Summary:
In this case report we describe the use of a 2-stage approach to treat severe recurrent vulvar lymphangiectasia in a patient with Noonan syndrome. First, 3 functional lymphatic vessels were identified and anastomosed to venules in an end-to-end fashion. Then, in a second surgical procedure, the vulvar lesions were resected as much as possible and the vulva was reconstructed. By the 12-month follow-up the patient had recovered well. Although there were still some small vesicles on the left labia there was no more ooze, itch, and pain. Lymphatic mapping using indocyanine green showed improvement of the edema of her vulva region and patent LVA. In addition to the demonstration of this 2-stage approach, this case report also demonstrates the benefits of preemptive LVA before performing surgery that may be at high risk for postoperative lymph edema.
Noonan syndrome (NS) is a relatively common autosomal-dominant disorder first described in 1963 by Dr. Noonan.1 Characteristics include minor facial dysmorphism, congenital heart defects, short posture, webbed neck, (minor) retardation, chest deformity (pectus excavatum, pectus carinatum), lymphatic dysplasia, and bleeding diathesis.1 Lymphatic dysplasia is described to occur in up to 20% of patients with NS, most frequently occurring in the dorsal limb.1 Cutaneous lymphangiectasia, also called acquired lymphangioma, is a benign cutaneous disorder involving the pathologic dilatation of dermal and subcutaneous lymphatic channels.2,3 The lesions formed by these malformed channels can have a “frogspawn”-like appearance.2,3 As a result of these damaged lymph channels, lymph edema can develop in the affected body part.2 Twenty-two cases of primary lymphangiectasia (also referred to as lymphangioma circumscriptum) have been described and confirmed by histopathology.3 Vulvar lymphangiectasia associated with NS is a rare phenomenon which, to our knowledge, has been described only once before.4 For the treatment of lymphangiectasia, no differentiation has been made between acquired and congenital lymphangiectasia. Observation and surgery seem to be the preferred strategy.3 We present a patient with recurrent vulvar lymphangiectasia and NS who has been treated successfully with a 2-stage approach using lymphovenous anastomosis (LVA) followed by excision of the vulvar lesion and reconstruction.
METHODS AND RESULTS
In this case study, a 25-year-old female patient with NS presented with major lymphangiectasia of both labia majora and minora. The patient was primarily concerned with the frequent oozing, itching, and severe pain the lesions were causing. In addition, the recurrence and uncertainty about the treatment were bothering her. Patient history revealed a growth retardation, atrial septal defect, pulmonary stenosis, excision of a tierfell nevus as an infant, and hearing loss with frequent ear infections. Vulval edema and also significant edema of her left leg were present. In the past, the lymphangiectasia was treated more than 15 times with cryotherapy, excision, coagulation, and shave excision. Unfortunately, these treatments relieved the symptoms only temporarily. Within 2 to 5 months postoperatively, her lesions recurred rapidly. It is suspected that the etiology of her vulval problem is related to former shave excisions of a tierfell nevus as an infant with damage of the superficial lymphatic vessels and the genetic susceptibility due to NS.
Lymphatic mapping using Photodynamic Eye (PULSION Medical Systems AG, Munich, Germany) with injection of 1 mL of indocyanine green in the feet and popliteal region showed pitting lymphedema of the vulvar region and tortuous lymphatic channels with 3 suitable lymph vessels for LVA (Fig. 1). To relieve the patient of her symptoms and to offer a sustained treatment for the lymphangiectasia, a surgery creating 3 end-to-end LVA procedures was performed 2 weeks after lymphatic mapping. The aim of this procedure was to facilitate adequate drainage of the lymphangiectasia and thereby preventing recurrence. The surgical procedure was performed in general anesthesia. The diameter of the lymphatic vessels was 0.5 mm. The patient recovered free of complications (Fig. 2). Thirteen weeks after the LVA surgery, the vulvar lesions were resected as much as possible in a second surgical procedure (Fig. 3). Reconstruction was performed with advancement flaps of the labia majora and wedge excision of the labia minora. The patient was given cefazoline for 3 days and was dismissed on the fourth day after surgery. Histopathology confirmed the diagnosis of lymphangiectasia. The patient recovered without complications. At the 12-month follow-up, the patient was doing well. Although there were still some small vesicles on the left labia (Fig. 4), there was no more ooze, itch, and pain. Lymphatic mapping using indocyanine green showed improvement of the edema of her vulva region and patent LVA.
