Supplemental Digital Content is available in the text.
Abstract
Background:
The application of bone tissue engineering for repairing bone defects has gradually shown some satisfactory progress. One of the concerns raising scientific attention is the poor supply of growth factors. A number of growth factor delivery approaches have been developed for promoting bone formation. However, there is no systematic comparison of those approaches on efficiency of neobone formation. In this study, the approaches using periosteum, direct supply of growth factors, or gene transfection of growth factors were evaluated to determine the osteogenic capacity on the repair of bone defect.
Methods:
In total, 42 male 21-week-old Sprague-Dawley rats weighing 250 to 400 g were used as the bone defect model to evaluate the bone repair efficiency. Various tissue engineered constructs of poly(ethylene glycol)-poly(l-lactic acid) (PEG-PLLA) copolymer hydrogel with periosteum, with external supply of bone morphogenetic protein-2 (BMP2), or with BMP2-transfected bone marrow–derived mesenchymal stem cells (BMMSCs) were filled in a 7-mm bone defect region. Animals were euthanized at 3 months, and the hydrogel constructs were harvested. The evaluation with histological staining and radiography analysis were performed for the volume of new bone formation.
Results:
The PEG-PLLA scaffold with BMMSCs promotes bone regeneration with the addition of periosteum. The group with BMP2-transfected BMMSCs demonstrated the largest volume of new bone among all the testing groups.
Conclusions:
Altogether, the results of this study provide the evidence that the combination of PEG-PLLA hydrogels with BMMSCs and sustained delivery of BMP2 resulted in the maximal bone regeneration.
The self-regeneration ability of bone tissue is only sufficient to repair small defects in bone. Large bone defects require bone grafts or other techniques to enhance healing. Over 1.5 million bone graft cases are performed annually.1 However, a major issue in bone reconstruction procedures is the shortage of donor autologous tissue.2 Recently, tissue-engineered bone has shown promise as an alternative source for donor tissue.3 The goal of tissue engineering is to identify the optimal combination of 3 elements, namely scaffolds, cells, and growth factors, to generate a functional bone graft.
Bone morphogenetic protein-2 (BMP2) has been studied extensively in osteogenesis, bone remodeling, and bone repair.4–6 In previous studies, BMP2 has been shown to stimulate the differentiation of bone marrow–derived mesenchymal stem cells (BMMSCs).7 However, the effects of soluble stimuli applied to increase bone formation decrease when they are inactivated because of environmental conditions. The time required for bone formation is much longer than the lifetime of these proteins in vivo. There are a large number of studies focusing on different growth factor delivery approaches for bone regeneration. Weng et al8 performed mucoperiosteal flaps to regenerate mandibular bone defect. BMP2 encapsulated within gelatin microparticles had shown the effect on promotion of bone formation by increasing bone sialoprotein expression.9 BMMSCs treated with either an adenovirus or a liposome to carry BMP2 complementary DNA had shown promising results on enhancing bone formation.10 Although many delivery approaches have been studied, there is no systematic comparison of those approaches to validate the efficiency on neobone formation.
In this study, we compared the effects of BMP2 delivered through autologous periosteum, direct supply of BMP2, or gene transfection approach on osteogenic ability to find the optimal condition for promoting bone formation. Copolymers of polyethylene glycol (PEG) and poly(l-lactic acid) (PLLA; PEG-PLLA) used in this study have been reported as biodegradable and biocompatible synthetic polymers that can be used in tissue-engineering applications.11,12 As for gene delivery approaches, a nonviral vector was selected for the BMP2 transfection. The liposome-mediated technique was developed over 20 years ago and has been used widely.13,14 The cationic lipids interact with the phosphate group of the nucleic acid to form a liposomal structure that transports the desired DNA into target cells through endocytosis.15 Lipofectamine transfection reagent (Invitrogen) was used in this study to facilitate the transfer of BMP2 gene into BMMSCs, allowing continuous expression of BMP2.16,17 By comparing different growth factor delivery approaches on bone formation, the result of this study could provide knowledge on tissue engineering for regeneration of osseous tissue.
