Abstract
Background:
Botulinum (neuro)toxin A (BoNT) is widely used in the field of plastic and reconstructive surgery. Among treatment of pain, hyperhidrosis, or aesthetic purposes, it is also used to enhance wound healing and prevent excessive scar formation. Some clinical data already exist, but only little is known on a cellular level. The aim of this study was to evaluate the effect of BoNT on cells essential for wound healing in vitro. Therefore, primary human keratinocytes and endothelial cells were treated with different concentrations of BoNT and tested on proliferation, migration, and angiogenic behavior.
Methods:
BoNT was exposed to human keratinocytes and endothelial cells in a low (1 IU/mL), medium (10 IU/mL), and high (20 IU/mL) concentrations in cell culture. Proliferation and migration of the 2 cell types were observed and also the angiogenic potential of endothelial cells in vitro.
Results:
BoNT 20 IU/mL negatively influenced proliferation and migration of keratinocytes but not those of endothelial cells. Angiogenesis in vitro was less effective with the highest BoNT concentrations tested. Low concentrations of BoNT supported sprouting of endothelial cells.
Conclusions:
High concentrations of botulinum toxin interfered with wound closure as keratinocytes’ proliferation and migration were deteriorated. Furthermore, BoNT concentrations of 20 IU/mL constrain in vitro vessel formation but do not influence proliferation or migration of endothelial cells.
Some of the most important cells in wound healing are keratinocytes, responsible for reepithelialization, and endothelial cells, which manage angiogenesis: to close the disrupted epidermal layer, keratinocytes migrate from the wound edge across the wound bed.1 On large-scale burn wounds, coverage with keratinocytes is often achieved by split skin grafts, keratinocyte sheets, or application of keratinocytes with scaffold-like fibrin sealant spray.2 Endothelial cells invade the granulation tissue, form new vessels, and thereby supply the newly formed extracellular matrix with oxygen and nutrients.
Botulinum (neuro)toxin A(BoNT) is a neurotoxin produced by the bacterium Clostridium botulinum, which induces chemodenervation of muscles by preventing the release of neurotransmitters such as acetylcholine and noradrenaline.3 It was primarily found in adulterated meat products by causing botulism. Nowadays, BoNT is commonly used in medical applications to treat chronic myofascial pain,4 headache,5 urinary incontinence,6 and hyperhidrosis7 or in cosmetic applications8,9 among others.
Recently, the effect of botulinum toxin on mature adipocytes, and also adipose-derived stem cells, and fibroblasts was evaluated in vitro.10 Botulinum toxin was shown to have no negative effect on adipose-derived stem cells, mature adipocytes, or fibroblasts. BoNT was also used as an adjuvant in autologous fat transfer in vivo where a higher fat graft survival could be achieved.11 Also, good results in wound healing were reported as BoNT had an effect on the tension in the wound edges with deleterious effects.12 A variety of predominantly clinical studies examined the impact of BoNT on wound healing, and most of them concluded that an early injection of botulinum toxin type A seems to enhance healing of facial wounds.13–15
Therefore, we wanted to evaluate the effect of botulinum toxin type A (BoNT) on wound healing on a cellular level. We tested the proliferation and migration of human keratinocytes and endothelial cells and the angiogenic behavior of endothelial cells in vitro.
MATERIALS AND METHODS
Cultivation of Human Umbilical Vein Endothelial Cells and Keratinocytes
Human umbilical vein endothelial cells (HUVECs) were kindly provided by the Cardiac Surgery Research Laboratories (Dr. Barbara Messner, Medical University of Vienna, Vienna, Austria). Umbilical cords were kindly donated by the Department of Gynecology, Medical University of Vienna, and isolation of cells is approved by the local ethics committee (EK number: 1280/2015). All donors gave written informed consent prior inclusion. Briefly, the umbilical cord vein was washed using sterile phosphate-buffered saline (PBS) to remove blood residues and afterward perfused with collagenase (type IV, 1 mg/mL) for 20 minutes at room temperature. After rinsing out the collagenase–endothelial cell suspension with sterile prewarmed RPMI medium (Lonza; Basel, Switzerland), HUVECs were collected by centrifugation. Cells were cultured on gelatin-coated (0.2% gelatin) cell culture flasks and using specialized endothelial cell culture medium (endothelial growth medium 2 [EGM-2], Lonza). Purity of cells reached at least 95% (checked by staining with anti-CD31 antibody and flow cytometry analyses) and was used for experiments within passages 1 and 5.
