Abstract
A new plant breeding method—the biotron breeding system (BBS)—can rapidly produce advanced generations in rice (Oryza sativa L.) breeding. This method uses a growth chamber (biotron) with CO2 control, accompanied by tiller removal and embryo rescue to decrease the period before seed maturity. However, tiller removal and embryo rescue are laborious and impractical for large populations. We investigated the influences of increased CO2, tiller removal, and root restriction on the days to heading (DTH) from seeding in growth chambers. The higher CO2 concentration significantly decreased DTH, but tiller removal and root restriction had little effect on DTH and drastically reduced seed yield. Based on these findings, we propose a simplified BBS (the sBBS) that eliminates the need for tiller removal and embryo rescue, but controls CO2 levels and day-length and maintains an appropriate root volume. Using the sBBS, we could reduce the interval between generations in ‘Nipponbare’ to less than 3 months, without onerous manipulations. To demonstrate the feasibility of the sBBS, we used it to develop isogenic lines using ‘Oborozuki’ as the donor parent for the low-amylose allele Wx1-1 and ‘Akidawara’ as the recipient. We were able to perform four crossing cycles in a year.
Keywords: isogenic lines, rice (Oryza sativa L.), rapid generation advancement, CO2 application, biotron
Introduction
Rapid generation-advancement techniques have been developed to accelerate breeding cycles and breeding progress in many crops (Bhattarai et al. 2009, Carandang et al. 2006, Depauw and Clarke 1976, Gaur et al. 2007, Heu et al. 1982, Ishigaki 2010, Kato 1959, Rizal et al. 2014, Tanaka 2006, Tanio et al. 2006, Wang et al. 2011). In Japanese rice breeding programs, a greenhouse-based rapid generation-advancement technique was the primary tool used to breed the cultivar ‘Nipponbare’ (Koumura 1972a, 1972b), which became Japan’s leading cultivar in the 1970s. Since then, such techniques have been utilized in the early generations of breeding programs to develop many new rice cultivars. The early generations of most modern cultivars released by Japan’s public sector underwent such rapid generation advancement (http://ineweb.narcc.affrc.go.jp/). In recent years, such techniques have also been used to develop experimental lines, such as recombinant inbred lines (RILs; Janwan et al. 2013, Sirithunya et al. 2002), backcrossed inbred lines (BILs; Robin et al. 2003), chromosome segment substitution lines (CSSLs; Toshio Yamamoto, personal communication), and isogenic cultivars (Sugiura et al. 2004, Tomita et al. 2006, Tsunematsu et al. 2015), thereby accelerating the progress of rice genomics research.
Recent advances in genotyping technology have permitted cost-effective scoring of genome-wide marker polymorphisms (Davey et al. 2011, Gupta et al. 2008, Li et al. 2008, Syvänen 2005), and offer a new avenue for novel breeding technologies that take advantage of detailed genomic information. Genomic selection has attracted attention as a new method for plant breeding (Heffner et al. 2009, Jannink et al. 2010, Lorenz et al. 2011). In genomic selection, researchers develop a model for predicting the genomic breeding value of a target trait on the basis of genotypes with dense, genome-wide markers, using both phenotype and marker genotype data from a training population. By using the model, it is possible to select individuals or lines from a breeding population on the basis of the genomic estimated breeding values predicted from their marker genotypes, without the need to obtain their phenotypic data. Because genomic selection does not require phenotypic data for the selection of candidates, rapid generation-advancement technologies can boost the rate of genetic improvement by reducing the interval between generations and accelerating selection cycles in a breeding program. Thus, efficient methods for rapid generation advancement are key technologies that can support the successful application of genomic selection in plant breeding.
Ohnishi et al. (2011) reported a revolutionary development in rapid generation advancement: the biotron breeding system (BBS), which can greatly shorten the generation cycle of ‘Nipponbare’ to about 2 months under controlled conditions. Combined use of genomic selection and the BBS can greatly boost the rate of genetic improvement in rice breeding owing to the accelerated generation cycles. It is, however, difficult to apply it to a large breeding population, because it requires laborious manipulations (tiller removal and embryo rescue). Therefore, for its practical use in rice breeding programs, modifications will be required to improve its efficiency.
The fundamental technologies used in the BBS are temperature control, regulation of day-length, CO2 application to enhance growth, tiller removal to develop compact rice plants, and embryo rescue to shortcut the seed maturation and dormancy-breaking periods (Ohnishi et al. 2011). However, there is not enough information about how each of these techniques contributes to a reduction of the generation interval (i.e., the days to heading, DTH, counted from sowing). In the present study, we first evaluated the effects of CO2 concentration control and tiller removal on DTH in two rice cultivars (‘Nipponbare’ and ‘Yamadawara’). We also investigated the contribution of root restriction, which causes precocious flowering in some shrub or tree species (Bar-Yosef et al. 1988, Ikeda and Kikuchi 2003, Imai et al. 1990, Tanaka 2006, Wang et al. 1997, Yahata et al. 1995). Then, on the basis of the observed effects of the CO2 application, tiller removal, and root restriction treatments on DTH, we tried to simplify the BBS (to produce the sBBS) to develop a more practical rapid generation-advancement system for rice breeding. Because cultivar-specific photoperiod sensitivities are expected to strongly influence the effectiveness of the sBBS, breeders need to consider the photoperiod sensitivities of the breeding materials before using the system. We investigated the variation in DTH among 48 rice accessions in two environments (high-temperature, short-day conditions in a phytotron, and field conditions) to confirm the capability and limitations of the new system for rapid development of advanced generations in rice culture. Finally, to evaluate its potential, we applied the sBBS to the rapid establishment of isogenic lines using ‘Oborozuki’ as the donor of the low-amylose allele Wx1-1 and ‘Akidawara’ as the recipient, through four crossing cycles in a single year, and thereby demonstrated the system’s efficiency in rice breeding.
Materials and Methods
Plant materials
This study consisted of three experiments. The first was an evaluation of the effect of various system elements on reducing, DTH and the use of the results to propose the sBBS. We evaluated the effects of CO2 application, tiller removal, and root restriction on DTH of two rice cultivars, ‘Nipponbare’ and ‘Yamadawara’. ‘Nipponbare’ is sensitive and ‘Yamadawara’ is insensitive to photoperiod, but they have almost equal heading periods under the general cultivation conditions in western Japan (http://ineweb.narcc.affrc.go.jp/). However, the heading period of ‘Yamadawara’ is known to fluctuate in response to the transplanting date and local climate conditions, whereas that of ‘Nipponbare’ is relatively stable.
The second investigated the potential applicability of the sBBS to diverse rice accessions. We characterized the DTH of 48 rice accessions (Table 1) under high-temperature and artificial short-day conditions, and under field conditions with natural day-length and without supplemental CO2 application.
Table 1.
