Abstract
Background:
For years, the safety and effectiveness of autologous fat grafting (AFG) for breast reconstruction have been in question, with particular concern over fat necrosis, calcifications, cyst formation, and interfering with the detection of breast cancer. However, increasing evidence suggests that the complication rates and clinical results are generally acceptable to both clinicians and patients. The emerging challenge is the numerous AFG techniques and systems, where there are limited knowledge and data. The objective of this study was to conduct a literature review that focuses on the safety, effectiveness, and efficiency of various AFG techniques as applied to the breast.
Methods:
A PubMed search using terms related to AFG was performed over a 5-year period (April 1, 2010–April 30, 2015). Original articles focused on AFG to the breast, with outcomes on safety, effectiveness, and efficiency, were included.
Results:
Five hundred ninety-eight articles were identified with 36 articles included (n = 4306 patients). Satisfaction rates were high although the prevalence of complications was low—similar to previous findings. Seven studies reported average operating room time with an overall mean of 125 minutes (range: 40–210). The mean volume of fat harvested was 558 mL (range: 120–1299), and fat injected was 145 mL (range: 20–607). A positive association between injection volume and operating time was observed.
Conclusions:
This review validates previous findings on the safety and effectiveness of AFG to the breast and highlights its efficiency. The efficiency data available, although limited, suggest that there is an opportunity to achieve time and cost savings while not sacrificing safety and effectiveness.
Autologous fat grafting (AFG) has been increasing in popularity among plastic surgeons as a means to provide soft-tissue augmentation, particularly for situations where there is no reasonable alternative. AFG is generally viewed favorably because it does not elicit hypersensitivity or a foreign body reaction,1 in contrast to the placement of a nonautologous material. It also provides a potential bonus of removing unwanted fat from other areas. Perhaps most importantly, it is a much less invasive alternative to breast supplementation than autologous tissue transfer or breast implants.
In the field of AFG, particular focus has been on applications such as breast reconstruction, where AFG can be used in conjunction with or instead of breast implants and even flaps. However, the use of AFG in the breast initially raised concerns about its safety and effectiveness. In 1987, the American Society of Plastic and Reconstructive Surgeons’ (ASPRS)2 Ad Hoc Committee on New Procedures released a position article raising concerns regarding the risk of postoperative fat necrosis and microcalcifications that could compromise the detection of breast cancer on subsequent mammography. Twenty years after the 1987 article, the ASPS position has evolved and in 2009, its task force made new recommendations for the safe and efficacious use of AFG to the breast as supportive evidence had become more readily available.3
Despite the wide range of described techniques for AFG, there are a generally low frequency of complications and a high level of clinical acceptance, which have been documented in a number of previous evidence reviews. Specifically, relatively low complication rates have been demonstrated, with the majority of patients and surgeons being satisfied with the results.3–6 A caveat is that many of the referenced studies focused on animal models and bench data.7,8 As such, patient characteristics associated with improved outcomes in AFG have rarely been explored. The ASPS Fat Graft Task Force3 recently reported that additional clinical studies were needed to identify potential risk factors to assist a surgeon in identifying patients best suited for AFG procedures.
Furthermore, there is limited knowledge on how various factors related to AFG correlate with both efficiency and safety. This is critical to both providers and payers because of the increasing demand for high-quality effective healthcare while lowering costs. In this cost- and safety-conscious environment, the operating room (OR) is often seen as the focus of change.9 The OR is one of the most expensive areas in an acute care hospital,10 and evidence suggests that despite their importance, ORs often exhibit considerable inefficiencies.11 Therefore, it is critical to understand the current level of AFG efficiency to determine how to increase it from both a time and cost perspective.
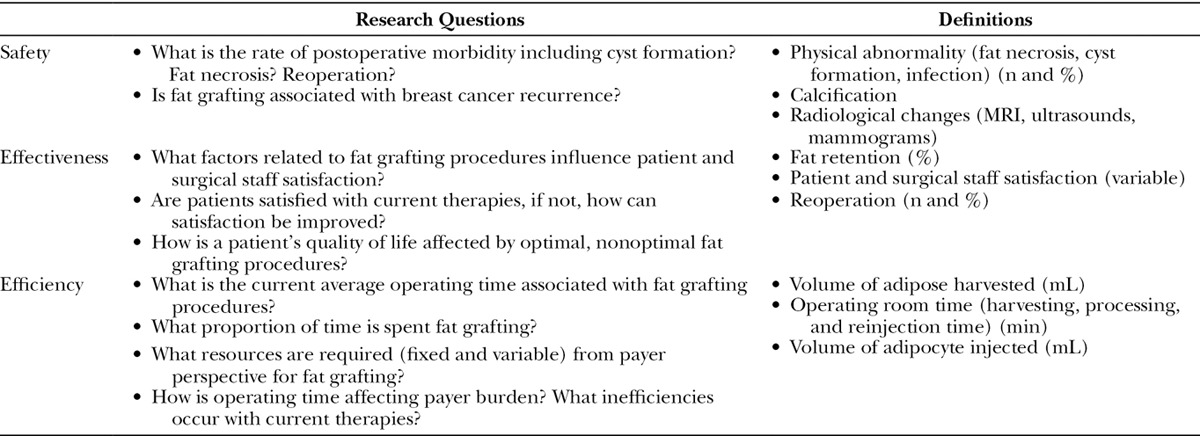
Because of the observed evidence gaps, our study focused on evaluating the real-world safety, effectiveness, and efficiency value proposition of AFG procedures. Table 1 summarizes the key outcomes and research questions of interest, which guided the inclusion of evidence for this review. Specifically, this literature review assessed the value of fat grafting in breast applications including cosmetic and reconstructive procedures using real-world clinical data, considering safety, effectiveness, and efficiency.
Table 1.
Research Questions and Definition of Outcomes of Interest
METHODS
Search Strategy
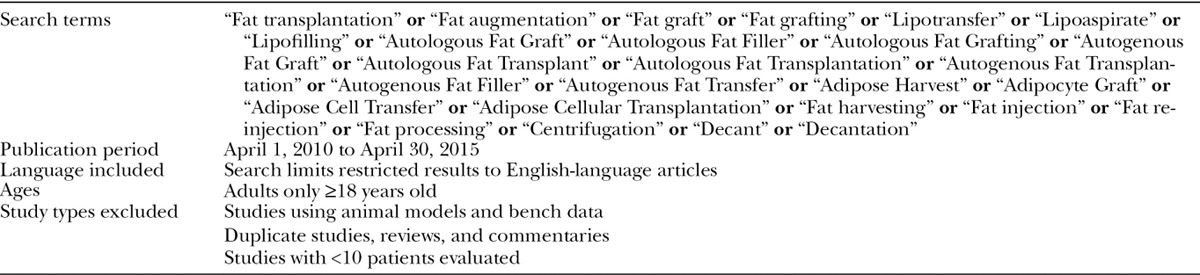
A comprehensive search of PubMed was performed using search terms combined with Boolean logical operators based on prior literature reviews.4,7,12 The main outcomes of interest were safety, effectiveness, and efficiency of AFG, and studies that reported data on these outcomes were included. Table 1 describes the components of each of the outcomes of interest. Search terms and criteria are described in Table 2. Given the dramatic increase in publications in the previous 5 years, the search focused on studies published during this time period (April 1, 2010–April 30, 2015).4
Table 2.