MATERIALS AND METHODS
Scaffold Fabrication
PEG-PLLA was synthesized by ring opening polymerization as described previously.18,19 Briefly, d,l-lactide (Purac, Corbion) and PEG (Fluka, Sigma-Aldrich) were stirred at 160°C for 6 hours after adding stannous octoate as a catalyst under a nitrogen purge. The copolymer was dissolved in dichloromethane and then precipitated in cold ether, followed by filtering and drying steps. The polymer was then acrylated at both ends to obtain PEG-PLLA-DA as follows. Ten grams of PEG-PLLA was dissolved in 100 mL dichloromethane and cooled to 0°C in an ice bath. Triethylamine and acryloyl chloride were added at 4 times the molar ratio. The reaction mixture was stirred for 12 hours at 0°C and then for 12 hours at room temperature. The solution was filtered to remove salt and then filtered in a large excess of diethyl ether. The white macromer obtained from this step was analyzed with nuclear magnetic resonance and gel permeation chromatography.
Gene Transfection and Analysis
Lipofectamine transfection reagent (Invitrogen) was introduced to incorporate the BMP2 gene in our gene delivery system. The transfection steps followed the manufacturer’s standard protocol. In brief, the rat BMP2 sequence (NM_017178) was customized for incorporation into the TrueORF cloning vector (pCMV6-AC-GFP) with green florescence protein (GFP) located at the C-terminal of the BMP2 gene (OriGene Technologies, Md.). Then, 4 μg of DNA extracted from the TrueORF clone and 40 μL of Lipofectamine Plus were separately diluted in 400 μL OptiMEM medium. The 2 dilutions were mixed and incubated for 30 minutes. Then, 200 μL of the mixture was transferred into the well of culture dish that contained approximately 105 BMMSCs and incubated at 37°C for 6 hours. Later, transfected BMMSCs were loaded into PEG-PLLA scaffolds. The BMP2-transfected BMMSCs were monitored by observing the GFP expression under a Leica TCS SP2 microscope.
Animal and Bone Defect Model
All animal procedures were performed according to the animal research guidelines of Chang Gung Memorial Hospital and Chang Gung University. Twenty-week-old Sprague-Dawley rats weighing 250 to 400 g were used in this study. The rat femoral bone segmental defect model described here is a modification of a previous model that has been used extensively to study the repair of long bones.20 Briefly, rats were anesthetized with isoflurane and prepared aseptically. A longitudinal skin incision was made along the lateral surface of the femoral diaphysis, and the overlying periosteum was removed from the defect area. A 7-mm segment of the central diaphysis was removed with an oscillating bone saw (Surgical-XT, NSK Nakanishi Inc., Tochigi-ken, Japan) with 0.9% normal saline irrigation, resulting in a femoral bone defect that was 7 mm in length in the mid-portion of the femoral diaphysis. A polyethylene fixation plate (4 × 4 × 23 mm) was secured to the anterolateral aspect of the femur by four 1.1-mm Kirschner wires and two 34-G circulated wires. Later, the defect was filled with a tissue-engineering construct (TEC) of compositions differing between experimental groups. The TECs were secured by placing two 4-0 vicryl sutures around the scaffold and the fixation plate. Six Sprague-Dawley rats were treated in each experimental group. The animals who encountered leg infections, mortality, or the broken fixation plate were excluded in this study. The final result analysis contained at least 3 biological replicates in each experimental group.
BMMSC Isolation and Cell Culture
Bone marrow was isolated from rat femurs. The red blood cells were removed by centrifugation at 500 rpm and 4°C, and the pellet was washed with Dulbecco modified Eagle medium (Gibco, Thermo Fisher Scientific, Inc.) and recentrifuged. The pellet was resuspended in α-minimum essential medium (Gibco) containing 20% fetal bovine serum (Gibco), 1% antibiotic–antimycotic (Gibco), and fibroblast growth factor-2 (4 ng/mL, PeproTech, Rehovot, Israel). Later, the cells are plated on a flask and incubated at 37°C with 5% humidified CO2. Cells of the third passage were used in this study. As for the scaffold + BMMSC group, the BMMSCs (approximately 105 cells) were added into the PEG-PLLA scaffold and placed in the bone defect area 1 week later. In the chondrogenic differentiation medium (CDM) group, the PEG-PLLA scaffold encapsulated with BMMSCs was cultured in the CDM medium for 1 week before being placed in the bone defect area (CDM: dexamethasone, 10–7 M; ascorbate, 50 mg/mL; 50 mg/mL, insulin–transferrin sodium selenite; sodium pyruvate, 100 mg/mL; proline, 40 mg/mL; l-glutamine, 2 mM; transforming growth factor-β3, 10 ng/mL; and bovine serum albumin, 1.25 mg/mL).