Days to heading (DTH) of 48 accessions under high-temperature and short-day conditions, and under field conditions
| Name of accessions | Origin | Estimated ecotypes | DTH under high-temperature, short-day conditions | n | DTH under the sBBS conditions | n | DTH under the field conditionsa | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| (Country) | (Area) | |||||||
| Nihonmasari | Japan | Kanto | japonica | 43.3 ± 0.6 | 3 | 109 | ||
| Nipponbare | Japan | Tokai | japonica | 46.5 ± 2.5 | 4 | 47.8 ± 0.5 | 4 | 113 |
| Ballila28 | Italy | japonica | 46.8 ± 1.0 | 4 | 99 | |||
| Ballila | Italy | japonica | 47.3 ± 1.0 | 4 | 102 | |||
| Akidawara | Japan | Kanto | japonica | 47.8 ± 0.5 | 4 | 114 | ||
| A-69b | Japan | indica | 47.9 ± 1.0 | 8 | – | |||
| Tachiaoba | Japan | Kyushu | japonica | 48.7 ± 1.5 | 3 | 50.7 ± 1.5 | 4 | 138 |
| Wa 2204 | Japan | Kanto | japonica | 48.8 ± 1.7 | 4 | 124 | ||
| Oboroduki | Japan | Hokkaido | japonica | 49.0 ± 2.0 | 3 | 75 | ||
| Benihime | Japan | Kanto | japonica | 49.0 ± 1.4 | 4 | 119 | ||
| Tanginbouzu | Japan | Hokuriku | japonica | 49.3 ± 0.5 | 4 | 120 | ||
| Hakuchomochi | Japan | Hokkaido | japonica | 50.0 ± 3.6 | 4 | 50.3 ± 0.5 | 4 | 69 |
| Bekogonomi | Japan | Tohoku | japonica | 51.8 ± 2.2 | 4 | 86 | ||
| Koshihikari | Japan | Hokuriku | japonica | 51.8 ± 2.4 | 4 | 53.8 ± 1.7 | 4 | 106 |
| Tachisuzuka | Japan | Chugoku | japonica | 52.0 ± 1.4 | 2 | 54.0 ± 1.4 | 4 | 135 |
| Reimei | Japan | Tohoku | japonica | 52.3 ± 1.2 | 3 | 94 | ||
| NERICA 3c | Nigeria (Africa Rice) | 53.0 ± 0.0 | 4 | 107 | ||||
| Tsuyahime | Japan | Tohoku | japonica | 53.0 ± 1.2 | 4 | 106 | ||
| Kusanohoshi | Japan | Chugoku | japonica | 53.8 ± 3.4 | 4 | 129 | ||
| Hoshijirushi | Japan | Kanto | japonica | 53.8 ± 1.3 | 4 | 113 | ||
| Tachisugata | Japan | Kanto | indica | 54.8 ± 2.4 | 4 | 112 | ||
| Chubu 132 | Japan | Tokai | japonica | 56.7 ± 1.5 | 3 | 108 | ||
| Yumeaoba | Japan | Hokuriku | japonica | 57.8 ± 0.5 | 4 | 60.0 ± 1.2 | 4 | 107 |
| Nona Bokra | India | indica | 59.0 ± 2.2 | 4 | 58.0 ± 1.4 | 4 | – | |
| Sasanishiki | Japan | Tohoku | japonica | 59.0 ± 1.4 | 4 | 99 | ||
| Bellmont | Spain | japonica | 59.0 ± 1.4 | 4 | 111 | |||
| Kanto-shi 258 | Japan | Kanto | indica | 58.5 ± 1.0 | 4 | 111 | ||
| Koyukimochi | Japan | Tohoku | japonica | 58.7 ± 1.2 | 3 | 105 | ||
| Kusahonami | Japan | Kanto | japonica | 59.5 ± 1.9 | 4 | 122 | ||
| Natsuaoba | Japan | Hokuriku | japonica | 61.5 ± 1.0 | 4 | 96 | ||
| NERICA 1c | Nigeria | 62.3 ± 2.5 | 3 | 112 | ||||
| Wa 2105 | Japan | Kanto | indica | 64.0 ± 4.2 | 4 | 124 | ||
| Arborio | Italy | japonica | 65.0 ± 0.0 | 2 | 100 | |||
| Mochidawara | Japan | Kanto | indica | 65.3 ± 0.5 | 4 | 113 | ||
| Yumetoiro | Japan | Hokuriku | indica | 66.5 ± 1.3 | 4 | 109 | ||
| Hokuriku 193 | Japan | Hokuriku | indica | 70.3 ± 4.5 | 4 | 72.3 ± 1.2 | 3 | 114 |
| Basmati 370 | India | indica | 73.0 ± 4.5 | 4 | 135 | |||
| Hiderishirazu D | Japan | Tohoku | japonica | 73.5 ± 1.0 | 4 | 76.0 ± 4.5 | 4 | 93 |
| Krnai | India | japonica | 74.0 ± 0.0 | 3 | 95 | |||
| Leafstar | Japan | Chugoku | japonica | 75.3 ± 2.5 | 4 | 76.5 ± 3.0 | 4 | 136 |
| Hoshiaoba | Japan | Chugoku | japonica | 81.0 ± 2.3 | 4 | 111 | ||
| spw-cls | Japan | japonica | 82.0 ± 0.0 | 2 | 115 | |||
| Makimizuho | Japan | Kyushu | japonica | 82.8 ± 2.9 | 4 | 112 | ||
| IR 8 | Philippines | indica | 84.5 ± 3.0 | 4 | 143 | |||
| Leah | USA | indica | 94.0 ± 1.4 | 4 | 113 | |||
| Mogumoguaoba | Japan | Kyushu | japonica | 106.0 ± 1.0 | 2 | 119 | ||
| Momiroman | Japan | Kanto | japonica | 103.8 ± 3.9 | 4 | 119 | ||
| Banten | Indonesia | japonica | 133.5 ± 3.3 | 4 | 114 | |||
At the Yawara paddy experimental field, Tsukubamirai, Ibaraki, Japan (36°01′N, 140°02′E). The materials were seeded on 25 April in the green house and replanted on 26 May 2012.
Near isogenic line of ‘Tachisugata’ with ‘Nona Bakra’ allele of Hd1.
Interspecific line derived from the cross between O. sativa and O. glaberrima, and with japonica genetic background.
Cleistogamous mutant of ‘Taichung 65’ (Yoshida et al. 2007).
n: Numbers of individuals.
The third was the application of the sBBS to the rapid establishment of isogenic lines. We evaluated the potential of the proposed sBBS by rapidly establishing isogenic lines using ‘Oborozuki’ as the donor of the low-amylose allele Wx1-1 and ‘Akidawara’ as the recipient. ‘Akidawara’ is a recently developed cultivar with good eating quality and high yield that is grown in the Kanto, Hokuriku, Chubu and western regions of Japan (Ando et al. 2011); ‘Oborozuki’, with a low amylose content and superior eating quality, is grown in Hokkaido, in northern Japan (Ando et al. 2007). The low amylose content of ‘Oborozuki’ is controlled by the wx locus, which encodes a granule-bound starch synthase gene on the short arm of chromosome 6 (Ando et al. 2010). In the backcrossing experiment, we selected individuals harboring the ‘Oborozuki’ allele of the wx locus (Wx1-1) by using a DNA marker described later in the methods.
Common growth conditions
Each seed was directly sown in a 50-mL horticultural plug tray (each plug is 4.4 cm wide, 3.9 cm long, and 6.9 cm high; RL-40PT, Tokai Chemical Industries, Ltd., Mino, Gifu, Japan). The seeds were germinated in a moist chamber at 30°C for 3 days, and subsequently transferred to a growth chamber. Two LH350-SP growth chambers were used (Nippon Medical & Chemical Instruments Co. Ltd., Osaka, Japan). The chambers originally had no CO2 application or humidity control functions. A CO2 regulator was introduced into one chamber, but not the other. It was set to create a concentration of 600 ppm, although the actual concentration of CO2 in the chamber fluctuated between 560 and 800 ppm. The temperature settings in both chambers were 27°C during the 10-h-light period and 25°C during the 14-h-dark period. The light intensities were 25 000 lx, which is equivalent to 230 pmol photons m−2 s−1. Up to 96 plants in their individual soil plug containers could be raised in each chamber.