PubMed Fat Grafting Search Terms and Criteria
Data Extraction
An initial list of articles was identified via the search strategy. Titles and abstracts of studies were initially screened for eligibility according to the inclusion and exclusion criteria in Table 2. Full-text articles of all references that could potentially meet the eligibility criteria were reviewed during the second screening, which consisted of a cursory review of the article for outcomes of interest. Articles were retained if they contained any information related to our outcomes of interest—as defined in Table 1. The eligibility criteria were again applied to ensure that all articles included after the second screening met the inclusion and exclusion criteria. Data were extracted by 2 reviewers using predefined evidence templates, and a third reviewer reconciled any articles uncertain for inclusion.
Analysis
We attempted to extract patient characteristics, surgical characteristics, and outcomes of interest related to safety, effectiveness, and efficiency from each of the selected primary articles. For continuous data, means, medians, and ranges were extracted. Both the sample size (n) and percentages were extracted when available. For studies where the percentage was not reported but could be calculated, the prevalence was calculated using the outcome sample size and the total number of participants in the study. If there were multiple study arms, weighted means were calculated using the sample size of each study arm.
RESULTS
Our initial search yielded a total of 598 peer-reviewed articles. Figure 1 illustrates how the final 36 studies were selected. After the screening of titles and abstracts to determine eligibility, 176 articles remained for further evaluation. After the cursory screening of full-text articles, a total of 80 articles remained. After the final screening, which was an in-depth review of the full-text articles and excluded those without primary data, the final list of included unique articles was 65. This literature review only reports information from the 36 articles reporting data on breast applications. A subsequent report will report findings on the remaining articles focused on other AFG applications to the face, buttocks, and other body locations.
Fig. 1.
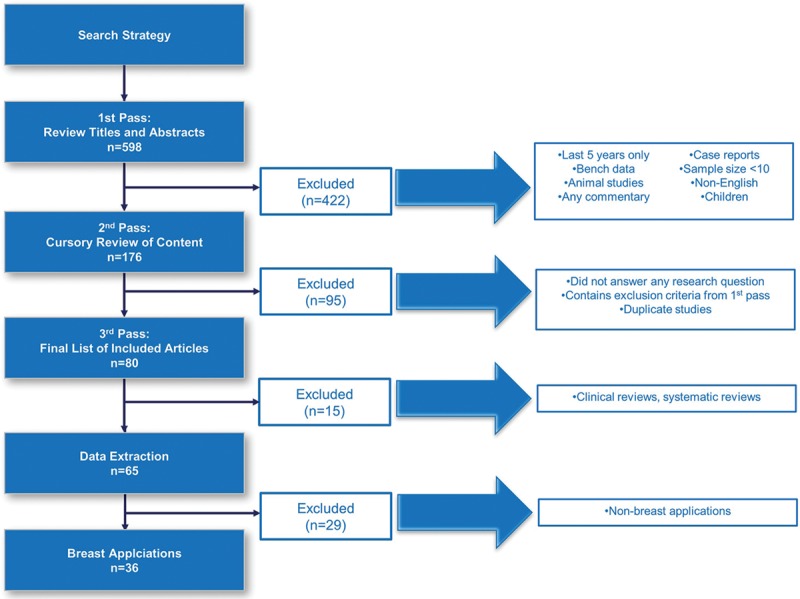
Illustration of how the final 36 studies were selected.
Study Characteristics
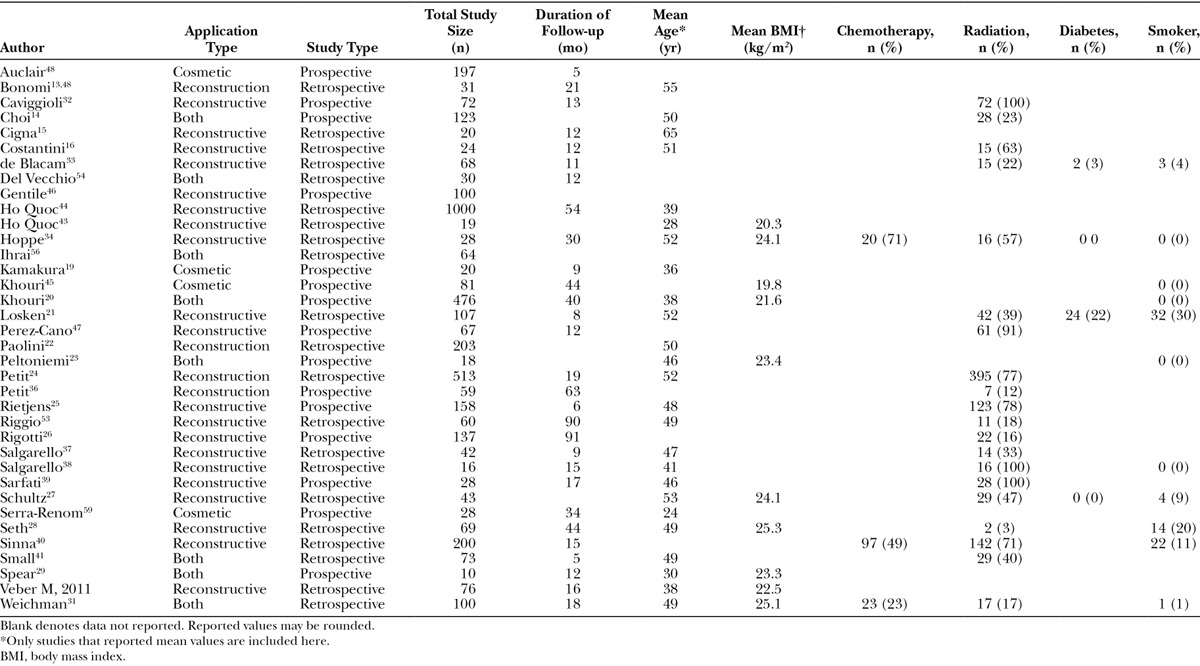
Table 3 provides a summary of study and patient characteristics of the included articles. There were 21 retrospective and 15 prospective studies and no randomized controlled trials. Fat grafting was utilized in a variety of applications; however, breast reconstruction after breast cancer was the most common application. This was followed by various aesthetic applications, such as breast augmentation or reconstruction of congenital deformities. There was little consistency in the follow-up time (mean across studies: 25.4 mo; range: 5–91); however, the most commonly reported follow-up time was 12 months. Additionally, there was a wide variability in sample sizes (mean across studies: 121.1; range: 18–1000) with a median sample size of 67.5 patients.