The Bone Defect Model and Preparation of Tissue-engineered Constructs
A 7-mm bone defect was created at the femoral diaphysis. Each end of the polyethylene fixation plate was fixed on the defective bone to provide physical support and a platform for holding the TECs (Fig. 1). The TEC contains various tissue-engineered constructs. The experiential grouping is as follows.
Fig. 1.
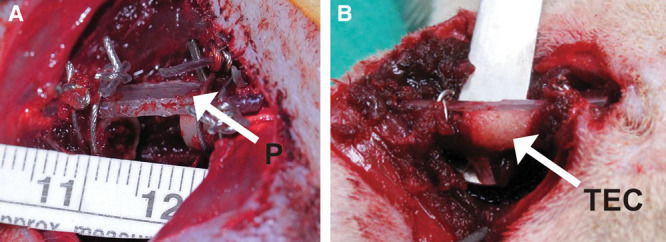
Bone defect model. Femoral bone was exposed after the incision was performed. Approximately 7 mm of the center portion of the femoral diaphysis was removed with an oscillating bone saw. A polyethylene fixation plate (4 × 4 × 23 mm) was applied to secure the anterolateral aspect of the femur (A). The arrow indicates the polyethylene fixation plate (P). The TEC (arrow) was placed in the gap of the bone defect (B).
A total of 7 groups were examined. The groups studied were as follows:
Control group: the gap in the bone defect was untreated.
Scaffold (S) group: the gap in the bone defect was filled with the PEG-PLLA scaffold alone.
Scaffold + BMMSC (S + C) group: the gap was filled with the PEG-PLLA scaffold and seeded with BMMSCs.
Periosteum (S + C + P) group: the gap was filled with the PEG-PLLA scaffold, seeded with BMMSCs, and then wrapped with a layer of preharvested periosteum.
Chondrogenic differentiation medium (S + C + CDM) group: the PEG-PLLA scaffold with BMMSCs was cultured in CDM for 1 week before being placed in the bone defect area.
BMP2 (S + C + BMP2) group: the PEG-PLLA scaffold with BMMSCs was treated with the growth factor BMP2 (10 ng/mL).
Gene-delivered BMP2 (S + C + gene BMP2): the PEG-PLLA hydrogel contained BMMSCs that had been transfected with the nonviral vector carrying the BMP2 gene.
The transfection efficiency (approximately 20%) was evaluated by observing the GFP expression that was tagged on C-terminal of the BMP2 gene (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A231).
Radiographic Analysis
Postoperative radiographs were used to verify the placement of the scaffold and served as a baseline for radiographic evaluation. Radiographs were taken using a high-resolution radiography system on the day of operation and at 12 weeks after sample harvesting. The exposure conditions were 40 kV, 1.2 mA, and 6.3 ms. The percentage areas of the femoral segmental defect occupied by newly formed bones were scored by manually selecting the new growth, and the sizes of bone or muscle tissues were quantified using ImageJ software.21 Bone regeneration was calculated as follows:
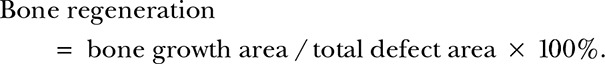 |
Histological and Histomorphometric Analysis
All specimens were harvested at 12 weeks. Before harvesting, the rats were killed with intravenous injection of potassium chloride (2 meq/kg). Tissues were dissected out of the chamber en bloc and weighed. The samples were stored in formalin, paraffin embedded, and sectioned at 4-µm thickness for histological analysis. Entire sections of hematoxylin and eosin–stained tissues were imaged (resolution = 0.66 μm per pixel). Masson trichrome staining was applied for more detailed morphological observation.
Statistical Analysis
Data are presented as the mean ± SD. Significant differences were evaluated by analysis of variance with Tukey post hoc test. Data were expressed as the means ± SE. A P value less than 0.05 was considered statistically significant.