Granulated nursery soil for rice seedlings (NPK: 1.5:2.7:2.2) was used. In the subsequent accelerated backcrossing experiment and in the investigation of DTH variations in the phytotron, we applied 100-mL of nutrient solution containing 5% (w/v) urea and 5% (w/v) Hyponex (NPK: 6.5:6:19) to each group of plants each time the leaves turned pale.
Evaluation of CO2 application, tiller removal, and root restriction
We used 14 experimental plots to evaluate the effect of CO2 application, tiller removal, and root restriction (Table 2). The plots were identified by the levels of four factors (cultivar, CO2 application, tiller removal, and root restriction), in the combinations shown in Table 2. Treatments were coded according to these factors: ‘Nipponbare’ = ‘N’ and ‘Yamadawara’ = ‘Y’; CO2 application = ‘C’ and its control (ambient CO2) = ‘a’; tiller removal = ‘R’ and its control (natural tillering) = ‘n’. The levels of root restriction were designated in accordance with the volume of the container (tray or pot) used to restrict root growth; for example, ‘NCR050’ indicates that ‘Nipponbare’ was cultivated with CO2 application and tiller removal in a 50-mL tray.
Table 2.
The effects of CO2 application, tiller removal, and root restriction on the traits of ‘Nipponbare’ and ‘Yamadawara’ rice grown in growth chambers
| Cultivar | CO2 application | Tiller removal | Root restriction volume (ml) | Experimental plot symbol | n | Days to Heading (DTH) | Culm length (cm) | Panicle number | Number of fertile spikelet | Percentage of ripening grain (%) | Total weight of unhulled grain (g) | Thousand unhulled grain weight (g) | Dry weight of aerial part (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nipponbare | + | − | 50 | NCn050 | 8 | 49.4 ± 0.9 | 44.6 ± 3.0 | 3.3 ± 0.5 | 74.9 ± 14.6 | 96.0 ± 2.2 | 1.72 ± 0.36 | 22.9 ± 0.5 | 3.92 ± 0.70 |
| + | − | 5 | NCn005 | 9 | 48.4 ± 1.4 | 52.9 ± 2.7 | 1.0 ± 0.0 | 35.2 ± 6.1 | 95.6 ± 3.8 | 0.85 ± 0.15 | 24.2 ± 1.4 | 1.97 ± 0.19 | |
| + | + | 50 | NCR050 | 8 | 49.6 ± 0.7 | 33.2 ± 6.1 | 1.4 ± 0.5 | 17.9 ± 6.2 | 95.5 ± 5.0 | 0.41 ± 0.15 | 22.6 ± 2.7 | 0.97 ± 0.33 | |
| − | − | 50 | Nan050 | 8 | 52.1 ± 0.8 | 37.4 ± 5.2 | 2.6 ± 0.9 | 52.1 ± 23.4 | 86.4 ± 11.2 | 0.98 ± 0.49 | 18.6 ± 3.7 | 2.38 ± 0.79 | |
| − | + | 50 | NaR050 | 9 | 51.4 ± 0.9 | 39.6 ± 4.3 | 1.0 ± 0.0 | 24.9 ± 4.3 | 86.6 ± 10.4 | 0.45 ± 0.14 | 18.0 ± 3.9 | 1.12 ± 0.16 | |
| − | − | 5 | Nan005 | 9 | 51.7 ± 0.9 | 26.1 ± 3.2 | 1.3 ± 0.5 | 15.0 ± 5.7 | 79.0 ± 12.3 | 0.23 ± 0.09 | 15.5 ± 4.2 | 0.74 ± 0.30 | |
|
| |||||||||||||
| Yamadawara | + | − | 260 | YCn260 | 10 | 71.4 ± 1.3 | 49.4 ± 4.2 | 6.8 ± 1.3 | 224.6 ± 21.0 | 95.6 ± 2.0 | 5.98 ± 0.52 | 26.6 ± 0.5 | 15.60 ± 1.26 |
| + | − | 50 | YCn050 | 8 | 71.3 ± 1.6 | 48.4 ± 3.9 | 2.5 ± 0.9 | 64.5 ± 24.1 | 94.9 ± 4.0 | 1.76 ± 0.65 | 27.3 ± 1.3 | 4.57 ± 1.67 | |
| + | + | 50 | YCR050 | 9 | 70.0 ± 1.4 | 49.0 ± 2.9 | 1.0 ± 0.0 | 33.3 ± 5.3 | 98.5 ± 1.8 | 0.90 ± 0.15 | 27.0 ± 0.7 | 2.19 ± 0.27 | |
| + | − | 5 | YCn005 | 9 | 74.4 ± 2.7 | 38.6 ± 5.4 | 1.0 ± 0.0 | 18.2 ± 5.1 | 96.7 ± 4.6 | 0.49 ± 0.13 | 27.2 ± 2.4 | 1.33 ± 0.35 | |
| − | − | 260 | Yan260 | 8 | 82.1 ± 2.2 | 43.3 ± 2.8 | 5.4 ± 0.7 | 162.8 ± 39.0 | 96.1 ± 6.5 | 4.54 ± 1.02 | 28.0 ± 0.6 | 11.79 ± 1.50 | |
| − | − | 50 | Yan050 | 9 | 80.6 ± 4.3 | 43.3 ± 3.5 | 2.3 ± 0.7 | 48.4 ± 11.6 | 98.5 ± 1.8 | 1.28 ± 0.32 | 26.4 ± 1.0 | 3.77 ± 0.92 | |
| − | + | 50 | YaR050 | 9 | 75.4 ± 2.5 | 40.1 ± 2.7 | 1.0 ± 0.0 | 25.2 ± 6.0 | 97.6 ± 2.9 | 0.68 ± 0.17 | 26.9 ± 1.2 | 1.85 ± 0.36 | |
| − | − | 5 | Yan005 | 10 | 87.9 ± 3.0 | 36.2 ± 2.7 | 1.0 ± 0.0 | 17.0 ± 7.8 | 96.4 ± 3.6 | 0.45 ± 0.24 | 26.4 ± 1.6 | 1.34 ± 0.41 | |
Values are means ± standard deviations.
Ten seeds were sown in each plot, and the resulting plants were used for the experiments. The plants in NCR050, NaR050, YCR050, and YaR050 were restricted to the main culm by removing all tillers. To evaluate the effect of root restriction, we used the abovementioned 50-mL plug trays, 5-mL cell trays (each cell is 2.1 cm wide, 2.1 cm long, and 3.2 cm high; Tokankosan Co. Ltd., Tokyo, Japan), and 260-mL horticultural pots (8.0 cm upper diameter, 5.0 cm bottom diameter, and 7.0 cm high; only in the ‘Yamadawara’ plots). A holder was used as twofold to sandwich the non-woven textile for restricting root volume.