Table 3.
Study and Population Demographics and Characteristics of Fat Grafting in Breast Applications
Patient Characteristics
Although we attempted to collect data on all patient characteristics, limited data were available for extraction (Table 3). The most commonly reported patient characteristic was mean age (69.4% of studies), which was 45.5 years across studies.13–31 The second most commonly reported patient characteristic was the percent of the population previously treated with radiation. An average of 50% (range: 3–100%) of study participants had radiation before undergoing AFG.14,16,21,24–28,31–41 Of the 20 studies that reported proportion of patients who underwent radiation, 3 studies reported that all patients had prior radiation therapy.27,38,39,42 There were no observed differences in patient characteristics, surgical characteristics, or outcomes between studies that reported higher proportions of patients who received radiation compared to studies with lower rates of radiation treatment. All other variables were consistently reported in <50% of studies. In general, the average body mass index was within the normal range (mean across studies = 23; range: 19.8–25.3).20,23,27–31,43–45 Most of the patients included in the studies were not smokers; however, one study reported the proportion of smokers as high as 30%.21 Of the 4 studies that reported the proportion of patients in the study with diabetes, the range was 0% to 22%.21,27,33,34 Only 3 studies reported the proportion of patients who underwent chemotherapy before their AFG procedure (range: 23–71%).31,34,40
Safety
Of the 36 studies included in this review, 18 reported the prevalence of complications such as cyst formation, fat necrosis, and/or infection after AFG procedures (Table 4). Although the majority of studies reported physical abnormalities associated with AFG, the actual prevalence of complications was relatively low. Among the studies (n = 9) that reported cyst formation, the overall prevalence during follow-up was about 4.5% (40 of 881 patients).13,14,30,33,40,43,46–48 It is also important to note that there was a wide variation for how cyst formation was evaluated. Studies reported the use of breast radiological images including mammography, ultrasound, and magnetic resonance imaging (MRI).
Table 4.
Safety Outcomes of Fat Grafting in Breast Reconstruction
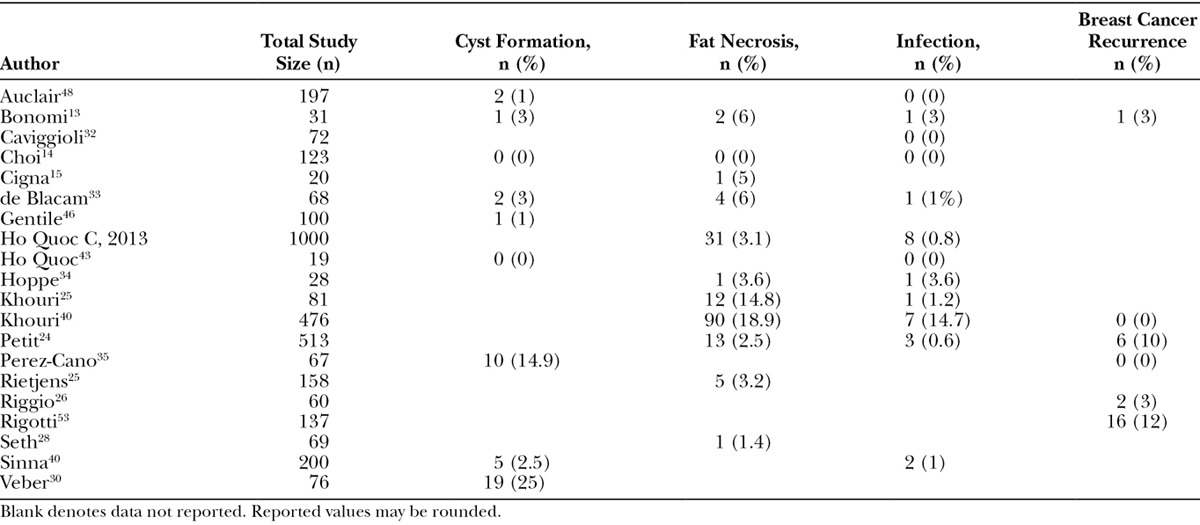
Fat necrosis was identified in 160 patients in 11 studies.14,20,24,28,33,34,44,45,49–51 This resulted in an overall prevalence of 6.2% (160 of 2567 patients). An increase in volume of fat injected was found to be associated with higher prevalence of fat necrosis. This association was not apparent with any other complication. The prevalence of infection was low (0.85%), with only 24 patients reported as having developed an infection after an AFG procedure.14,20,24,33,34,40,43–45,48,49,52 Other complications reported included granuloma (n = 1 patient),34 seroma (n = 1 patient),24 and pneumothorax (n = 2 patients).20,24 In addition, these low prevalence rates are consistent across the different fat processing techniques. Furthermore, we found relatively low prevalence rates of breast cancer recurrence. Among the 6 studies that reported breast cancer recurrence, follow-up times ranged from 12 to 91 months with a range of recurrence from 0% to 12%.13,20,26,35,36,53 There were no reported deaths.