Fig. 1.
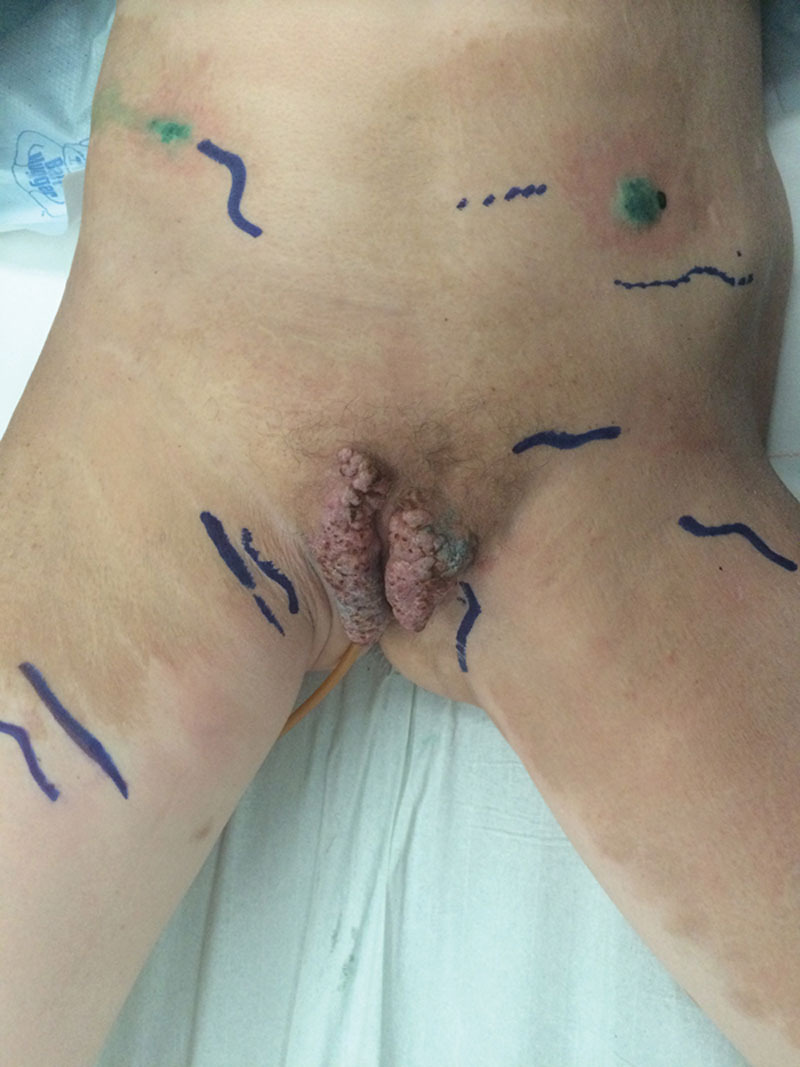
Marking of the lymphatic vessels before surgery using injections of indocyanine green and Photodynamic Eye.
Fig. 2.
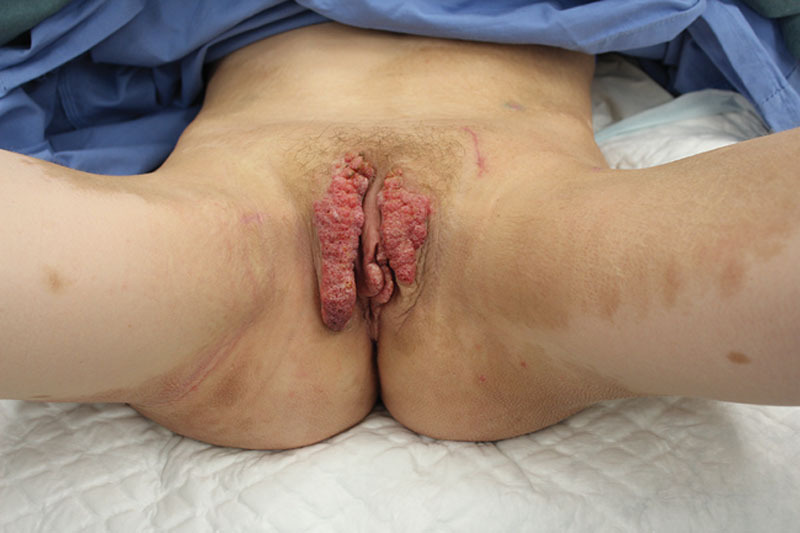
Lymphangiectasia of the vulva; scars at the site of the LVAs before excision.