Examination of DTH in a photoperiod-controlled phytotron and under field conditions
DTH was defined as the number of days from seeding to heading. To evaluate the variations in DTH under short-day conditions, we grew the 48 accessions both in the photoperiod-controlled phytotron (under high-temperature, short-day conditions) and under field conditions. The DTH values of 10 of these 48 cultivars were also measured under the sBBS conditions. In the phytotron, plants were grown at 35°C during the 10-h-light period and 28°C during the 14-h-dark period. The CO2 concentration was not controlled. The field experiment was conducted in an experimental paddy field in Yawara, Tsukubamirai, Ibaraki, Japan (36°01′N, 140°02′E). The materials were seeded on 25 April in the green house and replanted on 26 May for the field test.
Backcrossing
Backcrossing was carried out to develop isogenic line for low grain amylose content caused by the Wx1-1 allele derived from ‘Oborozuki’ (Ando et al. 2007) using the recurrent parent ‘Akidawara’. Spikes of the seed parent were emasculated in hot water (7 min at 43°C) and the florets were cut. Spikes of the pollen parent were bundled together with the seed parent’s spikes in the growth chamber, using garden ties, so that the flowers of the seed parent were pollinated when the pollen parent flowered. To elevate the height of the spikes and increase the fertilization rate, the pollen parents were transplanted into a larger pot 20 to 40 days before heading. After grain filling, the ripened seeds were harvested, and then dried for a few days at room temperature. The dormancy of the seeds was broken by treatment for 5 days at 50°C under dry conditions (Jennings and de Jesus 1964).
DNA was extracted from the germinated seedlings to screen for individuals harboring the target gene by using a DNA marker for the wx locus. DNA was extracted by using diatomaceous earth and a spin filter (Tanaka and Ikeda 2002) or a DNA Sui-Sui kit (Rizo Inc., Tsukuba, Ibaraki, Japan). We used the PCR primers Wx-U1 (5′-CAGGCTGGAGGAACAGAAGG-3′) and Wx-L3 (5′-TCACCTTGCCCGGATACTTC-3′) to detect the ‘Oborozuki’ allele of the wx locus (Ando et al. 2010). The PCR temperature conditions were based on the touchdown PCR technique (Don et al. 1991). The PCR program was as follows: 5 minutes at 94°C to completely denature the DNA; followed by 34 cycles of 30 s at 94°C, 60 s at the annealing temperature (described below), and 30 s at 72°C; followed by 10 minutes at 72°C to allow complete double-strand DNA synthesis. The annealing temperature, which was 62°C in the first cycle, was reduced by 0.5°C per cycle during cycles 2 to 14 and then maintained at 55°C for the last 20 cycles.
Results
Effects of CO2 application, tiller removal, and root restriction
Of the 10 seeds of ‘Nipponbare’ and ‘Yamadawara’ sown in each plot, an average of 8.8 germinated and were used in the experiment. Table 2 shows the variations in DTH, culm length, panicle number, number of fertile spikelets, percentage of ripe grains, total weight of unhulled grains, weight per thousand unhulled grains, and dry weight of the aboveground parts. We performed ANOVA to test for significant effects of cultivar, CO2 application, tiller removal, and root restriction on these eight traits (Table 3). We found significant relationships between all four factors and DTH. Cultivar had the greatest influence. The DTH of ‘Yamadawara’ was significantly greater than that of ‘Nipponbare’ under any combination of the other factors.
Table 3.
ANOVA results for the effects of the cultivar, CO2 application, tiller removal, and root restriction on the eight rice parameters
| Degrees of freedom | Sum of squares | Mean square | F value | Significance1) | ||
|---|---|---|---|---|---|---|
| Number of days to flowering (NDF) | Cultivar | 1 | 20,604.3 | 20,604.3 | 1,838.5 | *** |
| CO2 application | 1 | 1,451.8 | 1,451.8 | 129.5 | *** | |
| Root restriction | 2 | 334.2 | 167.1 | 14.9 | *** | |
| Tiller removal | 1 | 69.8 | 69.8 | 6.2 | * | |
| Residuals | 117 | 1,311.2 | 11.2 | |||
|
| ||||||
| Culm length (cm) | Cultivar | 1 | 462.2 | 462.2 | 24.2 | *** |
| CO2 application | 1 | 1,528.7 | 1,528.7 | 80.0 | *** | |
| Root restriction | 2 | 2,747.3 | 1,373.6 | 71.9 | *** | |
| Tiller removal | 1 | 217.1 | 217.1 | 11.4 | ** | |
| Residuals | 114 | 2,178.2 | 19.1 | |||
|
| ||||||
| Panicle number | Cultivar | 1 | 25.7 | 25.7 | 65.6 | *** |
| CO2 application | 1 | 5.2 | 5.2 | 13.3 | *** | |
| Root restriction | 2 | 321.0 | 160.5 | 410.0 | *** | |
| Tiller removal | 1 | 47.4 | 47.4 | 121.1 | *** | |
| Residuals | 114 | 44.6 | 0.4 | |||
|
| ||||||
| Number of fertile spikelets | Cultivar | 1 | 43,214 | 43,214 | 127 | *** |
| CO2 application | 1 | 14,214 | 14,214 | 42 | *** | |
| Root restriction | 2 | 376,497 | 188,248 | 553 | *** | |
| Tiller removal | 1 | 15,310 | 15,310 | 45 | *** | |
| Residuals | 114 | 38,784 | 340 | |||
|
| ||||||
| Ripe grains (%) | Cultivar | 1 | 2,486.4 | 2,486.4 | 25.9 | *** |
| CO2 application | 1 | 1,116.3 | 1,116.3 | 11.6 | *** | |
| Root restriction | 2 | 236.6 | 118.3 | 1.2 | ||
| Tiller removal | 1 | 13.8 | 13.8 | 0.1 | ||
| Residuals | 114 | 10,945.2 | 96.0 | |||
|
| ||||||
| Total weight of unhulled grains (g) | Cultivar | 1 | 45.7 | 45.7 | 220.6 | *** |
| CO2 application | 1 | 11.3 | 11.3 | 54.3 | *** | |
| Root restriction | 2 | 274.1 | 137.1 | 661.8 | *** | |
| Tiller removal | 1 | 8.5 | 8.5 | 41.2 | *** | |
| Residuals | 114 | 23.6 | 0.2 | |||
|
| ||||||
| Weight per thousand unhulled kernels (g) | Cultivar | 1 | 645.6 | 645.6 | 157.4 | *** |
| CO2 application | 1 | 40.5 | 40.5 | 9.9 | ** | |
| Root restriction | 2 | 9.5 | 4.8 | 1.2 | ||
| Tiller removal | 1 | 0.4 | 0.4 | 0.1 | ||
| Residuals | 114 | 467.7 | 4.1 | |||
|
| ||||||
| Dry weight of aerial parts (g) | Cultivar | 1 | 356.2 | 356.2 | 370.4 | *** |
| CO2 application | 1 | 57.1 | 57.1 | 59.3 | *** | |
| Root restriction | 2 | 1,833.9 | 917.0 | 953.4 | *** | |
| Tiller removal | 1 | 60.0 | 60.0 | 62.4 | *** | |
| Residuals | 114 | 109.6 | 1.0 | |||
*** P < 0.001; ** P < 0.01; * P < 0.05.
Two cultivars, Nipponbare and Yamadawara were used.