Effectiveness
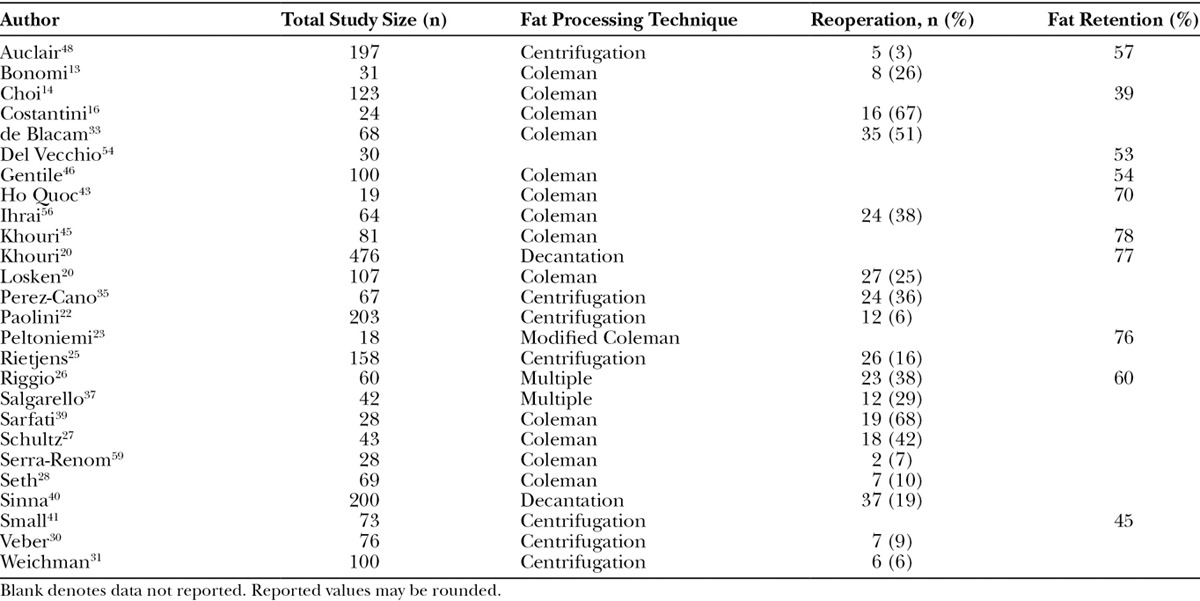
Objective characteristics highlighting the effectiveness of AFG (ie, mean fat retention and reoperation rates) were assessed (Table 5). Fat retention was reported in 10 articles (range: 39–77%), and there was consistency in the length of time when it was measured,14,20,23,25,26,41,43,45,46,48,54 which, in general, was after either 6 or 12 months. Fat retention was most commonly reported as an estimated volume over time as evaluated by various imaging techniques. Effectiveness was also evaluated in 2 studies using stem-cell enrichment and results were mixed, with a study by Gentile et al showing a meaningful difference of 69% versus 39% for those with and without stem-cell enrichment, respectively. Conversely, another study showed no differences with 74.2% and 78.8% with and without stem-cell enrichment, respectively.22,46 Eighteen studies reported reoperation rates. Reoperation was common with 7 studies reporting at least 30% of subjects having a subsequent AFG procedure (range: 3–68%).13,25–28,30,31,33,35,37,40,48,55–59
Table 5.
Objective Effectiveness Outcomes of Fat Grafting in Breast Reconstruction
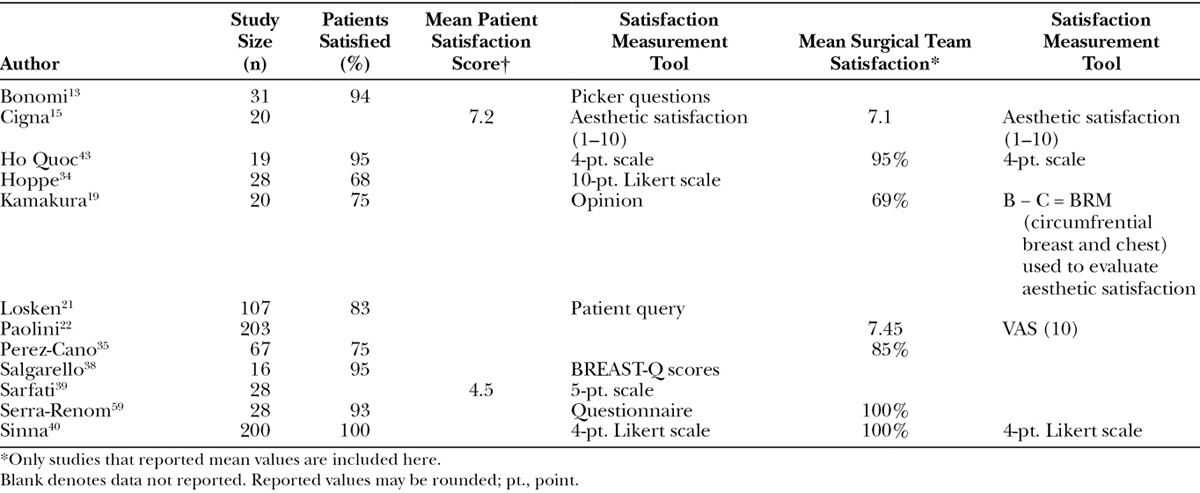
A total of 12 studies reported subjective characteristics highlighting the effectiveness of AFG (Table 6). Overall, both patients and surgeons reported high rates of satisfaction where data were reported.19,21,22,34,43,44,47,49,50,57–59 Although there was a considerable amount of variation in the tools used to evaluate satisfaction, the most commonly used was a simple 4-, 5-, or 10-point Likert scale (4 studies).34,40,43,58 Other studies used the Picker Patient Experience Questionnaire (1 study),13 Value of Aesthetic Satisfaction (1 study),50 the Visual Analog Scale (1 study),22 or the BREAST-Q (1 study),38 a patient-reported outcome (PRO) instrument.
Table 6.
Subjective Effectiveness Outcomes of Fat Grafting in Breast Reconstruction
Efficiency
As shown in Table 7, little data related to the efficiency of fat grafting procedures were identified. An attempt was made to collect data on the time associated with the 3 major steps of fat grafting (harvesting, processing, and reinjection). Because of the paucity of data, we were unable to extract data on fat processing time. Nine studies reported total OR time, and there was a large range reported (mean = 125 min; range: 40–210) across processing techniques.19,22,23,29,34,40,47,50,60 It is important to note that it was unclear if the OR time reported from each study consisted of the total OR time or only the time for AFG procedures. Also, the study that reported an OR time of only 40 minutes reported an average volume of fat injected of only 20 mL.60 When looking at AFG procedures that utilized an additional step, specifically stem-cell enrichment, the OR time was at the upper end, ranging from 192 to 240 minutes.19,22,47 Only one study reported the number of staff required for a fat grafting procedure.22 Specifically, the study found that compared with the simplified liposuction technique, additional surgical staff was needed for AFG procedures utilizing the Coleman technique.22
Table 7.