Keratinocytes (Evercyte GmbH; Vienna, Austria) were cultured in DermaLife K Complete Medium (CellSystems; Troisdorf, Germany). Cells between passages 3 and 8 were used for experiments.
Treatment of Cells with BoNT
Cells were seeded into multiwell plates, depending on the cell assay. The medium was removed, and cells were incubated with 1-, 10-, and 20-IU/mL onabotulinumtoxin A (Botox, Allergan Inc.; Irvine, Calif.), respectively. To obtain different concentrations, a stock solution (200 IU/mL) was diluted with the corresponding proliferation medium. As control, cells were incubated with medium only.
Proliferation
Keratinocytes and HUVECs were seeded in 96-well plates (8 × 104 cells per well). After 24 hours of cultivation, medium was changed to BoNT-containing proliferation medium. As a control, normal proliferation medium without BoNT was added to the cells. The cell number on days 1, 2, and 3 was evaluated using a CellTiter96 nonradioactive proliferation assay (MTT-Assay, Promega; Madison, Wis.) according to the manufacturer’s protocol, and absorbance was measured on a Wallac 1420 VICTOR2 plate reader (PerkinElmer; Waltham, Mass.).
Migration: In Vitro Scratch Assay
Cells were cultured in 12-well plates until they reached 100% confluence and were then scratched with a 1-mL plastic micropipette tip. Cells were rinsed with PBS to remove detached cells and incubated with BoNT-containing proliferation medium. Control cells were cultivated in proliferation medium without BoNT. Cells were analyzed using an inverted Nikon Eclipse Ti-E microscope (Nikon; Tokyo, Japan). Wound area was measured with the NIS-Elements AR 3.0 program (Nikon; Tokyo, Japan) directly after scratching and after 24, 48, 72, and 118 hours (until wound closure).
Angiogenesis: In Vitro Tube-Forming Assay
Matrigel (basement membrane matrix, growth factor reduced, BD Biosciences; Bedford, Mass.) was filled into µ-slide angiogenesis chambers (ibidi; Martinsried, Germany) and was polymerized for 30 minutes at 37°C. HUVECs were seeded onto the Matrigel and were incubated with different concentrations of BoNT mixed with the EGM-2 (Lonza; Basel, Switzerland) containing vascular endothelial growth factor (VEGF). After 6 hours of incubation, the cells were photographed with an inverted Nikon Eclipse Ti-E microscope (Nikon; Tokyo, Japan), and the number of tubes, the total tube length, the number of loops and branching points, and the covered area in percent were calculated by Wimasis (Wimasis GmbH; Munich, Germany).
Angiogenesis: Spheroid-based In Vitro Sprouting Assay
Cell spheroids were generated as described by Korff et al.16 For the sprouting assay, spheroids were embedded into collagen gels as described in Testori et al.17 In short, cells were grown overnight in spheroids in hanging drops containing 0.25% methylcellulose. Approximately 450 cells formed a spheroid, which was mixed with rat collagen and 80% of Methocel/20% of fetal calf serum. The hydrogels with the spheroids were incubated with different concentrations of BoNT. Endothelial basal medium 2 (Lonza; Basel, Switzerland) without supplements was used as negative control, and medium containing 30-ng/mL VEGF was used as positive control. After 20 hours of incubation, the cumulative sprout length for each spheroid was calculated with the cellSens Entry 1.8 program (Olympus Tokyo, Japan).