The factor with the second-greatest influence on DTH was CO2 application. Higher CO2 shortened the DTH by 2.7 days in ‘Nipponbare’ and by 9.3 days in ‘Yamadawara’ in the plots with a 50-mL root volume and without tiller removal (Fig. 1). The smaller reduction in ‘Nipponbare’ was confirmed in plots with container volumes other than 50-mL. CO2 application generally increased the culm length, panicle number, number of fertile spikelets, percentage of ripe grains, total weight of unhulled grains, weight per thousand unhulled grains, and dry weight of aboveground parts relative to ambient CO2. The CO2 concentration inside the growth chamber with ambient CO2 was around 500 ppm throughout the dark period and <100 ppm throughout the light period, when there was rapid plant growth. The leaves of these plants visibly sagged, unlike those in the growth chamber with CO2 application.
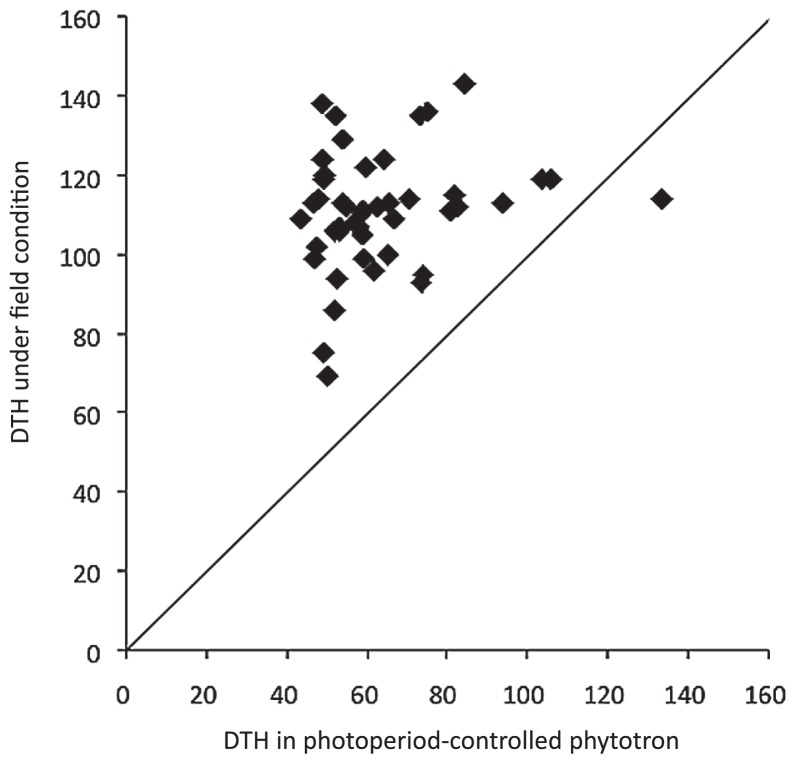
Fig. 1.
Relationship between days to heading (DTH) in a photoperiod-controlled phytotron (high temperature, short day-length) and under field conditions for the 46 accessions. The diagonal line represents y = x. Photoperiod-controlled phytotron: 35°C during the 10-h-light period and 28°C during the 14-h-dark period. The CO2 concentration was not controlled. Field conditions: an experimental paddy field in Yawara, Tsukubamirai, Ibaraki, Japan (36°01′N, 140°02′E). The seeds were sown on 25 April and transplanted on 26 May 2011.
Tiller removal affected DTH in the YaR050 treatment, where DTH was 5.2 days less than in Yan050. In the other pairs of comparisons (i.e., NCR050 vs. NCn050, NaR050 vs. Nan050, and YCR050 vs. YCn050), however, the effect of tiller removal was statistically significant but too small to have practical importance (i.e., decreases of 1.0, 0.7, and 1.3 days, respectively; P < 0.05). However, tiller removal reduced the number of grains in all treatments to around 50% of the control value, and also reduced the dry weight of the aboveground parts (Table 2).
There was no significant difference in DTH between the 50-mL and 260-mL root restriction levels in ‘Yamadawara’. However, in both cultivars, the DTH of the 5-mL root restriction treatment was far larger than at the other root restriction levels. Plant growth was significantly suppressed in the 5-mL root volume, as indicated by the smaller dry weight of the aerial parts and a smaller seed yield in both cultivars (Table 2). In addition, the culm length in the 5-mL root volume was so small that we could grow two layers of plants, one above the other, in a single growth chamber (data not shown).
The results indicate that the DTH of ‘Nipponbare’ could be reduced to less than 50 days. This could be achieved by CO2 application combined with a 10-h-light period at 27°C and a 14-h-dark period at 25°C, without requiring tiller removal. This suggests that it would take less than 90 days to advance one generation; in addition to the DTH of 50 days, the preparation of seeds for the next generation also requires a grain-filling period (25 days), seed drying (2 days), and a dormancy-breaking period at 50°C (5 days). We defined these optimized conditions (i.e., day-length, high CO2 concentration, and appropriate temperature) and treatments (i.e., without embryo rescue or tiller removal) as a rapid generation-advancement technique using growth chambers, and named it the simplified BBS (sBBS) method.
DTH in a photoperiod-controlled phytotron and under field conditions
The DTH under sBBS conditions seem to be slightly larger than that under high-temperature, short-day conditions (Table 1). The DTH values of the 48 accessions were measured in the photoperiod-controlled phytotron and field conditions. Fig. 1 shows the relationship between the DTH of 46 accessions under phytotron and field conditions (excluding two that did not flower under field conditions). Under high-temperature, short-day conditions, ‘Nihonmasari’ had the smallest DTH (43.3 days) and ‘Banten’ had the largest (133.5 days). The DTH of ‘Banten’ under high-temperature short-day condition was clearly larger than that under fieled conditions. ‘Nona Bokra’ and ‘A-69’ did not flower under field conditions because of late floral differentiation, but they had a smaller DTH under the high-temperature, short-day conditions than ‘Hiderishirazu D’ and ‘Krunai’, which both flowered relatively early (small DTH) under field conditions. We found no statistically significant correlation between DTH under high-temperature, short-day conditions and the corresponding DTH under field conditions (Fig. 1).
Backcrossing for breeding isogenic lines
Four backcrossing cycles per year could be carried out to establish isogenic lines using ‘Oborozuki’ as the donor of the Wx1-1 allele and ‘Akidawara’ as the recipient (Table 4). ‘Oborozuki’ flowers extremely early under any conditions (Table 1), but under sBBS conditions, the DTH of ‘Akidawara’ was similar to that of ‘Oborozuki’, and a cross between the two cultivars was possible even when the seeds of both cultivars were sown at the same time. The color of the crossed F1 seeds (dehulled rice grains) turned from green to white about 20 days after crossing. The seeds were then harvested, and after 2 days of drying and 5 days of heat treatment at 50°C (dry) to break dormancy, they germinated normally. Therefore, in the sBBS, the duration from crossing to sowing was 27 days.
Table 4.