RESULTS
PEG-PLLA Biomaterial Mainly Promotes Chondrogenesis by BMMSCs in 12 Weeks
First, the effects of the biomaterials on bone regeneration were examined. The bone defect space was filled with or without PEG-PLLA scaffolds (Supplemental Digital Content 2, http://links.lww.com/PRSGO/A232). In the control group containing no scaffold, the space in the bone defect was filled with muscle tissue. The histological image shows that the muscle tissue might be pushed inward to fill the gap within the area of the bone defect. With the implantation of the PEG-PLLA scaffold (S group), the proportion of muscle tissue was reduced. Either in the center or at the border between the old bone and the scaffold, the scaffold with porous structures allows the formation of blood vessels. Some of the PEG-PLLA scaffolds were observed biomaterial degraded, but part of it remained.
Next, we encapsulated the BMMSCs within the PEG-PLLA hydrogel (S + C group) or cultured the hydrogel loaded with BMMSCs in the CDM medium (S + C + CDM group) to examine the effects of nonspecific osteogenic growth factors for neobone formation. When BMMSCs were encapsulated within the PEG-PLLA scaffold, chondrogenesis and few osteocytes were observed at the border between the old and new bone but not in the center area. In S + C + CDM group, both chondrocytes and vessels were detected in the scaffold incubated with CDM. The cartilage formation was more significant at the border than in the center area. The chondrogenesis was more promising by the supply of chondrogenic growth factors in CDM.
The PEG-PLLA Scaffold with BMP2-transfected BMMSCs Promotes Bone Regeneration with the Addition of Periosteum
Despite the space and mechanical structures provided by PEG-PLLA scaffolds, the differentiation of BMMSCs in bone tissue was not very efficient without the simulation of specific osteogenic growth factors, such as BMP2. Several approaches of growth factor delivery were tested for their effects on promoting bone formation. The PEG-PLLA scaffold with BMMSCs was treated separately with periosteum (S + C + P group), direct supply of BMP2 (S + C + BMP2 group), or gene-transfected BMP2 (S + C + gene BMP2 group) to determine which treatment is most efficient for bone regeneration. BMMSCs promote cartilage formation and may direct development toward bone formation in longer incubations. Because the periosteum is known to provide nutrition and blood supply to the bone, the scaffold with BMMSCs was wrapped with tibia periosteum to study the effect of periosteum on promoting bone regeneration. After wrapping with periosteum, both osteocytes and vessels were detected (Fig. 2). Numerous chondrocytes were generated along the border, and vessel formation occurred in the center of the scaffold. Compared to the central part of the scaffold, the border between the old bone and the scaffold began to show areas combining cartilage formation with the presence of chondrocyte lacunae and some osteocytes. However, the bone matrix and haversian canal were not identified here.
Fig. 2.
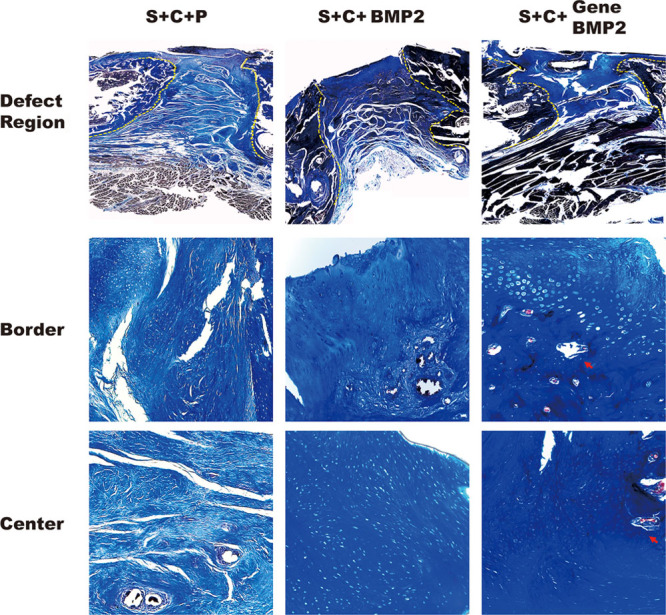
Histological analysis of new bone formation. CDM group: BMMSCs were encapsulated in the PEG-PLLA scaffold and cultured in CDM. The border between old and new bone showed chondrocytes and little vessel formation. S + C + BMP2 group: chondrocytes were present at both the border and the center of the bone defect area. S + C + gene BMP2 group: Cartilage formation was present everywhere. The ossification occurred in the border and center of the defect area. New bone was observed both in the center and border of the defect. The bone defect area lies between the yellow dashed lines. The images of border and center region were at larger magnification (magnification, 100×).