Real-time Polymerase Chain Reaction Analysis
HUVECs were seeded into 6-well plates and incubated with different concentrations of BoNT mixed with the EGM-2. Cells with proliferation medium without BoNT were used as control. After 6 hours of incubation, total RNA was prepared by homogenizing cells in lysis buffer containing 0.1% 2-mercaptoethanol followed by RNA extraction according to a standard protocol (RNeasy Mini Kit, Qiagen GmbH; Hilden, Germany). RNA was transcribed into cDNA by QuantiTect Reverse Transcription Kit (Qiagen). Quantitative real-time polymerase chain reaction (qPCR) was performed using the KAPA SYBR FAST qPCR Kit (peqlab, VWR; Darmstadt, Germany) with primers for VEGF-A, angiopoietin-2 (ANGPT2), and matrix metalloproteinase 9 (MMP9) normalized to glyceraldehyde 3-phosphate dehydrogenase expression as endogenous control (all Microsynth; Balgach, Switzerland). Expression of specific mRNA in each sample was quantitated in duplicates on an ABI PRISM 7000 Cycler (Applied Biosystems, Foster City, Calif.).
Statistic
Statistical analysis was performed with the GraphPad Prism statistic software (GraphPad Software, Inc.; La Jolla, Calif.). Data are presented as mean ± SEM of at least 3 independent experiments. Statistical comparisons were based on paired t test and 1-way analysis of variance followed by Dunnett’s multiple comparison test with P < 0.05 considered as significant.
RESULTS
High Concentrations of BoNT Have a Negative Effect on Keratinocytes’ Proliferation and Migration
Proliferation of keratinocytes and HUVECs was observed by measuring the cell number after 24, 48, and 72 hours of incubation with BoNT. Proliferation of keratinocytes was decelerated when incubated with 20 IU/mL BoNT. This effect was significant after 24 and 48 hours but was compensated after 72 hours of cell proliferation (Fig. 1A). In contrast, endothelial cells showed no significant differences in cell proliferation over 3 days (Fig. 1B).
Fig. 1.
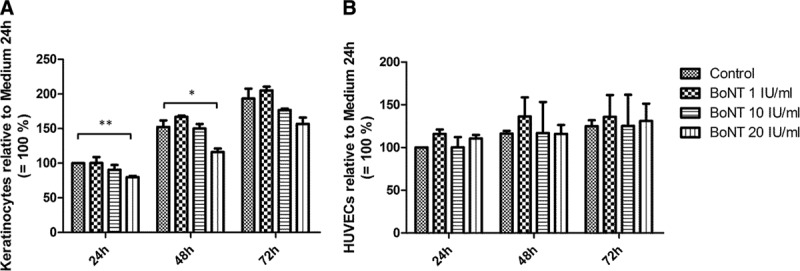
Proliferation of keratinocytes (A) and HUVEC (B) exposed to BoNT after 24, 48, and 72 h. Cell numbers were normalized and related to control (24 h = 100%); n = 3, *P < 0.05 and **P < 0.01.
In an in vitro scratch assay, high BoNT concentrations of 20 IU/mL had a negative effect on keratinocytes’ migration as the scratch is closed significantly slower compared with the medium control (Fig. 2A). No significant effect could be observed with HUVECs (Fig. 2B).
Fig. 2.