Fig. 3.
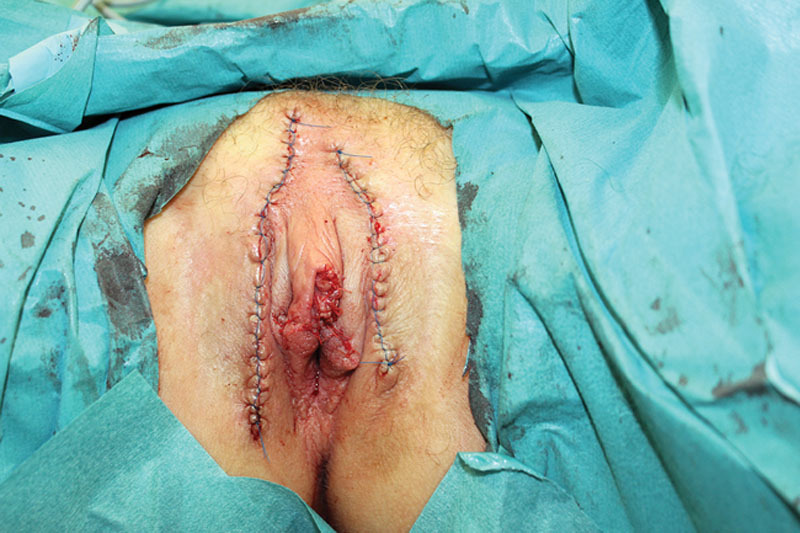
Immediate postoperative result after resection of the lymphangiectasia and reconstruction using advancement flaps and wedge excision of the labia minora.
Fig. 4.
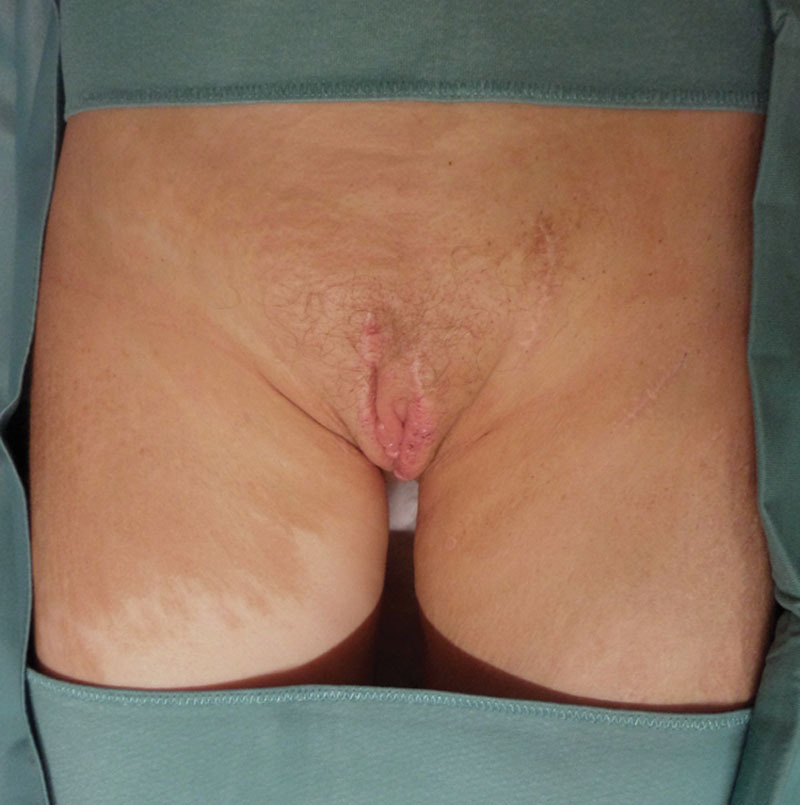
Postoperative result at 12-month follow-up.