In S + C + BMP2 group, osteocytes were detected, and cartilage formation was also observed in the center area. Another gene deliver approach was also examined here. As predicted, the S + C + Gene BMP2 group increased the efficiency of bone regeneration. In this condition, the border area reached the stage of bone formation; not only were osteocytes detected but a patch of bone matrix was also formed.
The Expression of BMP2 by Gene Delivery Approach Enhances the Bone Repair Efficiency
To monitor the formation of new bone, alkaline phosphatase activity was commonly performed in this study. The cartilage formation was observed first after the addition of the scaffolds. With the stimulation of the BMMSCs, osteocytes were detected at the border area (Fig. 3). Ossification was shown after treatment with periosteum and BMP2. The function of the periosteum is to supply blood and nutrients, as well as appropriate osteogenic growth factors, for bone formation. Both in S + C + BMP2 group and S + C + Gene BMP2 group, the supply of specific BMP2 resulted in more promising results on formation of new bone when compared with the S + C + P group.
Fig. 3.
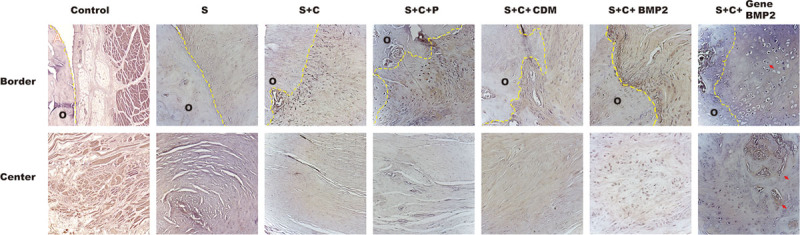
Alkaline phosphate activity in all the groups. Alkaline phosphate activity was the marker for osteoblasts. Control group: no osteoblasts were present in the control group. S group: chondrogenesis was observed at the border area, but no osteoblasts were found. S + C group: alkaline phosphate showed positive activity at the border but not in the center. Scaffold, cell, and periosteum group: osteoblast proliferation occurred at the border. CDM group: chondrogenesis was observed. S + C + BMP2 group: osteoblasts were detected at the border. Chondrogenesis also appears in the center. S + C + gene BMP2 group: bone formation was observed at the border and the center. The arrow indicates the new bone tissue. O indicate old bone area.
The morphology of different types of cells and tissues could be clearly identified using hematoxylin and eosin and Masson trichrome staining. However, the small patch of neobone could have easily been overlooked. Consequently, radiographic analysis was also performed to verify the formation of new bone. In the group treated with biomaterial or cells, the neobone formation was not prominent in the control, S, and BMMSC groups (Fig. 4). With the addition of periosteum, the neobone formation detected in the radiographic image was significant compared with the control group (P = 0.048) (Fig. 4). CDM promotes the differentiation of BMMSCs into chondrocytes. Cartilage then requires time to transform into bone. Consequently, the BMP2 group shows more neobone formation than the CDM group. The volume of the newly grown bone tissue was very prominent in the S + C + BMP2 group with compared with control group (P = 0.04). The S + C + Gene BMP2 group showed the most significant increase in the volume of neobone formation, even showed more promising result compared with S + C + P and S + C + BMP2 group (both P < 0.0001). The quantification of neobone formation indicated that the increasing volume of neobone tissue resulted from the addition of periosteum and BMP2.
Fig. 4.