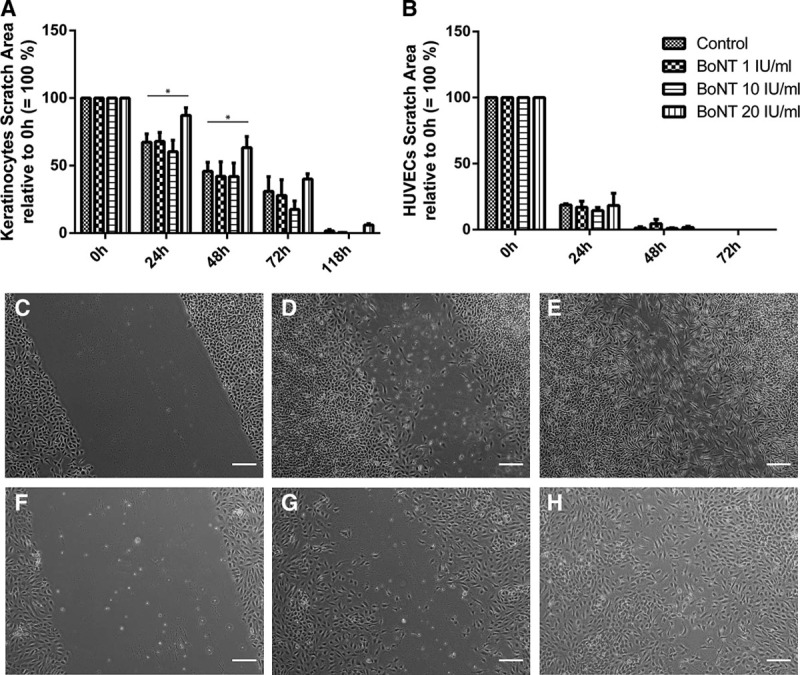
In vitro wound healing assay of keratinocytes (A and C–E) and HUVECs (B and F–H) exposed to BoNT for 0 h (C and F), 24 h (G), 48 h (D and H), and 72 h (E). Data were normalized and related to baseline (0 h = 100%); n = 3, *P < 0.05; magnification, 40×.
High Concentrations of BoNT Have a Negative Effect on Neoangiogenesis
HUVECs were seeded in a basal membrane and incubated with different concentrations of BoNT (Fig. 3). Compared with medium control, HUVEC built significant fewer tubes when incubated with 20-IU/mL BoNT. Also the total tube length was less with the highest BoNT concentration tested (Fig. 4A, B). Significant differences could also be shown in the quality of the tubes: both the number of built vessel loops and the branching points between the vessels were fewer with 20-IU/mL BoNT compared with the control (Fig. 4C, D). Concerning the cell-covered area, no differences between the samples could be observed.
Fig. 3.
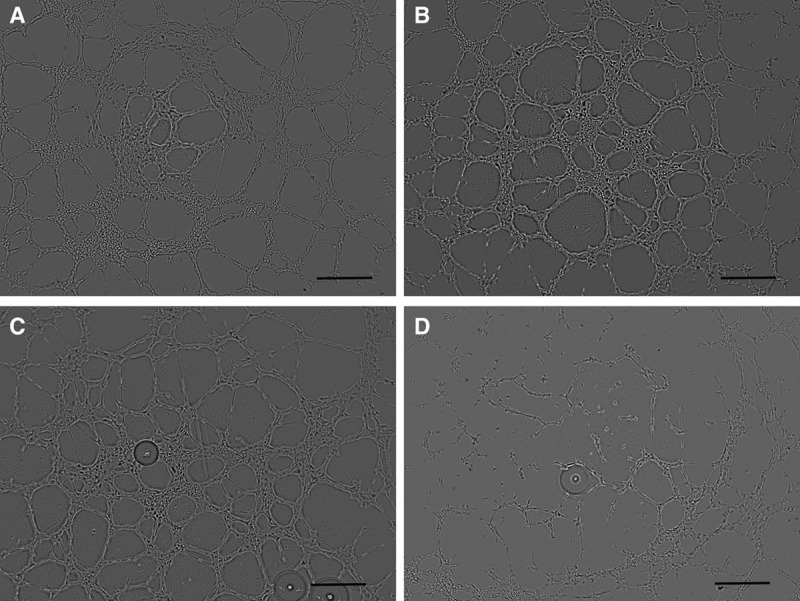
Tube-forming assay of HUVEC on Matrigel incubated with growth medium (A), BoNT 1 IU/mL (B), 10 IU/mL (C), and 20 IU/mL (D); magnification, 40×.
Fig. 4.