DISCUSSION
Vulval lymphangiectasia is a rare disease and is usually reported after surgery/radiotherapy for carcinoma of the cervix or vulva, tubercular inguinal lymphadenitis, or Crohn’s disease of the vulva.2,5,6 It can be asymptomatic, pruritic, burning, or painful. It is an unpleasant but benign condition, with a severe impact on the patient’s quality of life.7 Histologically, dilated lymphatic channels are present in the superficial and mid-dermis; few dilated lymphatics are seen in the deep dermis.8 As the lesions progress, functional impairment regarding urination and sexual intercourse can develop, in addition to cosmetic problems. However, the treatment of lymphangiectasia can be very challenging because of high recurrence rate after surgery, which is reported to be 23.1%.3,9 The prognosis for patients with diffuse lymphangiomatosis is poor if the condition is resistant to standard therapies.8 More conservative treatments such as skin care compression are difficult to apply considering the location of the lesions.10 Carbon dioxide lasers causing superficial vaporization of the lesions have been also described in the treatment.7 However, this treatment may lead to pain, aggravation of the lesions, and keloid formation. Ghaemmaghami et al conclude that major labiaectomy appears to be more successful than other methods including LVA. However, the evidence cited to support this is 29 years old, and the technique to create LVAs has changed dramatically.9 Recently, Yamamoto et al evaluated the use of multiple site LVAs for the treatment of genital lymphedema complicated with severe lymphorrhea. Their approach was successful and they stated that multisite LVAs can serve as the most effective therapy for lymphedema.10 The initial treatment with LVA was performed to prevent recurrence of lymphangiectasia after resection of the lesions. Because the lymphangiectasia did indeed not recur, this case study suggests that preoperative LVA might be worthwhile before performing any surgery that may be at high risk for postoperative lymph edema.
SUMMARY
This case report is, to our knowledge, the first to describe this 2-stage approach in the treatment of severe vulvar lymphangiectasia. The treatment consists of creating LVAs followed by reconstruction of the vulva. In our opinion, adequate drainage facilitated by LVAs might eradicate the underlying cause of the lymphangiectasia and lymph edema and thereby prevent recurrence after the reconstruction of the vulva. The combination of LVA and secondary resection seems to be a therapeutic option in patients with these rare problems. In addition, this case demonstrates the benefits of preemptive LVA before performing surgery that may be at high risk for postoperative lymph edema.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Mendez HM, Opitz JM. Noonan syndrome: a review. Am J Med Genet. 1985;21:493–506. doi: 10.1002/ajmg.1320210312. [DOI] [PubMed] [Google Scholar]
- 2.Chang M, Shiao G, Tseng C. Lymphangiectasia—Report of one case and review of the literature. Dermatol Sinica. 1997;15:275–279. [Google Scholar]
- 3.Kokcu A, Sari S, Kefeli M. Primary vulvar lymphangioma circumscriptum: a case report and review of literature. J Low Genit Tract Dis. 2015;19:e1–e5. doi: 10.1097/LGT.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 4.Pootrakul L, Nazareth MR, Cheney RT, et al. Lymphangioma circumscriptum of the vulva in a patient with Noonan syndrome. Cutis. 2014;93:297–300. [PubMed] [Google Scholar]
- 5.Singh N, Kumari R, Thappa D. Vulval lymphangiectasia secondary to tubercular lymphadenitis. Indian J Sex Transm Dis. 2007;28:38–39. [Google Scholar]
- 6.Haneef NS, Ramachandra S, Metta AK, et al. Lymphangiectasias of vulva. Indian Dermatol Online J. 2011;2:40–42. doi: 10.4103/2229-5178.79854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okur MI, Köse R, Yildirim AM, et al. Lymphangiectasia of the vulva accompanying congenital lymphedema. Dermatol Online J. 2009;15:13. [PubMed] [Google Scholar]
- 8.Bhat RM, Saldanha CS, Kambil SM, et al. Cutaneous lymphangiectasia of the vulva secondary to tuberculosis. Indian J Sex Transm Dis. 2012;33:35–37. doi: 10.4103/2589-0557.93817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghaemmaghami F, Karimi Zarchi M, Mousavi A. Major labiaectomy as surgical management of vulvar lymphangioma circumscriptum: three cases and a review of the literature. Arch Gynecol Obstet. 2008;278:57–60. doi: 10.1007/s00404-007-0440-3. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Koshima I, Yoshimatsu H, et al. Simultaneous multi-site lymphaticovenular anastomoses for primary lower extremity and genital lymphoedema complicated with severe lymphorrhea. J Plast Reconstr Aesthet Surg. 2011;64:812–815. doi: 10.1016/j.bjps.2010.10.011. [DOI] [PubMed] [Google Scholar]