Efficiency Outcomes of Fat Grafting in Breast Reconstruction
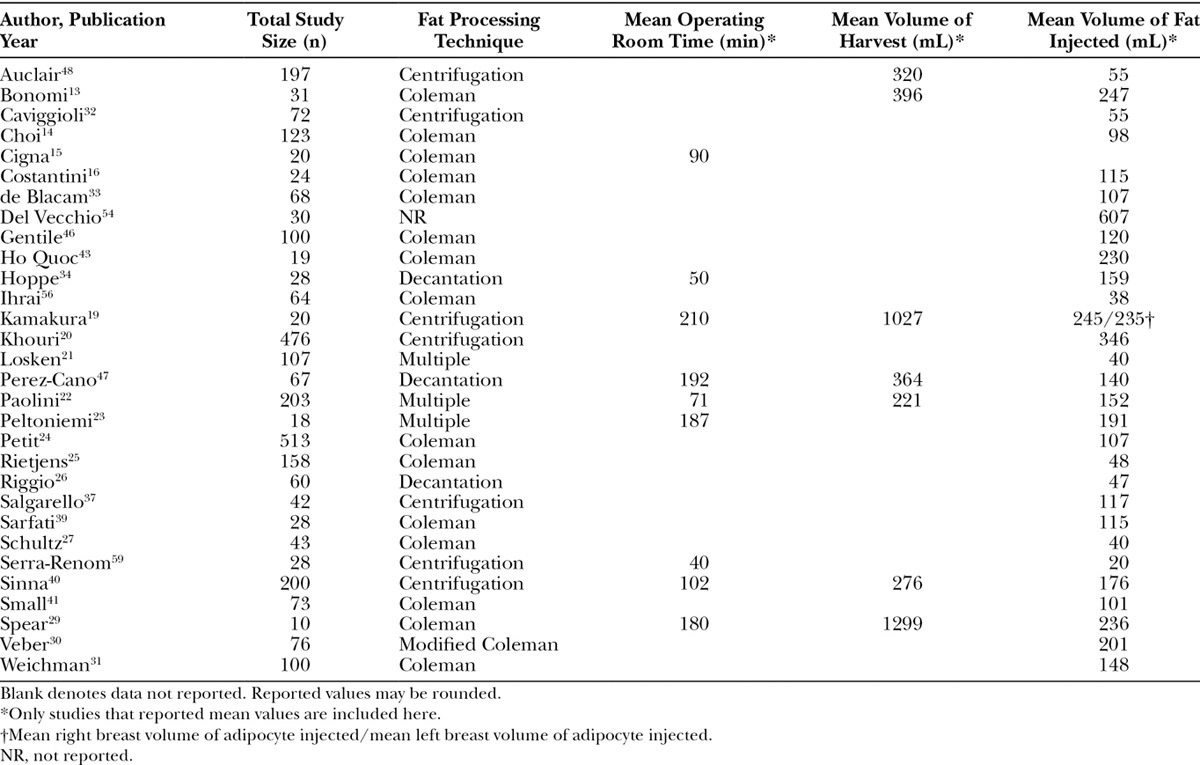
Although we did not find any studies that reported the mean volume of fat processed, 7 studies reported the mean volume of fat harvested (mean across studies = 558 mL; range: 120–1299).13,19,22,29,40,47,48 Most studies (35 of 36 studies) reported the mean volume of fat reinjected (mean across studies = 145 mL; range: 20–607). Both of these factors may be fair proxies for the volume of fat processed. There was a difference in the volume of fat reinjected between patients who had an AFG procedure done in conjunction with receiving breast implants and those who did not. Specifically, the mean volume of fat injected for implant-based fat grafting procedures was 137 mL. Conversely, the mean volume for pure AFG procedures to the breast was considerably higher at 285 mL. Overall, the majority (66.6%) of studies represented large volume procedures with injections >100 mL (Table 3).
As shown in Figure 2, there is a positive association between injection volume and OR times among the seven studies that reported both variables.19,22,23,29,34,35,40 Additionally, the amount of fat harvested and injected was highly variable across the different fat processing techniques, specifically centrifugation and decantation, and the data were lacking to assess differences in OR time by technique (Table 7).
Fig. 2.
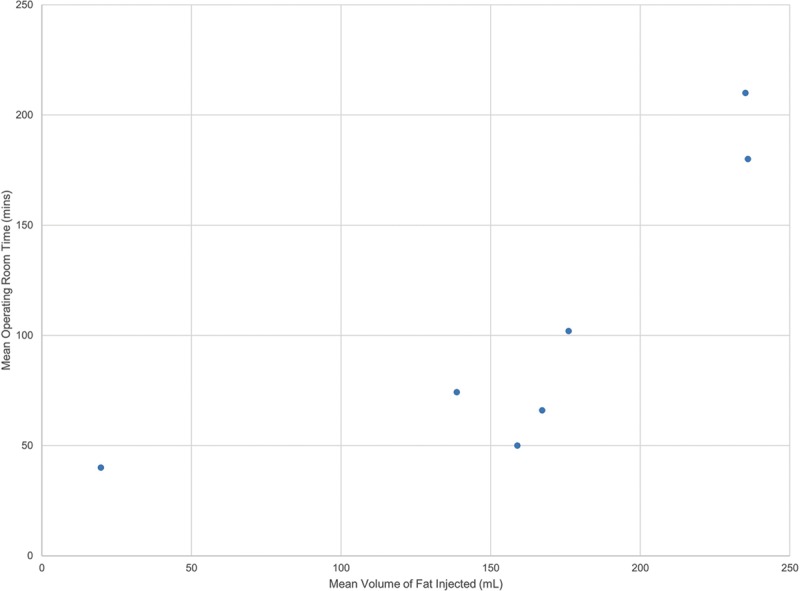
Association between mean volume of fat injected and mean operating room time.
DISCUSSION
Although limited data related to patient and surgical characteristics were available, this study included all information that was available in these articles, which suggests that the information presented is generalizable and representative as it relates to clinical practice. Results from this study confirm previous studies’ assertion that fat grafting is associated with relatively low complication rates regardless of processing technique. It also supports the assertion that fat grafting is associated with generally high levels of patient and clinical staff satisfaction. Reoperation and fat retention rates have been reported, but the data highlight the need for a more reproducible “gold standard” technique that could help improve outcomes while reducing overall costs. Although reported complication rates are low (prevalence of 5–6% for cyst formation and fat necrosis), the possibility to reduce those rates further and still enhance the efficacy of the procedure should help ameliorate concerns about cost and diagnostic issues for tumor detection. Our findings also indicate that the efficiency of AFG may be limited, with the majority of breast-related applications averaging over 100 mL injected per procedure and an average OR time of over 1.5 hours.