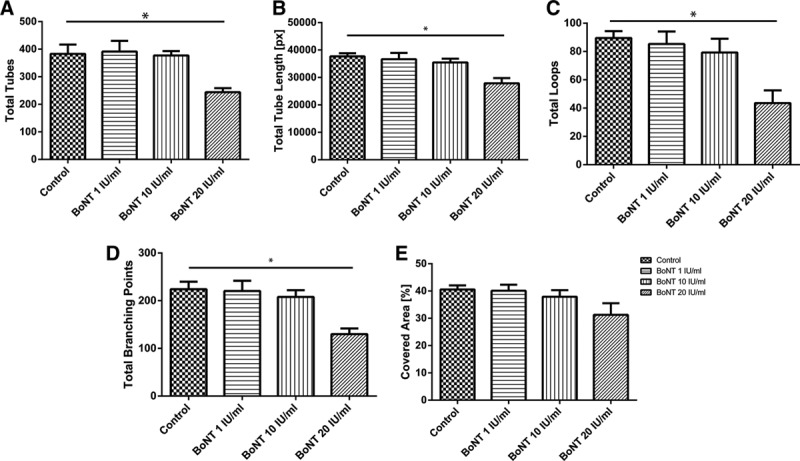
Evaluation of tube-forming assay. Number of tubes (A), total tube length (B), number of loops (C), and branching points (D), and also the cell-covered area (E) were measured; n = 4, *P < 0.05.
In sprouting assay experiments, cell spheroids were encapsulated into a hydrogel and had to migrate and invade into the surrounding to build vessel-like structures. Compared with the negative control, low BoNT concentrations of 1 and 10 IU/mL enhanced sprouting of endothelial cells, and more vessels were formed. Spheroids treated with 20-IU/mL BoNT did not build more sprouts than the negative control (Fig. 5).
Fig. 5.
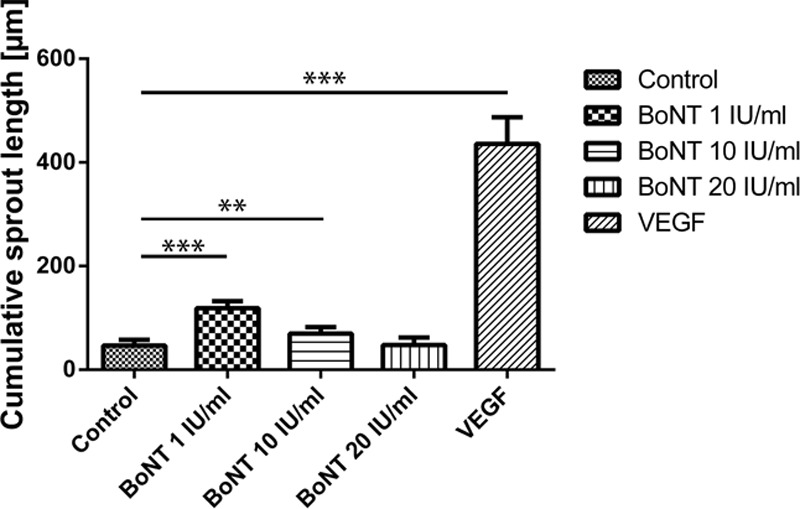
In vitro angiogenesis assay/sprouting assay: spheroids of HUVEC were incubated with basal medium (control), BoNT 1, 10, 20 IU/mL, and 30 ng/mL VEGF for 20 h. A minimum of 30 randomly selected spheroids were used for quantification of the cumulative sprout length. **P < 0.01 and ***P < 0.001.
Real-time PCR analysis showed significantly lower expressions of VEGF-A, ANGPT2, and MMP9 in higher BoNT levels (Fig. 6).
Fig. 6.
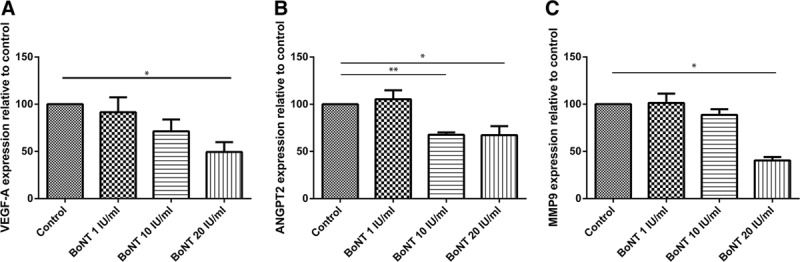
Expression of genes relevant for neoangiogenesis: HUVECs were incubated with basal medium (control), BoNT 1, 10, and 20 IU/mL for 6 h. Expression of VEGF-A (A), ANGPT2 (B), and MMP9 (C) is shown relative to control (100%); n = 3, *P < 0.05 and **P < 0.01.