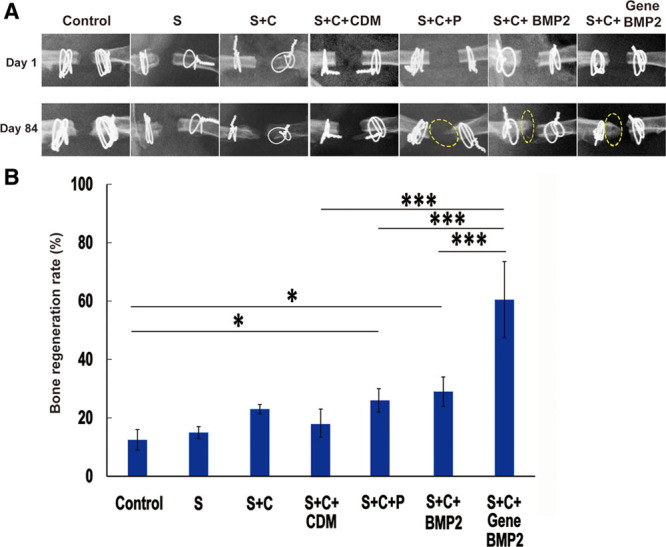
The radiographic analysis of biomaterial and growth factor group (A) and the graph showing bone formation (B). A, The bone volume increased, as shown by the ImageJ comparison of the radiographic images from day 1 and day 84. Yellow circles indicate newly formed bone. B, The histogram shows the percentage of newly formed bone tissue. Data are presented as mean ± SD. The analysis of variance test was performed, and P values of less than 0.05 were considered statistically significant. Control group, n = 4; S + C group, n = 4; CDM group, n = 5; gene BMP2, n = 8; and the rest of group, n = 3.
DISCUSSION
In the United States, approximately 500,000 people undergo bone defect repair.22 With the increasing need for bone replacement in the medical field, engineered bone tissue has received more attention as an alternative option for bone replacement. In this project, we used a murine model to investigate several tissue-engineering strategies to enhance the formation of vascularized bone tissues. Growth factors such as BMP2, vascular endothelial growth factor, and fibroblast growth factor have been proven to have major impacts on bone formation.23–25 However, whether or how to incubate those osteogenic growth factors with the PEG-PLLA scaffold to enhance bone regeneration or how effective the different delivery system of the growth factors is remains unclear. In this study, we first examine the effect of scaffold or supply of nonspecific osteogenic growth factors for bone regeneration. The formation of new bone tissue was not observed in the control group, indicating that the self-regeneration of the old bone was insufficient to repair the bone defect that we created. In both S + C group and S + C + CDM group, the center region still retained the remodeling tissue morphology and had not yet been identified as any type of tissue. At the border between the old and new bone, chondrogenesis was observed, and vessels were also detected but no sign of osteogenesis. These results indicate that the combination of scaffolds and cells exhibits more promising results on the formation of neocartilage after 12-week in vivo culture. It is possible that those groups followed the chondrogenic ossification pathway when no or few nonspecific osteogenic growth factors were provided. By supplying with more specific osteogenic growth factors, we used the method of the scaffold wrapped with periosteum, direct supply of BMP2, or gene transfection to determine the optimal conditions for bone regeneration. The supply of S + C + CDM and nutrition from the periosteum accelerated the vascularization and bone formation. The S + C + CDM group was clearly less promising for bone formation compared to the BMP2 group, which is specialized for bone differentiation. Many studies indicated that BMP2 in combination with other growth factors, such as transforming growth factor-β or BMP7, exhibited enhancement on bone formation.26 However, the group with periosteum, which provides multiple growth factors other than BMP2, presented less bone formation when compared with the S + C + Gene BMP2 group, in which only 1 growth factor was provided. It is possible that the dosage or sustaining time of the growth factors strongly affects the result of bone regeneration.
Furthermore, the gene delivery technique for the supply of BMP2 with gene transfection approach greatly enhanced the formation of new bone. The result was more promising than with the group with direct supply of BMP2. The result might be contributed from the dosage or the longer sustention of the growth factor, which was confirmed in other studies.27 The scaffold properties, BMMSCs, periosteum, and growth factors all affect the formation of osseous tissue. Growth factors combined with the scaffold and BMMSCs are the most influential factors for promoting and accelerate the formation of new bone.
In this study, we compared different growth factor delivery approaches in a bone defect model to evaluate the effects of those approaches. When BMMSCs were cultured with the PEG-PLLA scaffold on bone regeneration, a supply of BMP2 with gene transfection approach indeed promoted bone formation to a significant degree. Overall, this system could be an optimal option for bone regeneration.
Supplementary Material
Footnotes
Drs. Hsiao and Yang contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.US Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/oralhealth/infectioncontrol/faq/allografts.htm. Accessed October 25, 2013.