As the volume of fat injected increases, the operating time and operating costs increase. In terms of the injected volume and OR time, the majority of evidence published to date has been focused on centrifugation with some on decantation. In general, the calculated injection rates for centrifugation ranged from 0.5 to 2.5 mL/min,19,22,29,40,59 although a recent study using decantation had an injection rate of 3.2 mL/min.34 Methods to increase this injection rate, which may act as a data proxy for fat processing speed, have the potential to reduce OR time and consequently reduce procedure-related in-hospital costs. Two studies recently published after the end date (April 30, 2015) of the publication search period evaluated the efficiency of a new fat processing system. Brzezienski and Jarrell retrospectively evaluated 37 breast reconstruction patients using either the REVOLVE system (n = 24; LifeCell Corporation, Bridgewater, N.J.) or the Coleman Centrifugation technique (n = 13).61 The resulting injection rates were significantly different at 4.69 mL/min (range: 1.9–10) and 1.77 mL/min (range: 0.84–2.57) for the REVOLVE system and centrifugation, respectively (P < 0.0001). In addition, a second larger retrospective review of patients who underwent AFG to the breast was also conducted.62 The mean volume of fat harvested and injected were significantly higher in the REVOLVE group (n = 103 patients; P < 0.0001), and the time to complete fat grafting was significantly shorter (30 vs 85 min; P < 0.0001) when compared with the centrifugation group (n = 118 patients). Given current fat processing techniques have low fixed costs, the primary factor to reduce OR cost relates to the variable time required for fat processing. Some of the newly developed systems have a higher fixed cost, but this can be offset by their potential reduction in processing time, suggesting that use of these systems has the potential to contribute to more efficient use of OR resources.
Gaps in the evidence reviewed were present, limiting the ability to aggregate and quantify the results to serve as a basis for evidence-based recommendations. The primary limitation was the quality of evidence. Although some large prospective observational studies were found, there was a lack of randomized control trials or clinical studies with a control arm (ie, without AFG). There was also a lack of rigor with the majority of studies being retrospective chart reviews. Future evidence must attempt to validate the effectiveness of AFG seen in the real world through controlled studies assessing the clinical and humanistic benefits of AFG, especially as newer technologies and techniques evolve the treatment landscape.
Limited data available on effectiveness outcomes with regards to reoperation rates, targeted breast size, and the variability in follow-up time further enhance the large heterogeneity in the evidence. The recent launch of the General Registry of Autologous Fat Transfer (GRAFT) registry by the Plastic Surgery Foundation is attempting to help improve data consistency and robustness in the United States through a national registry. Although a meta-analysis was not completed because of the heterogeneous methodology and patient populations among studies included, future evaluations of the GRAFT registry may help overcome these hurdles. Other evidence gaps may be addressed through the GRAFT registry including evaluating the benefits of stem-cell enrichment and assessing outcomes using a single standardized and validated PRO instrument, the BREAST-Q.
Although efficiency outcomes from this review were the most significant findings, many potential variables of interest were lacking from the published literature. There was uncertainty surrounding the definitions of OR time, a lack of reported fat processing times, and limited collection of data on operative resource use including OR equipment and staff. Further research is needed to assess the association between injection volume and OR time and the effect of fat processing time directly on OR time.
CONCLUSIONS
This literature review attempted to evaluate the real-world value—safety, effectiveness, and efficiency—of AFG procedures to the breast. The findings as it relates to safety and effectiveness are consistent and validate the previous and recent research published. The findings also provide a foundation for the current efficiency of AFG such that newer techniques and systems may have a basis for assessing their value.
The evidence as it stands is limited and of low quality. The results from a recently published systematic review suggest that “despite some differences in harvest and implantation technique in the laboratory, these findings have not translated into a universal protocol for fat grafting. Furthermore, no Level I or Level II data exist to warrant a consensus recommendation for clinical practice. Therefore, additional human studies are necessary….”63 This study supports these findings and also suggests existing uncertainty surrounding fat grafting, such as the benefits of stem-cell enrichment on fat retention, the value of fat processing systems, and long-term patient satisfaction using standardized PRO instruments.
Of the available data, there is a need for standardization of data collection and reporting on key variables and outcomes to allow for the ability to make evidence-based recommendations from the literature. Specifically, there is a need for future studies to use validated measurements including follow-up at 1 year, study admission criteria regarding relevant patient characteristics, such as body mass index and diabetes, and standardized testing for complications, such as MRI, mammography, or ultrasound. Fat retention should be measured by volume increase or percent retention. Lastly, when determining efficiency, time should be reported as a function of cc/min for harvesting, processing, and injection.
With recent advances in the field of breast reconstruction, it is likely that AFG will become more widely used either alone or in conjunction with implants and/or meshes such as acellular matrices. As these advances in clinical practice occur, it is paramount that more standardized, reproducible, and efficient procedures are developed in the field of AFG to provide maximum cost and clinical benefits to healthcare providers, payers, and patients.
ACKNOWLEDGMENTS
We thank Lauren Lee, PharmD, for initial comments and guidance in the design and protocol development as well as Arrash Darban and Brian Duffant for their assistance in data abstraction.
Footnotes
Disclosure: Braden Leung, Mousam Parekh, and David Macarios are employees of Acelity. Courtney Coles and Matthew Gitlin received funding to conduct research. Scott Spear is a consultant to LifeCell/Acelity Corporation, Allergan, Establishment labs, Novadaq, and Endurance labs. The Article Processing Charge was paid for by Acelity.