DISCUSSION
A variety of studies show that botulinum toxin type A injections induce temporary muscular paralysis and relieve the tension on wound edges. This relief of tension may help prevent the widening, hypertrophy, and hyperpigmentation of facial scars.12,13 Only few studies have been conducted that investigate the biological effects of BoNT in terms of wound healing beyond the effect of immobilization. However, it has been shown that BoNT inhibits fibroblast proliferation and in addition promotes apoptosis of fibroblasts derived from hypertrophic scars.18 BoNT has also been reported to reduce the expression of transforming growth factor beta-1.19 Therefore, it has even been suggested that BoNT might be used to decrease fibrosis. Clinical results suggest that it would benefit in early stages of scar formation when fibroblasts have stronger proliferative and apoptotic activity.
In a preceding study, we observed the effect of BoNT regarding fat transplantation. In that course, we still tested BoNT on normal human fibroblasts and could show that there is no negative influence on these cells.10 Nevertheless, a comparison between normal human fibroblasts and scar-derived fibroblasts would be interesting.
In our present study, we focused on the behavior of keratinocytes and endothelial cells under the influence of BoNT in different concentrations to observe its impact on wound healing. An in vitro scratch assay imitates wounding in vivo by damaging a confluent cell layer and destruct cell–cell contact. Therefore, cell migration is triggered. We were able to show that in the highest concentration of 20 IU/mL, BoNT had a diminishing effect on keratinocyte migration and proliferation. Lower concentrations of BoNT did not show such an effect. Therefore, a low BoNT concentration is suggested to be used for fast reepithelialization.
In several in vivo studies, it was shown that BoNT increases blood supply, which accelerates the process of healing20 and prevents the collapse of the peripheral vessels in the cutaneous flap. Additionally, increased diameters of arterioles and venules were observed histologically.21
In our study, we observed the effect of BoNT on a cellular basis. We used tube-forming assays and spheroid-sprouting assays to evaluate the impact of different concentrations of BoNT on angiogenesis. We were able to detect reduced vessel formation with endothelial cells in vitro in the tube-forming assay with high BoNT concentrations of 20 IU/mL. The tube formation assay is one of the most widely used in vitro assays to model the early stages of angiogenesis.22 Endothelial cells were plated on a hydrogel of basement matrix, which supports the cells to form capillary-like structures. Tube formation is typically quantified by measuring the number, length, complexity, or area of these structures. The assay can be used to identify inhibitors or stimulators of angiogenesis. This assay involves endothelial cell adhesion, migration, protease activity, and tubule formation.23 In gene expression level, endothelial cells had a significantly reduced VEGF-A and ANGPT2 expression after 6 hours when incubated with high BoNT concentrations. This indicates a reduced potency for neoangiogenesis. Furthermore, a sprouting assay can identify proangiogenic and antiangiogenic effects of test substances. The assay simulates the entire angiogenesis process in vitro in 3 dimensions: proliferation, invasion, migration, and proteolytic activity of endothelial cells. In contrast to the tube formation assay, also three-dimensional networks can be observed. The number and length of the sprouts correspond with the pro- or antiangiogenicity of the test substance. Again, in our experimental setting, we were able to observe an inhibition of angiogenesis when spheroids were treated with high BoNT concentrations of 20 IU/mL. This corresponds with a significant downregulation of MMP9 in endothelial cells, which were incubated with 20-IU/mL BoNT for 6 hours (Fig. 6). Low concentrations of 1 and 10 IU/mL enhanced sprouting of endothelial cells with more vessels being formed compared with the negative control. In contrast, no influence of BoNT on proliferation or migration of endothelial cells could be observed.
