive (HBsAg negative) profile reflects past HBV infection, it could also reflect passively acquired antibody from recent blood products or may rarely be because of nonspecific reactivity or HBsAg mutants. Additional testing is critical to clarify the true HBV status to avoid unnecessary antiviral prophylaxis. As the frequency of HBV testing increases in patients receiving immunosuppression/chemotherapy, there is likely to be an associated increase in false-positive results, as observed in our cohort with the patient who received intravenous immunoglobulin. This means that there needs to be close collaboration between virology and clinical teams to ensure that all test results are interpreted accurately.
There are limitations with this study. The national guidelines were in evolution during the study period and some of the improvement in screening practices may be because of the dissemination of the NICE guidelines and FDA guidance as well as our implementation methods. In addition, the reasons for the low rates of HBV screening before implementation of the policy were not formally assessed.
This work clearly shows that simple strategies (introduction of a local guideline, targeted education and close liaison with the virologists) can markedly improve appropriate HBV screening. In our cohort, 6% of patients (of whom only 43% had recognized risk factors for HBV) required antiviral prophylaxis, which emphasizes the importance of universal screening in patients before rituximab. However, reinforcement of the guidelines and ongoing education is needed to further increase testing rates. Education should be across the broader medical audience, but with specifically targeted sessions to specialities who are known to be users of rituximab. Screening for HBV in patients receiving rituximab is now clearly defined, but the magnitude of the risk associated with other chemotherapeutic and immunosuppressive regimens is more controversial 43 and further work is needed to define this.
Acknowledgements
The authors acknowledge all members of the virology team at the Newcastle upon Tyne Hospitals NHS Foundation Trust who have implemented the changes to the laboratory protocols.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO. Hepatitis B factsheet. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/. [Accessed 8 November 2015].
- 2.Cooke GS, Main J, Thursz MR. Treatment for hepatitis B. BMJ 2010; 340:b5429. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Travelers’ health; yellow book. Atlanta, GA: Services UDoHaH; 2008. [Google Scholar]
- 4.Uddin G, Shoeb D, Solaiman S, Marley R, Gore C, Ramsay M, et al. Prevalence of chronic viral hepatitis in people of south Asian ethnicity living in England: the prevalence cannot necessarily be predicted from the prevalence in the country of origin. J Viral Hepat 2010; 17:327–335. [DOI] [PubMed] [Google Scholar]
- 5.McPherson S, Valappil M, Moses SE, Eltringham G, Miller C, Baxter K, et al. Targeted case finding for hepatitis B using dry blood spot testing in the British-Chinese and South Asian populations of the North-East of England. J Viral Hepat 2013; 20:638–644. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Clinical Excellence (NICE). Hepatitis B (chronic): diagnosis and management of chronic hepatitis B in children, young people and adults; 2013. Available at: guidance.nice.org.uk/cg165. [Accessed 8 November 2015].
- 7.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR [online serial]. 2008; 57:RR-8. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5708.pdf. [Accessed 8 November 2015]. [Google Scholar]
- 9.Lee R, Vu K, Bell CM, Hicks LK. Screening for hepatitis B surface antigen before chemotherapy: current practice and opportunities for improvement. Curr Oncol 2010; 17:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wi CI, Loo NM, Larson JJ, Moynihan TJ, Madde NR, Grendahl DC, et al. Low level of hepatitis B virus screening among patients receiving chemotherapy. Clin Gastroenterol Hepatol 2015; 13:970–975. quiz e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang JP, Fisch MJ, Lok AS, Zhang H, Vierling JM, Suarez-Almazor ME. Trends in hepatitis B virus screening at the onset of chemotherapy in a large US cancer center. BMC Cancer 2013; 13:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148:215–219. quiz e16–e17. [DOI] [PubMed] [Google Scholar]
- 13.Lee GW, Ryu MH, Lee JL, Oh S, Kim E, Lee JH, et al. The prophylactic use of lamivudine can maintain dose-intensity of adriamycin in hepatitis-B surface antigen (HBs Ag)-positive patients with non-Hodgkin’s lymphoma who receive cytotoxic chemotherapy. J Korean Med Sci 2003; 18:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology 1991; 100:182–188. [DOI] [PubMed] [Google Scholar]
- 15.Chen KL, Chen J, Rao HL, Guo Y, Huang HQ, Zhang L, et al. Hepatitis B virus reactivation and hepatitis in diffuse large B-cell lymphoma patients with resolved hepatitis B receiving rituximab-containing chemotherapy: risk factors and survival. Chin J Cancer 2015; 34:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol 2014; 32:3736–3743. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai K, Takagi T, Nakamura S, Sawada U, Kura Y, Kodama F, et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol 1997; 8 (Suppl 1):107–109. [PubMed] [Google Scholar]
- 18.Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology 1999; 46:2925–2930. [PubMed] [Google Scholar]
- 19.FDA Prescribing Information. Label information for rituximab. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103705s5367s5388lbl.pdf. [Accessed 8 November 2015].