REFERENCES
- 1.Tan Q, Tian C, Chen J, et al. Autologous fat grafting for breast reconstruction. The Cochrane Library. 2013 [Google Scholar]
- 2.ASPRS. Report on autologous fat transplantation. Plast Surg Nurs. 1987;7(4):140–1. [PubMed] [Google Scholar]
- 3.Gutowski KA ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;124:272–280. doi: 10.1097/PRS.0b013e3181a09506. [DOI] [PubMed] [Google Scholar]
- 4.Agha RA, Fowler AJ, Herlin C, et al. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J Plast Reconstr Aesthet Surg. 2015;68:143–161. doi: 10.1016/j.bjps.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Claro F, Jr, Figueiredo JC, Zampar AG, et al. Applicability and safety of autologous fat for reconstruction of the breast. Br J Surg. 2012;99:768–780. doi: 10.1002/bjs.8722. [DOI] [PubMed] [Google Scholar]
- 6.Leopardi D, Thavaneswaran P, Mutimer KL, et al. Autologous fat transfer for breast augmentation: a systematic review. ANZ J Surg. 2014;84:225–230. doi: 10.1111/ans.12202. [DOI] [PubMed] [Google Scholar]
- 7.Gir P, Brown SA, Oni G, et al. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–258. doi: 10.1097/PRS.0b013e318254b4d3. [DOI] [PubMed] [Google Scholar]
- 8.Strong AL, Cederna PS, Rubin JP, et al. The current state of fat grafting: a review of harvesting, processing, and injection techniques. Plast Reconstr Surg. 2015;136:897–912. doi: 10.1097/PRS.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanich DG, Byrd JR. How to increase efficiency in the operating room. Surg Clin North Am. 1996;76:161–173. doi: 10.1016/s0039-6109(05)70429-1. [DOI] [PubMed] [Google Scholar]
- 10.Wright JG, Roche A, Khoury AE. Improving on-time surgical starts in an operating room. Can J Surg. 2010;53:167–170. [PMC free article] [PubMed] [Google Scholar]
- 11.Malangoni MA. Assessing operating room efficiency and parallel processing. Ann Surg. 2006;243:15–16. doi: 10.1097/01.sla.0000193601.57597.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saint-Cyr M, Rojas K, Colohan S, et al. The role of fat grafting in reconstructive and cosmetic breast surgery: a review of the literature. J Reconstr Microsurg. 2012;28:99–110. doi: 10.1055/s-0031-1287675. [DOI] [PubMed] [Google Scholar]
- 13.Bonomi R, Betal D, Rapisarda IF, et al. Role of lipomodelling in improving aesthetic outcomes in patients undergoing immediate and delayed reconstructive breast surgery. Eur J Surg Oncol. 2013;39:1039–1045. doi: 10.1016/j.ejso.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Choi M, Small K, Levovitz C, et al. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 15.Cigna E, Ribuffo D, Sorvillo V, et al. Secondary lipofilling after breast reconstruction with implants. Eur Rev Med Pharmacol Sci. 2012;16:1729–1734. [PubMed] [Google Scholar]
- 16.Costantini M, Cipriani A, Belli P, et al. Radiological findings in mammary autologous fat injections: a multi-technique evaluation. Clin Radiol. 2013;68:27–33. doi: 10.1016/j.crad.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Ho Quoc C, Delay E. [How to treat fat necrosis after lipofilling into the breast?]. Ann Chir Plast Esthet. 2015;60:179–183. doi: 10.1016/j.anplas.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Ho Quoc C, Fakiha M, Meruta A, et al. Breast lipofilling: a new treatment of Becker nevus syndrome. Ann Chir Plast Esthet. 2015;60:336–339. doi: 10.1016/j.anplas.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Kamakura T, Ito K. Autologous cell-enriched fat grafting for breast augmentation. Aesthetic Plast Surg. 2011;35:1022–1030. doi: 10.1007/s00266-011-9727-7. [DOI] [PubMed] [Google Scholar]
- 20.Khouri RK, Khouri RK, Jr, Rigotti G, et al. Aesthetic applications of Brava-assisted megavolume fat grafting to the breasts: a 9-year, 476-patient, multicenter experience. Plast Reconstr Surg. 2014;133:796–807. doi: 10.1097/PRS.0000000000000053. discussion 808. [DOI] [PubMed] [Google Scholar]
- 21.Losken A, Pinell XA, Sikoro K, et al. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg. 2011;66:518–522. doi: 10.1097/SAP.0b013e3181fe9334. [DOI] [PubMed] [Google Scholar]
- 22.Paolini G, Amoroso M, Longo B, et al. Simplified lipostructure: a technical note. Aesthetic Plast Surg. 2014;38:78–82. doi: 10.1007/s00266-013-0254-6. [DOI] [PubMed] [Google Scholar]
- 23.Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66:1494–1503. doi: 10.1016/j.bjps.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Petit JY, Lohsiriwat V, Clough KB, et al. The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: a multicenter study–Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg. 2011;128:341–346. doi: 10.1097/PRS.0b013e31821e713c. [DOI] [PubMed] [Google Scholar]
- 25.Rietjens M, De Lorenzi F, Rossetto F, et al. Safety of fat grafting in secondary breast reconstruction after cancer. J Plast Reconstr Aesthet Surg. 2011;64:477–483. doi: 10.1016/j.bjps.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Riggio E, Bordoni D, Nava MB. Oncologic surveillance of breast cancer patients after lipofilling. Aesthetic Plast Surg. 2013;37:728–735. doi: 10.1007/s00266-013-0166-5. [DOI] [PubMed] [Google Scholar]
- 27.Schultz I, Lindegren A, Wickman M. Improved shape and consistency after lipofilling of the breast: patients’ evaluation of the outcome. J Plast Surg Hand Surg. 2012;46:85–90. doi: 10.3109/2000656X.2011.653256. [DOI] [PubMed] [Google Scholar]
- 28.Seth AK, Hirsch EM, Kim JY, et al. Long-term outcomes following fat grafting in prosthetic breast reconstruction: a comparative analysis. Plast Reconstr Surg. 2012;130:984–990. doi: 10.1097/PRS.0b013e318267d34d. [DOI] [PubMed] [Google Scholar]
- 29.Spear SL, Pittman T. A prospective study on lipoaugmentation of the breast. Aesthet Surg J. 2014;34:400–408. doi: 10.1177/1090820X13520449. [DOI] [PubMed] [Google Scholar]
- 30.Veber M, Tourasse C, Toussoun G, et al. Radiographic findings after breast augmentation by autologous fat transfer. Plast Reconstr Surg. 2011;127:1289–1299. doi: 10.1097/PRS.0b013e318205f38f. [DOI] [PubMed] [Google Scholar]
- 31.Weichman KE, Broer PN, Tanna N, et al. The role of autologous fat grafting in secondary microsurgical breast reconstruction. Ann Plast Surg. 2013;71:24–30. doi: 10.1097/SAP.0b013e3182920ad0. [DOI] [PubMed] [Google Scholar]
- 32.Caviggioli F, Maione L, Forcellini D, et al. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg. 2011;128:349–352. doi: 10.1097/PRS.0b013e31821e70e7. [DOI] [PubMed] [Google Scholar]