As already discussed in a previous study,10 a wide range of concentrations of BoNT is used in the clinical settings. In the literature, concentrations between 1 and 10 IU/mL were used11,24–26; according to the package inserts, a dose of 5 IU per injection for overactive bladder, 1.25 to 2.5 units per injection for blepharospasm or strabismus (Botox),27 and 4 IU per intramuscular injection (Botox Cosmetic, Allergan Inc., Irvine, Calif.)28 are recommended. Furthermore, medical use generally involves significantly higher doses than cosmetic treatment.29 Therefore, we tested 1 and 10 units per mL, and also 20 IU as a high dose of BoNT.
Chang et al30 revealed that several clinical studies showed that BoNT can improve facial scars,14,31,32 but so far no proven explanation for the benefit of BoNT on a cellular level could be provided.
In this study, we showed an important effect of BoNT on cells important for wound healing: High doses of BoNT (20 IU/mL) had a diminishing effect on wound closure by decelerating proliferation and migration of keratinocytes, thereby inhibiting reepithelialization. Additionally, those high concentrations diminished angiogenesis by showing a negative effect on vessel formation by endothelial cells.
Footnotes
Supported partly by the European Union Seventh Framework Programme (FP7) through the ArtiVasc 3D project under grant agreement number 263416 and by the Medical University of Vienna. The study sponsors had no involvement in study design or writing the manuscript.
Presented at 45. Jahrestagung der DGPRÄC 2014 (Munich, Germany).
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Grose R, Hutter C, Bloch W, et al. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–2315. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- 2.Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713–1726. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 3.Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109:10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nix WA. Botulinum toxin in chronic myofascial pain. Schmerz. 2007;21:467–468. doi: 10.1007/s00482-007-0567-y. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Silberstein S. Botulinum toxin type A for the treatment of headache: why we say yes. Arch Neurol. 2008;65:146–149. doi: 10.1001/archneurol.2007.20. [DOI] [PubMed] [Google Scholar]
- 6.Santos-Silva A, da Silva CM, Cruz F. Botulinum toxin treatment for bladder dysfunction. Int J Urol. 2013;20:956–962. doi: 10.1111/iju.12188. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler T. Sweat and tears: treating the patient with primary hyperhidrosis. Br J Nurs. 2012;21:408, 410–412. doi: 10.12968/bjon.2012.21.7.408. [DOI] [PubMed] [Google Scholar]
- 8.Beer K, Waibel J. Botulinum toxin type A enhances the outcome of fractional resurfacing of the cheek. J Drugs Dermatol. 2007;6:1151–1152. [PubMed] [Google Scholar]
- 9.Lee H, Saladi RN, Fox JL. Cohort study on patient response to botulinum toxin cosmetic therapy. J Cosmet Dermatol. 2008;7:39–42. doi: 10.1111/j.1473-2165.2008.00359.x. [DOI] [PubMed] [Google Scholar]
- 10.Gugerell A, Kober J, Schmid M, et al. Botulinum toxin A and lidocaine have an impact on adipose-derived stem cells, fibroblasts, and mature adipocytes in vitro. J Plast Reconstr Aesthet Surg. 2014;67:1276–1281. doi: 10.1016/j.bjps.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Baek RM, Park SO, Jeong EC, et al. The effect of botulinum toxin A on fat graft survival. Aesthetic Plast Surg. 2012;36:680–686. doi: 10.1007/s00266-011-9864-z. [DOI] [PubMed] [Google Scholar]
- 12.Al-Qattan MM, Al-Shanawani BN, Alshomer F. Botulinum toxin type A: implications in wound healing, facial cutaneous scarring, and cleft lip repair. Ann Saudi Med. 2013;33:482–488. doi: 10.5144/0256-4947.2013.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziade M, Domergue S, Batifol D, et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66:209–214. doi: 10.1016/j.bjps.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg. 2006;117:1758–1766. doi: 10.1097/01.prs.0000209944.45949.d1. discussion 1767–1768. [DOI] [PubMed] [Google Scholar]