- 2.Kneser U, Schaefer DJ, Polykandriotis E, et al. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 4.Abe E. Function of BMPs and BMP antagonists in adult bone. Ann N Y Acad Sci. 2006;1068:41–53. doi: 10.1196/annals.1346.007. [DOI] [PubMed] [Google Scholar]
- 5.James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013;2013:684736. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappuis V, Gamer L, Cox K, et al. Periosteal BMP2 activity drives bone graft healing. Bone. 2012;51:800–809. doi: 10.1016/j.bone.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Guo D, Harris MA, et al. Bmp2 in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J Cell Sci. 2013;126(Pt 18):4085–4098. doi: 10.1242/jcs.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng D, Hürzeler MB, Quiñones CR, et al. Contribution of the periosteum to bone formation in guided bone regeneration. A study in monkeys. Clin Oral Implants Res. 2000;11:546–554. doi: 10.1034/j.1600-0501.2000.011006546.x. [DOI] [PubMed] [Google Scholar]
- 9.Solorio L, Zwolinski C, Lund AW, et al. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med. 2010;4:514–523. doi: 10.1002/term.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J, Ries J, Gelse K, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 11.Kim HD, Bae EH, Kwon IC, et al. Effect of PEG-PLLA diblock copolymer on macroporous PLLA scaffolds by thermally induced phase separation. Biomaterials. 2004;25:2319–2329. doi: 10.1016/j.biomaterials.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B, Akar B, Waller TM, et al. Design of a composite biomaterial system for tissue engineering applications. Acta Biomater. 2014;10:1177–1186. doi: 10.1016/j.actbio.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitt M, Buonocore L, Rose JK. Liposome-mediated transfection. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im1016s03. Chapter 10:Unit 10.16. [DOI] [PubMed] [Google Scholar]
- 15.Ewert KK, Ahmad A, Bouxsein NF, et al. Non-viral gene delivery with cationic liposome-DNA complexes. Methods Mol Biol. 2008;433:159–175. doi: 10.1007/978-1-59745-237-3_10. [DOI] [PubMed] [Google Scholar]
- 16.Chesnoy S, Huang L. Structure and function of lipid-DNA complexes for gene delivery. Annu Rev Biophys Biomol Struct. 2000;29:27–47. doi: 10.1146/annurev.biophys.29.1.27. [DOI] [PubMed] [Google Scholar]
- 17.Hirko A, Tang F, Hughes JA. Cationic lipid vectors for plasmid DNA delivery. Curr Med Chem. 2003;10:1185–1193. doi: 10.2174/0929867033457412. [DOI] [PubMed] [Google Scholar]
- 18.Chiu YC, Kocagöz S, Larson JC, et al. Evaluation of physical and mechanical properties of porous poly (ethylene glycol)-co-(L-lactic acid) hydrogels during degradation. PLoS One. 2013;8:e60728. doi: 10.1371/journal.pone.0060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu Y-C, Larson JC, Perez-Luna VH. Formation of microchannels in poly(ethylene glycol) hydrogels by selective degradation of patterned microstructures. Chem Mater. 2009;21:1677–1682. [Google Scholar]
- 20.Chakkalakal DA, Strates BS, Mashoof AA, et al. Repair of segmental bone defects in the rat: an experimental model of human fracture healing. Bone. 1999;25:321–332. doi: 10.1016/s8756-3282(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 21.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baroli B. From natural bone grafts to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J Pharm Sci. 2009;98:1317–1375. doi: 10.1002/jps.21528. [DOI] [PubMed] [Google Scholar]
- 23.Yun YR, Jang JH, Jeon E, et al. Administration of growth factors for bone regeneration. Regen Med. 2012;7:369–385. doi: 10.2217/rme.12.1. [DOI] [PubMed] [Google Scholar]
- 24.Du X, Xie Y, Xian CJ, et al. Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol. 2012;227:3731–3743. doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 25.Kaigler D, Wang Z, Horger K, et al. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 26.Kempen DH, Creemers LB, Alblas J, et al. Growth factor interactions in bone regeneration. Tissue Eng Part B Rev. 2010;16:551–566. doi: 10.1089/ten.teb.2010.0176. [DOI] [PubMed] [Google Scholar]
- 27.Wegman F, Geuze RE, van der Helm YJ, et al. Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation in a large animal model. J Tissue Eng Regen Med. 2014;8:763–770. doi: 10.1002/term.1571. [DOI] [PubMed] [Google Scholar]