Four successive backcrosses per year could be achieved using the sBBS method described in this study, with ‘Akidawara’ as the pollen recipient and ‘Oborozuki’ as the pollen donor
| Cross combination | Seeding date of parent plants | Date when crossing started | Number of days from seeding to crossing | Seed harvest date | Number of days from crossing to seed harvesting | Length of a generation | Generation of the harvested seeds | Number of seeds | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female parent | Male parent harvested | ||||||||
| Oborozuki | Akidawara | 26-Jan-11 | 24-Mar-11 | 57 | 15-Apr-11 | 22 | 79 | F1 | 24 |
| Akidawara | F1 individuals | 22-Apr-11 | 20-Jun-11 | 59 | 11-Jul-11 | 21 | 80 | BC1F1 | 53 |
| Akidawara | BC1F1 individuals | 26-Jul-11 | 22-Sep-11 | 58 | 15-Oct-11 | 23 | 81 | BC2F1 | 286 |
| Akidawara | BC2F1 individuals | 27-Oct-11 | 27-Dec-11 | 61 | 20-Jan-12 | 24 | 85 | BC3F1 | 15 |
| Akidawara | BC3F1 individuals | 27-Jan-12 | 23-Mar-12 | 56 | 14-Apr-12 | 22 | 78 | BC4F1 | 16 |
|
| |||||||||
| Average | – | – | 58.2 | – | 22.4 | 80.6 | – | 78.8 | |
|
| |||||||||
| Total | – | – | 291 | – | 112 | 403 | – | 394 | |
Backcrosses were performed under the sBBS conditions; CO2 concentration 560 to 800 ppm, 27°C during the 10-h-light period and 25°C during the 14-h-dark period.
Discussion
Evaluation of the factors that are important in the BBS and the sBBS
In our evaluation of the effects of CO2 application, tiller removal, and root restriction, under a 10-h-day-length, the DTH of ‘Nipponbare’ under all treatment conditions was much smaller than that of ‘Yamadawara’ under the equivalent treatment conditions (Table 2), even though the cultivars have almost equal heading times under the general field cultivation conditions in western Japan (http://ineweb.narcc.affrc.go.jp/). Cultivar had a highly significant effect on DTH in the experiment with the 48 accessions, because the effects of other BBS and sBBS factors on DTH depended greatly on the photoperiod-sensitivity of the cultivars as shown in Table 3.
CO2 application was the second-most important factor for DTH. Even though the growth chambers were not sealed, the CO2 concentration in the chamber with ambient CO2 fell below 100 ppm during the active growth phase of the plants. The plants in that growth chamber exhibited sagging leaves, suggesting that their growth was impeded by the low CO2 concentration; this was confirmed by the aboveground biomass values (Table 2). The observed increase in DTH (Table 2) and decrease in other quantitative traits (e.g., culm length, panicle number) could have been caused by this growth reduction. Therefore, this shows that CO2 application is essential to the growth of rice plants in the growth chambers, probably because rice is a C3 photosynthetic species.
The tiller removal treatment did not change DTH drastically in the photoperiod-sensitive cultivar ‘Nipponbare’. Moreover, it reduced the number of harvested seeds. These findings suggest that the laborious step of tiller removal is unnecessary and possibly counterproductive, especially when the BBS method is applied to a large population.
In many woody perennial species, root restriction promotes juvenile flowering and fruiting (Bar-Yosef et al. 1988, Ikeda and Kikuchi 2003, Imai et al. 1990, Tanaka 2006, Wang et al. 1997, Yahata et al. 1995). However, in the photoperiod-insensitive rice, it was less effective than we expected. Cultivation in a 5-mL root volume increased DTH (Table 2) and was accompanied by a large reduction in the number of seeds harvested per plant. The increase in DTH appears to be due to delayed growth. In salvia (Salvia splendens), excessive restriction of root volume extends the vegetative growth period (van Iersel 1997). Using a 5-mL volume would, however, let us grow up to 1152 individuals per growth chamber. This was achieved by using double-decker shelving, which was possible because the height of each plant was low (data not shown). Thus, the root restriction treatment will be useful for growing a large population, such as in a single-seed-descent population used to produce RIL and BIL populations. On the other hand, in an experiment that requires a large number of seeds per plant, as in the case of crosses between two cultivars, the root volume should be greater than 50-mL to ensure the production of enough seeds.
Applicability of the sBBS to various rice materials
The sBBS system was more efficient than growing plants under field conditions, especially for photosensitive late-heading rice accessions, which had DTH values of 120 days or more under field conditions.
When ranked by DTH, the order of the 48 accessions grown in the phytotron under high-temperature, short-day conditions differed from the order under field conditions. The accessions with a small DTH in the phytotron and under field conditions, such as ‘Nona Bokra’, are expected to have strong photoperiod sensitivity. On the other hand, accessions that have a large DTH under short-day conditions and early heading (small DTH) under field conditions, such as ‘Hiderishirazu D’, are expected to be less or not photosensitive.
The DTH of ‘Banten’ under high-temperature short-day condition was larger than the DTH under field conditions. Since ‘Banten’ is an Indonesian tropical japonica cultivar with large biomass, the 50-mL root volume is too small for it. Delayed growth may have been due to an insufficient root volume, as in the case of rice growth in the 5-mL root volume and in salvia (van Iersel 1997).
Wide variations of DTH were found in conditions both of high-temperature short-day and natural fields (Fig. 1, Table 1), and these results indicated that DTH of rice accessions used in this study were genetically controlled by complex genetic mechanisms. The duration of the generation of each accession under sBBS conditions is affected by various factors such as day-length, temperature during the daytime and the night, and root volume. When sBBS is used for specific genotypes, the altering these factors is effective in shortening the duration of the generation more.
In the photoperiod-controlled phytotron, the DTH of ‘Nipponbare’ was smaller than that under the sBBS conditions. This probably resulted from the higher temperature and stronger light in the phytotron. Therefore, further reduction of DTH could be achieved through further optimization of the environmental conditions. However, under the phytotron conditions in this experiment, it was difficult to obtain cross-pollinated seed (data not shown): that is, the high temperature (35°C) during the light period could have decreased pollen viability, as reported by Matsui et al. (1997).
When the sBBS approach was used, it was difficult to promote early heading of non-photosensitive accessions, except for cultivars developed in Hokkaido, such as ‘Oborozuki’. In this study, the cultivars from Hokkaido showed extremely early heading under both short-day conditions in the sBBS and natural day-length conditions in the field. On the other hand, under sBBS conditions, it was easy to stimulate early heading of photosensitive cultivars such as ‘Nona Bokra’ and the progeny of line ‘A-69’, which did not flower in the field. The photosensitivity of rice cultivars and lines is strongly controlled by the Hd1 locus (Takahashi et al. 2009, Yano et al. 2000). Thus, information on the Hd1 allele will be useful for selecting materials that are most suitable for the sBBS approach. When applying the sBBS, the synchronization of heading will be the key to increasing the crossing rate. The functional alleles of Hd1 could be used to adjust the timing and synchronization of heading among many materials.
Use of the sBBS for rapid establishment of isogenic lines
The sBBS enabled us to perform four crossings in a year (i.e., one generation every 3 months), which would be useful for developing isogenic lines from the donor parent ‘Oborozuki’ and the recipient parent ‘Akidawara’. In the sBBS method, the time from crossing to sowing was only 27 days, because the cross-pollinated seeds could be harvested about 20 days after heading, and then germinated after 2 days of drying and 5 days of heat treatment to break dormancy. In contrast, in the original BBS system, embryos were rescued 7 days after crossing, and this step required an additional 4 days compared with normal germination, thus requiring a total of 11 days from crossing to sowing. Therefore, the sBBS required an extra 16 days compared with the BBS. The length of the 50°C treatment to break seed dormancy could be decreased from 5 days to 1 day by replacing this step with a 1-day H2O2 treatment (Takagi et al. 1986), suggesting that the difference in the cycle length between the BBS and sBBS systems could be reduced to 12 days. The establishment of a isogenic line normally requires 6 generations. For example, generating a BC4F2 population requires 6 generations: the first cross to generate the F1 individuals, followed by four backcrosses and one selfing. Using the sBBS, a near-isogenic line could therefore be established within 1.5 years.