- 33.de Blacam C, Momoh AO, Colakoglu S, et al. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg. 2011;128:411e–418e. doi: 10.1097/PRS.0b013e31822b669f. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe DL, Ueberreiter K, Surlemont Y, et al. Breast reconstruction de novo by water-jet assisted autologous fat grafting–a retrospective study. Ger Med Sci. 2013;11:Doc17. doi: 10.3205/000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 36.Petit JY, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol. 2013;24:1479–1484. doi: 10.1093/annonc/mds660. [DOI] [PubMed] [Google Scholar]
- 37.Salgarello M, Visconti G, Rusciani A. Breast fat grafting with platelet-rich plasma: a comparative clinical study and current state of the art. Plast Reconstr Surg. 2011;127:2176–2185. doi: 10.1097/PRS.0b013e3182139fe7. [DOI] [PubMed] [Google Scholar]
- 38.Salgarello M, Visconti G, Barone-Adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg. 2012;129:317–329. doi: 10.1097/PRS.0b013e31822b6619. [DOI] [PubMed] [Google Scholar]
- 39.Sarfati I, Ihrai T, Kaufman G, et al. Adipose-tissue grafting to the post-mastectomy irradiated chest wall: preparing the ground for implant reconstruction. J Plast Reconstr Aesthet Surg. 2011;64:1161–1166. doi: 10.1016/j.bjps.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Sinna R, Delay E, Garson S, et al. Breast fat grafting (lipomodelling) after extended latissimus dorsi flap breast reconstruction: a preliminary report of 200 consecutive cases. J Plast Reconstr Aesthet Surg. 2010;63:1769–1777. doi: 10.1016/j.bjps.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Small K, Choi M, Petruolo O, et al. Is there an ideal donor site of fat for secondary breast reconstruction? Aesthet Surg J. 2014;34:545–550. doi: 10.1177/1090820X14526751. [DOI] [PubMed] [Google Scholar]
- 42.Caviggioli F, Vinci V, Maione L, et al. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg. 2013;70:119. doi: 10.1097/SAP.0b013e31823cd7b8. [DOI] [PubMed] [Google Scholar]
- 43.Quoc CH, Delaporte T, Meruta A, et al. Breast asymmetry and pectus excavatum improvement with fat grafting. Aesthet Surg J. 2013;33(6):822–829. doi: 10.1177/1090820X13493907. [DOI] [PubMed] [Google Scholar]
- 44.Quoc CH, Sinna R, Gourari A, et al. Percutaneous fasciotomies and fat grafting. Aesthet Surg J. 2013;33(7):995–1001. doi: 10.1177/1090820X13501511. [DOI] [PubMed] [Google Scholar]
- 45.Khouri RK, Eisenmann-Klein M, Cardoso E, et al. Brava and autologous fat transfer is a safe and effective breast augmentation alternative: results of a 6-year, 81-patient, prospective multicenter study. Plast Reconstr Surg. 2012;129:1173–1187. doi: 10.1097/PRS.0b013e31824a2db6. [DOI] [PubMed] [Google Scholar]
- 46.Gentile P, Di Pasquali C, Bocchini I, et al. Breast reconstruction with autologous fat graft mixed with platelet-rich plasma. Surg Innov. 2013;20:370–376. doi: 10.1177/1553350612458544. [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 48.Auclair E, Blondeel P, Del Vecchio DA. Composite breast augmentation: soft-tissue planning using implants and fat. Plast Reconstr Surg. 2013;132:558–568. doi: 10.1097/PRS.0b013e31829ad2fa. [DOI] [PubMed] [Google Scholar]
- 49.Bonomi R, Betal D, Rapisarda IF, et al. Role of lipomodelling in improving aesthetic outcomes in patients undergoing immediate and delayed reconstructive breast surgery. Eur J Surg Oncol. 2013;39:1039–1045. doi: 10.1016/j.ejso.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Cigna E, Ribuffo D, Sorvillo V, et al. Secondary lipofilling after breast reconstruction with implants. Eur Rev Med Pharmacol Sci. 2012;16:1729–1734. [PubMed] [Google Scholar]
- 51.Rietjens M, De Lorenzi F, Rossetto F, et al. Safety of fat grafting in secondary breast reconstruction after cancer. J Plast Reconstr Aesthet Surg. 2011;64:477–483. doi: 10.1016/j.bjps.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Caviggioli F, Vinci V, Codolini L. Autologous fat grafting: an innovative solution for the treatment of post-mastectomy pain syndrome. Breast Cancer. 2013;20:281–282. doi: 10.1007/s12282-013-0468-0. [DOI] [PubMed] [Google Scholar]
- 53.Rigotti G, Marchi A, Stringhini P, et al. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg. 2010;34:475–480. doi: 10.1007/s00266-010-9481-2. [DOI] [PubMed] [Google Scholar]
- 54.Del Vecchio DA, Del Vecchio SJ. The graft-to-capacity ratio: volumetric planning in large-volume fat transplantation. Plast Reconstr Surg. 2014;133:561–569. doi: 10.1097/01.prs.0000438471.23249.6e. [DOI] [PubMed] [Google Scholar]
- 55.Constantine RS, Harrison B, Davis KE, et al. Fat graft viability in the subcutaneous plane versus the local fat pad. Plast Reconstr Surg Glob Open. 2014;2:e260. doi: 10.1097/GOX.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ihrai T, Georgiou C, Machiavello JC, et al. Autologous fat grafting and breast cancer recurrences: retrospective analysis of a series of 100 procedures in 64 patients. J Plast Surg Hand Surg. 2013;47:273–275. doi: 10.3109/2000656X.2012.759583. [DOI] [PubMed] [Google Scholar]
- 57.Losken A, Pinell-White X, Hodges M, et al. Evaluating outcomes after correction of the breast conservation therapy deformity. Ann Plast Surg. 2015;74:S209–S213. doi: 10.1097/SAP.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 58.Sarfati I, Ihrai T, Kaufman G, et al. Adipose-tissue grafting to the post-mastectomy irradiated chest wall: preparing the ground for implant reconstruction. J Plast Reconstr Aesthet Surg. 2011;64:1161–1166. doi: 10.1016/j.bjps.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Serra-Renom JM, Muñoz-Olmo J, Serra-Mestre JM. Treatment of grade 3 tuberous breasts with Puckett’s technique (modified) and fat grafting to correct the constricting ring. Aesthetic Plast Surg. 2011;35:773–781. doi: 10.1007/s00266-011-9686-z. [DOI] [PubMed] [Google Scholar]
- 60.Serra-Renom JM, Serra-Mestre JM. Periorbital rejuvenation to improve the negative vector with blepharoplasty and fat grafting in the malar area. Ophthal Plast Reconstr Surg. 2011;27:442–446. doi: 10.1097/IOP.0b013e318224b0d5. [DOI] [PubMed] [Google Scholar]
- 61.Brzezienski AL, Jarrell JA. Autologous fat grafting to the breast using REVOLVE system to reduce clinical costs. Ann Plast Surg. 2015 doi: 10.1097/SAP.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 62.Gabriel A, Champaneria MC, Patrick Maxwell G. Fat grafting and breast reconstruction: tips for ensuring predictability. Gland Surg. 2015;4(3):232–243. doi: 10.3978/j.issn.2227-684X.2015.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strong AL, Bowles AC, MacCrimmon CP, et al. Adipose stromal cells repair pressure ulcers in both young and elderly mice: potential role of adipogenesis in skin repair. Stem Cells Transl Med. 2015;4:632–642. doi: 10.5966/sctm.2014-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]