- 15.Tollefson TT, Senders CM, Sykes JM, et al. Botulinum toxin to improve results in cleft lip repair. Arch Facial Plast Surg. 2006;8:221–222. doi: 10.1001/archfaci.8.3.221. [DOI] [PubMed] [Google Scholar]
- 16.Korff T, Krauss T, Augustin HG. Three-dimensional spheroidal culture of cytotrophoblast cells mimics the phenotype and differentiation of cytotrophoblasts from normal and preeclamptic pregnancies. Exp Cell Res. 2004;297:415–423. doi: 10.1016/j.yexcr.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Testori J, Schweighofer B, Helfrich I, et al. The VEGF-regulated transcription factor HLX controls the expression of guidance cues and negatively regulates sprouting of endothelial cells. Blood. 2011;117:2735–2744. doi: 10.1182/blood-2010-07-293209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhibo X, Miaobo Z. Botulinum toxin type A affects cell cycle distribution of fibroblasts derived from hypertrophic scar. J Plast Reconstr Aesthet Surg. 2008;61:1128–1129. doi: 10.1016/j.bjps.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z, Zhang F, Lin W, et al. Effect of botulinum toxin type A on transforming growth factor beta1 in fibroblasts derived from hypertrophic scar: a preliminary report. Aesthetic Plast Surg. 2010;34:424–427. doi: 10.1007/s00266-009-9423-z. [DOI] [PubMed] [Google Scholar]
- 20.Aydin A, Memisoglu K, Cengiz A, et al. Effects of botulinum toxin A on fracture healing in rats: an experimental study. J Orthop Sci. 2012;17:796–801. doi: 10.1007/s00776-012-0269-x. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Roh TS, Lee WJ, et al. The effect of botulinum toxin A on skin flap survival in rats. Wound Repair Regen. 2009;17:411–417. doi: 10.1111/j.1524-475X.2009.00477.x. [DOI] [PubMed] [Google Scholar]
- 22.Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 2009;90:195–221. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5:628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 24.Haubner F, Leyh M, Ohmann E, Sadick H, Gassner HG. Effects of botulinum toxin A on patient-specific keloid fibroblasts in vitro. The Laryngoscope. 2014;124:1344–1351. doi: 10.1002/lary.24456. [DOI] [PubMed] [Google Scholar]
- 25.Jung MK, Song SB, Cheon SY, et al. Botulinum toxin enhances the implantation effect of adipocytes in C57/BL6 mice. Aesthetic Plast Surg. 2009;33:722–729. doi: 10.1007/s00266-009-9394-0. [DOI] [PubMed] [Google Scholar]
- 26.Bagheri M, Jahromi BM, Bagheri M, et al. A pilot study on lipolytic effect of subcutaneous botulinum toxin injection in rabbits. Anal Quant Cytol Histol. 2010;32:186–191. [PubMed] [Google Scholar]
- 27.Allergan I. BOTOX® Product Information. 2013. Available at: http://www.allergan.com/assets/pdf/botox_pi.pdf.
- 28.Allergan I. BOTOX® Cosmetic Product Information. 2013. Available at: http://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf.
- 29.Berry MG, Stanek JJ. Botulinum neurotoxin A: a review. J Plast Reconstr Aesthet Surg. 2012;65:1283–1291. doi: 10.1016/j.bjps.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Chang C-S, Wallace CG, Hsiao Y-C, Chang C-J, Chen PK-T. Botulinum toxin to improve results in cleft lip repair: a double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE. 2014;9(12):e115690. doi: 10.1371/journal.pone.0115690. doi:10.1371/journal.pone.0115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gassner HG, Brissett AE, Otley CC, et al. Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study. Mayo Clin Proc. 2006;81:1023–1028. doi: 10.4065/81.8.1023. [DOI] [PubMed] [Google Scholar]
- 32.Flynn TC. Use of intraoperative botulinum toxin in facial reconstruction. Dermatol Surg. 2009;35:182–188. doi: 10.1111/j.1524-4725.2008.34407.x. [DOI] [PubMed] [Google Scholar]