Potential of the sBBS for constructing experimental populations and conducting efficient genomic selection
In molecular genetics, large experimental populations (e.g., RILs, BILs, and CSSLs) that include a target phenotype are indispensable. However, several generations are needed to achieve this goal, and this takes a long time. In addition, managing such a large population in the BBS system is laborious because of the need for embryo rescue and tiller removal. In contrast, the sBBS can be applied more easily to a large population because it does not require either of these manipulations. In addition, the sBBS will be useful for crossing genetically modified materials, because it can be completed in a laboratory. Therefore, the sBBS technique will be a powerful tool to construct experimental populations.
However, it would be difficult to apply the sBBS directly in practical breeding programs using phenotype-based screening, because the agronomic phenotypes under sBBS conditions can be quite different from the phenotypes under field conditions. In a study of QTLs using BILs derived from crosses between the Japanese rice cultivars ‘Nipponbare’ and ‘Koshihikari’, almost half of the QTLs that control agronomic traits were detected near the heading date QTLs (Hori et al. 2012). In this study, the relationship between DTH under sBBS conditions and DTH under field conditions was very weak and not significant (Fig. 1). Therefore, selection based on the agronomic phenotypes of extremely early-heading individuals under sBBS conditions would be meaningless for practical breeding.
However, using genomic selection, we could select plants cultivated with the sBBS method on the basis of genomic estimated breeding values predicted from their marker genotypes, without requiring any phenotypic evaluations of the plants. Using genomic selection and the sBBS in combination, it would be possible to accelerate genetic improvement by reducing the intervals between generations and thereby shortening the selection cycles. Because genomic selection can increase the genetic gain per unit time by shortening breeding cycles (Heffner et al. 2010), the sBBS can be a promising way to implement genomic selection in plant breeding.
Acknowledgments
We thank Mrs. J. Shioda, Mrs. Y. Niizeki, and Mrs. E. Odajima, who are assistants at the NARO Institute of Crop Science, for their help. We also thank Dr. T. Kinoshita and Dr. T. Ohnishi of Nara Institute of Science and Technology for their helpful instructions for performing the BBS. We also thank Dr. Nobuhiro Suzuki in Sophia University for revision of English in the manuscript. This work was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation GMA-0002).
Literature Cited
- Ando, I., Araki, H., Shimizu, H., Kuroki, M., Miura, K., Nagano, K. and Konno, K. (2007) “Oborozuki”, a new rice cultivar with low amylose content and superior eating quality”. Res. Bull. Natl. Agric. Res. Cent. for Hokkaido Reg. 186: 31–46. [Google Scholar]
- Ando, I., Sato, H., Aoki, N., Suzuki, Y., Hirabayashi, H., Kuroki, M., Shimizu, H., Ando, T. and Takeuchi, Y. (2010) Genetic analysis of the low-amylose characteristics of rice cultivars Oborozuki and Hokkai-PL9. Breed. Sci. 60: 187–194. [Google Scholar]
- Ando, I., Nemoto, H., Kato, H., Ohta, H., Hirabayashi, H., Takeuchi, Y., Sato, H., Ishii, T., Maeda, H., Imbe, T.et al. (2011) A new rice cultivar “Akidawara” with high yield, good grain appearance, and good eating quality. Breed. Res. 13: 35–41. [Google Scholar]
- Bar-Yosef, B., Schwartz, S., Markovich, T., Lucas, B. and Assaf, R. (1988) Effect of root volume and nitrate solution concentration on growth, fruit yield, and temporal N and water uptake rates by apple trees. Plant Soil 107: 49–56. [Google Scholar]
- Bhattarai, S.P., De La Pena, R.C., Midmore, D.J. and Palchamy, K. (2009) In vitro culture of immature seed for rapid generation advancement in tomato. Euphytica 167: 23–30. [Google Scholar]
- Carandang, F.M., Shanmugasundaram, S. and Carpena, A.L. (2006) Rapid generation advancement in soybeans using immature seeds. Philipp. J. Crop Sci. 31: 53–59. [Google Scholar]
- Davey, J.W., Hohenlohe, P.A., Etter, P.D., Boone, J.Q., Catchen, J.M. and Blaxter, M.L. (2011) Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat. Rev. Genet. 12: 499–510. [DOI] [PubMed] [Google Scholar]
- Depauw, R.M. and Clarke, J.M. (1976) Acceleration of generation advancement in spring wheat. Euphytica 25: 415–418. [Google Scholar]
- Don, R.H., Cox, P.T., Wainwright, B.J., Baker, K. and Mattick, J.S. (1991) ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19: 4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur, P.M., Srinivasan, S., Gowda, C.L.L. and Rao, B.V. (2007) Rapid generation advancement in chickpea. J. SAT Agric. Res. 3. [Google Scholar]
- Gupta, P.K., Rustgi, S. and Mir, R.R. (2008) Array-based high-throughput DNA markers for crop improvement. Heredity (Edinb) 101: 5–18. [DOI] [PubMed] [Google Scholar]
- Heffner, E.L., Sorrells, M.E. and Jannink, J.L. (2009) Genomic selection for crop improvement. Crop Sci. 49: 1–12. [Google Scholar]
- Heffner, E.L., Lorenz, A.J., Jannink, J.-L. and Sorrells, M.E. (2010) Plant breeding with genomic selection: gain per unit time and cost. Crop Sci. 50: 1681–1690. [Google Scholar]
- Heu, M.H., Chung, G.S., Kim, D.K., Sasaki, T. and Vergara, B.S. (1982) Rapid generation advance in breeding rice for low temperature tolerance. Proc. Rice Res. Conf., Los Baños, Philippines. [Google Scholar]
- Hori, K., Kataoka, T., Miura, K., Yamaguchi, M., Saka, N., Nakahara, T., Sunohara, Y., Ebana, K. and Yano, M. (2012) Variation in heading date conceals quantitative trait loci for other traits of importance in breeding selection of rice. Breed. Sci. 62: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, H. and Kikuchi, H. (2003) Effect of root restriction by using non-woven fabric pots made of different materials on the growth and yield of Japanese persimmon ‘Hachiya’. Tohoku Agric. Res. 56: 175–176. [Google Scholar]
- Imai, S., Shidawara, M., Furui, S. and Fujiwara, T. (1990) Tree growth and fruit production of ‘Kyoho’ grapes under root zone restriction. Bull. Natl. Agric. Res. Cent. West. Reg. 79: 44–50. [Google Scholar]