- 20.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013; 31:2765–2772. [DOI] [PubMed] [Google Scholar]
- 21.Ho EY, Yau T, Rousseau F, Heathcote EJ, Lau GK. Preemptive adefovir versus lamivudine for prevention of hepatitis B reactivation in chronic hepatitis B patients undergoing chemotherapy. Hepatol Int 2015; 9:224–230. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA 2014; 312:2521–2530. [DOI] [PubMed] [Google Scholar]
- 23.Dyson JK, Hudson M, McPherson S. Lesson of the month 2: severe reactivation of hepatitis B after immunosuppressive chemotherapy. Clin Med (Lond) 2014; 14:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Standards for microbiology investigations. Available at: https://www.gov.uk/government/collections/standards-for-microbiology-investigations-smi. [Accessed 14 February 2016].
- 25.Liang R. How I treat and monitor viral hepatitis B infection in patients receiving intensive immunosuppressive therapies or undergoing hematopoietic stem cell transplantation. Blood 2009; 113:3147–3153. [DOI] [PubMed] [Google Scholar]
- 26.Hoofnagle JH, Dusheiko GM, Schafer DF, Jones EA, Micetich KC, Young RC, Costa J. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Intern Med 1982; 96:447–449. [DOI] [PubMed] [Google Scholar]
- 27.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology 2008; 47:844–853. [DOI] [PubMed] [Google Scholar]
- 28.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuss MN, Desch CE, McNiff KK, Eisenberg PD, Gesme DH, Jacobson JO, et al. A process for measuring the quality of cancer care: the Quality Oncology Practice Initiative. J Clin Oncol 2005; 23:6233–6239. [DOI] [PubMed] [Google Scholar]
- 30.Sun WC, Hsu PI, Yu HC, Lin KH, Tsay FW, Wang HM, et al. The compliance of doctors with viral hepatitis B screening and antiviral prophylaxis in cancer patients receiving cytotoxic chemotherapy using a hospital-based screening reminder system. PLoS One 2015; 10:e0116978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 2012; 10:1412–1415. [DOI] [PubMed] [Google Scholar]
- 32.Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, Feld JJ. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol 2012; 30:3167–3173. [DOI] [PubMed] [Google Scholar]
- 33.Uhm JE, Kim K, Lim TK, Park BB, Park S, Hong YS, et al. Changes in serologic markers of hepatitis B following autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13:463–468. [DOI] [PubMed] [Google Scholar]
- 34.Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology 2006; 131:59–68. [DOI] [PubMed] [Google Scholar]
- 35.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996; 2:1104–1108. [DOI] [PubMed] [Google Scholar]
- 36.Li JM, Wang L, Shen Y, Xia ZG, Chen Y, Chen QS, et al. Rituximab in combination with CHOP chemotherapy for the treatment of diffuse large B cell lymphoma in Chinese patients. Ann Hematol 2007; 86:639–645. [DOI] [PubMed] [Google Scholar]
- 37.Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009; 27:605–611. [DOI] [PubMed] [Google Scholar]
- 38.Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 2009; 90:13–23. [DOI] [PubMed] [Google Scholar]
- 39.Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol 2011; 22:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 2007; 110:2924–2930. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsumi Y, Tanaka J, Kawamura T, Miura T, Kanamori H, Obara S, et al. Possible efficacy of lamivudine treatment to prevent hepatitis B virus reactivation due to rituximab therapy in a patient with non-Hodgkin’s lymphoma. Ann Hematol 2004; 83:58–60. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC). PRAC recommendations on signals. EMAPRAC (PRAC); 2016. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/PRAC_recommendation_on_signal/2016/04/WC500204264.pdf. [Accessed 14 February 2015]. [Google Scholar]
- 43.Keam B, Lee JH, Im SA, Yoon JH. Why, when, and how to prevent hepatitis B virus reactivation in cancer patients undergoing chemotherapy. J Natl Compr Canc Netw 2011; 9:465–477. [DOI] [PubMed] [Google Scholar]