- Ishigaki, Y. (2010) Establishment of cultivation technique with rapid generation advancement of Cyclamen persicum by sowing seeds right after picking seeds. Bull. Gifu Pref. Res. Inst. Agr. Sci. in Hill. Mount. Are. 6: 7–12. [Google Scholar]
- Jannink, J.L., Lorenz, A.J. and Iwata, H. (2010) Genomic selection in plant breeding: From theory to practice. Brief. Funct. Genomics 9: 166–177. [DOI] [PubMed] [Google Scholar]
- Janwan, M., Sreewongchai, T. and Sripichitt, P. (2013) Rice breeding for high yield by advanced single seed descent method of selection. J. Plant Sci. 8: 24–30. [Google Scholar]
- Jennings, P.R. and de Jesus, J. (1964) Effect of heat on breaking seed dormancy in rice. Crop Sci. 4: 530–533. [Google Scholar]
- Kato, Y. (1959) Stimulation of differentiation of flower buds in Sugi (Cryptomeria japonica) by gibberellin (2). J. Jpn. For. Soc. 4: 138–141. [Google Scholar]
- Koumura, T. (1972a) Breeding of new rice variety ‘Nipponbare’ (1). Agric. Tech. 27: 112–116. [Google Scholar]
- Koumura, T. (1972b) Breeding of new rice variety ‘Nipponbare’ (2). Agric. Tech. 27: 159–161. [Google Scholar]
- Li, C., Li, M., Long, J.R., Cai, Q. and Zheng, W. (2008) Evaluating cost efficiency of SNP chips in genome-wide association studies. Genet. Epidemiol. 32: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, A.J., Chao, S., Asoro, F.G., Heffner, E.L., Hayashi, T., Iwata, H., Smith, K.P., Sorrells, M.E. and Jannink, J.L. (2011) Genomic selection in plant breeding: Knowledge and prospects. Adv. Agron. 110: 77–123. [Google Scholar]
- Matsui, T., Omasa, K. and Horie, T. (1997) High temperature-induced spikelet sterility of japonica rice at flowering in relation to air temperature, humidity and wind velocity conditions. Jpn. J. Crop Sci. 66: 449–455. [Google Scholar]
- Ohnishi, T., Yoshino, M., Yamakawa, H. and Kinoshita, T. (2011) The biotron breeding system: a rapid and reliable procedure for genetic studies and breeding in rice. Plant Cell Physiol. 52: 1249–1257. [DOI] [PubMed] [Google Scholar]
- Rizal, G., Karki, S., Alcasid, M., Montecillo, F., Acebron, K., Larazo, N., Garcia, R., Slamet-Loedin, I.H. and Quick, W.P. (2014) Shortening the breeding cycle of sorghum, a model crop for research. Crop Sci. 54: 520–529. [Google Scholar]
- Robin, S., Pathan, M.S., Courtois, B., Lafitte, R., Carandang, S., Lanceras, S., Amante, M., Nguyen, H.T. and Li, Z. (2003) Mapping osmotic adjustment in an advanced back-cross inbred population of rice. Theor. Appl. Genet. 107: 1288–1296. [DOI] [PubMed] [Google Scholar]
- Sirithunya, P., Tragoonrung, S., Vanavichit, A., Pa-In, N., Vongsaprom, C. and Toojinda, T. (2002) Quantitative trait loci associated with leaf and neck blast resistance in recombinant inbred line population of rice (Oryza sativa). DNA Res. 9: 79–88. [DOI] [PubMed] [Google Scholar]
- Sugiura, N., Tsuji, T., Fujii, K., Kato, T., Saka, N., Touyama, T., Hayano, Y.S. and Izawa, T. (2004) Molecular marker-assisted selection in a recurrent backcross breeding for the incorporation of resistance to rice stripe virus and panicle blast in rice (Oryza sativa L.). Breed. Res. 6: 143–148. [Google Scholar]
- Syvänen, A.C. (2005) Toward genome-wide SNP genotyping. Nat. Genet. 37: S5–S10. [DOI] [PubMed] [Google Scholar]
- Takagi, Y., Samoto, S., Kishikawa, H. and Motomura, T. (1986) Effect of hydrogen peroxide on dormancy breaking in rice seed. Bull. Fac. Agr. Saga Univ. 61: 55–59. [Google Scholar]
- Takahashi, Y., Teshima, K.M., Yokoi, S., Innan, H. and Shimamoto, K. (2009) Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc. Natl. Acad. Sci. USA 106: 4555–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, J. and Ikeda, S. (2002) Rapid and efficient DNA extraction method from various plant species using diatomaceous earth and a spin filter. Breed. Sci. 52: 151–155. [Google Scholar]
- Tanaka, J. (2006) Breeding system combining of juvenile selection using DNA markers and acceleration of generations, aiming for the significant improvement of the tea breeding in Makurazaki Tea Research Station, NIVTS. Breed. Res. 8: 119–126. [Google Scholar]
- Tanio, M., Kato, K., Ishikawa, N., Tabiki, T., Nishio, Z., Nakamichi, K., Tamura, Y., Sato, M., Takagi, H. and Matsuoka, M. (2006) Effect of shuttle breeding with rapid generation advancement on heading traits of Japanese wheat. Breed. Sci. 56: 311–320. [Google Scholar]
- Tomita, K., Horiuchi, H., Terada, K., Kobayashi, A., Tanaka, I., Tanoi, M., Minobe, T., Furuta, H., Yamamoto, A., Masaki, N.et al. (2006) “Hanaechizen BL1”, “Hanaechizen BL2”, “Hanaechizen BL3”, “Hanaechizen BL4”, new rice isogenic lines with true resistant genes to blast”. Bull. Fukui Agr. Exp. Stn. 43: 1–27. [Google Scholar]
- Tsunematsu, H., Ando, I., Nemoto, H., Sunohara, Y., Kato, H., Hirabayashi, H., Takeuchi, Y., Maeda, H., Sato, H., Tanaka, J.et al. (2015) Breeding of “Koshihikari Kanto BL1”, a near isogenic line of “Koshihikari” with blast resistance gene Pi9. Bull. NARO Inst. Crop Sci. 15: 75–93. [Google Scholar]
- van Iersel, M. (1997) Root restriction effects on growth and development of salvia (Salvia splendens). HortScience 32: 1186–1190. [Google Scholar]
- Wang, S., Okamoto, G. and Hirano, K. (1997) Vine growth and fruit development of ‘Pione’ grapes planted in root-restricted buried and raised beds. J. Japan. Soc. Hort. Sci. 66: 253–259. [Google Scholar]
- Wang, X.F., Wang, Y.X., Zhang, G.Y. and Ma, Z.Y. (2011) An integrated breeding technology for accelerating generation advancement and trait introgression in cotton. Plant Breed. 130: 569–573. [Google Scholar]
- Yahata, D., Oba, Y., Kuwahara, M. and Matsumoto, K. (1995) Effects of crop load on water stress, fruit quality, yield and flower bud formation on root-restricted wase satsuma mandarin trees grown indoors. J. Japan. Soc. Hort. Sci. 63: 745–752. [Google Scholar]
- Yano, M., Katayose, Y., Ashikari, M., Yamanouchi, U., Monna, L., Fuse, T., Baba, T., Yamamoto, K., Umehara, Y., Nagamura, Y.et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H., Itoh, J., Ohmori, S., Miyoshi, K., Horigome, A., Uchida, E., Kimizu, M., Matsumura, Y., Kusaba, M., Satoh, H.et al. (2007) superwoman1-cleistogamy, a hopeful allele for gene containment in GM rice. Plant Biotechnol. J. 5: 835–846. [DOI] [PubMed] [Google Scholar]