Abstract
Introduction
The circadian clock plays an important role in several aspects of female reproductive biology. Evidence linking circadian clock-related genes to pregnancy outcomes has been inconsistent. We sought to examine whether variations in single nucleotide polymorphisms (SNPs) of circadian clock genes are associated with PA risk.
Methods
Maternal blood samples were collected from 470 PA case and 473 controls. Genotyping was performed using the Illumina Cardio-MetaboChip platform. We examined 119 SNPs in 13 candidate genes known to control circadian rhythms (e.g., CRY2, ARNTL, and RORA). Univariate and penalized logistic regression models were fit to estimate odds ratios (ORs); and the combined effect of multiple SNPs on PA risk was estimated using a weighted genetic risk score (wGRS).
Results
A common SNP in the RORA gene (rs2899663) was associated with a 21% reduced odds of PA (P<0.05). The odds of PA increased with increasing wGRS (Ptrend< 0.001). The corresponding ORs were 1.00, 1.83, 2.81 and 5.13 across wGRS quartiles. Participants in the highest wGRS quartile had a 5.13-fold (95% confidence interval: 3.21–8.21) higher odds of PA compared to those in the lowest quartile. Although the test for interaction was not significant, the odds of PA was substantially elevated for preeclamptics with the highest wGRS quartile (OR=14.44, 95%CI: 6.62–31.53) compared to normotensive women in the lowest wGRS quartile.
Discussion
Genetic variants in circadian rhythm genes may be associated with PA risk. Larger studies are needed to corroborate these findings and to further elucidate the pathogenesis of this important obstetrical complication.
1. Introduction
Placental abruption (PA), the premature separation of the placenta, is a life threatening obstetrical condition that complicates approximately 1% of all pregnancies. Pathophysiologic mechanisms involved in PA include utero-placental ischemia, underperfusion, oxidative stress, chronic hypoxia, and infarctions. On this basis, investigators have begun to conceptualize abruption as an “ischemic placental disorder” characterized by acute and chronic pathophysiological features [1]. As a multi-factorial disorder of complex origin, PA aggregates in families of women with the condition [2], suggesting a strong role for genetic predisposition, a thesis supported by a number of candidate gene studies [3–4]. Findings from recent PA-related genome-wide association studies (GWAS) and candidate gene association studies (mitochondrial biogenesis and oxidative phosphorylation pathway genes) in the maternal genome by our group provided suggestive evidence supporting associations of variation in maternal cardio-metabolic genes with risk of PA [5–7]. On balance, findings from family studies suggest that the heritability of PA is approximately 16% [8]. Despite considerable effort, however, the precise genetic factors that predispose to PA remain unknown.
The circadian clock plays an important role in several aspects of female reproductive biology, including ovulation, embryonic implantation, and parturition. For example, in premenopausal women the luteinizing hormone (LH) surge generally occurs immediately prior to the start of the active period, while the onset of parturition generally occurs during inactive period as a result of the circadian secretion of the pineal hormone melatonin [9–11]. Investigators have reported that circadian rhythm disruption attributable to rotating and night shiftwork or jetlag is associated with an increase in the frequency of irregular, extended menstrual cycles, alterations in serum LH and follicle stimulating hormone (FSH) concentrations, and reduced fecundity [12–14]. However, evidence linking circadian clock-related genes to pregnancy outcomes has been inconsistent [15–16]. Notably, polymorphisms in ARNTL and NPAS2 have been associated with the risk of miscarriages [16]. Furthermore, we recently noted some evidence of diurnal circadian periodicity among PA cases [17]. Given these findings indicative of the importance of circadian rhythm disruption in reproductive biology and data suggesting genetic susceptibility factors in miscarriages and preterm delivery [15, 17], we hypothesized that genetic variations in the maternal genome, and particularly those variants in circadian clock gene pathways are associated with PA risk. Furthermore, given that available evidence suggest individual genetic variants are likely to contribute small effects and/or be weakly associated with PA, we also assessed weighted genetic risk scores to evaluate the influence of accumulation of variants in genes regulating circadian rhythms on PA risk.
2. Materials and Methods
2.1. Study Setting and Population
The current analyses were conducted using data from two case-control studies completed in the setting of the Peruvian Abruptio Placentae Epidemiology (PAPE) study group. The studies have been previously described [5, 7]. Briefly, PAPE study participants were recruited and enrolled among patients admitted for obstetrical services to the Hospital Nacional Dos de Mayo, Instituto Especializado Materno Perinatal, and Hospital Madre-Nino San Bartolome in Lima, Peru. There were two enrollment periods (August 2002 and May 2004 and September 2006 and September 2008). Study protocols are the same for the two study periods. Hospital admission and delivery logs were monitored daily to identify PA cases among new admissions to antepartum, emergency room, and labor and delivery wards of participating hospitals. PA was diagnosed based on evidence of retro-placental bleeding (fresh blood) entrapped between the decidua and the placenta or blood clots behind the placental margin and accompanied by any two of the following: (i) vaginal bleeding in late pregnancy not due to placenta previa or cervical lesions; (ii) uterine tenderness and/or abdominal pain; and, (iii) non-reassuring fetal status or fetal death. Controls were randomly selected from among pregnant women who delivered at participating hospitals during the study period and did not have a diagnosis of PA in the current pregnancy. PA cases and controls were not matched on any maternal characteristics. A total of 517 PA cases and 524 controls provided maternal blood specimens.
Ethical approval for the study (both study periods) was granted by the Institutional Review Boards (IRB) of Hospital Nacional Dos de Mayo, Instituto Especializado Materno Perinatal, Hospital Madre-Nino San Bartolome in Lima, Peru and the IRB of Swedish Medical Center, Seattle, WA. All participants provided written informed consent in accordance with the principles of the declaration of Helsinki.
2.2. Data Collection, DNA Extraction and Genotyping
Standardized structured questionnaires administered by trained research personnel were used to collect information on socio-demographic characteristics, and medical history. Medical records were reviewed to abstract information on course and outcomes of the pregnancy. The Gentra PureGene Cell kit for DNA preparations (Qiagen, Hilden, Germany) was used to extract DNA from blood specimens. Genotyping was conducted using the Illumina Cardio-MetaboChip (Illumina Inc., San Diego, CA) platform [5], a high-density custom array designed to include 217,697 SNPs that represent DNA variations at regions previously related to diseases and traits relevant to metabolic and atherosclerotic-cardiovascular endpoints [18]. During the assay manufacturing process 20,972 SNPs (9.6%) failed, resulting in 196,725 SNPs available for genotyping, downstream quality control and statistical analyses [18].
2.3. Candidate Gene, SNP Selection & Data Quality Control
For the candidate association study, 13 genes that were involved in circadian clock gene regulation (based on literature review) and a total of 119 SNPs belonging to these genes and found in the Cardio-MetaboChip were included in the candidate gene association analyses. Quality control and preprocessing were performed on the genotype data as described previously [5, 7]. A total of 470 PA cases and 473 controls with genotyping data that passed quality control tests were included in the present study.
2.4. Statistical Analysis
Univariate logistic regression model was used to estimate odds ratio (OR) and 95% confidence interval (95% CI) relating each SNP with risk of PA. For multiple testing corrections, a false discovery rate (FDR) procedure was used [19]. In multivariable analyses, we used a penalized logistic regression model to identify sets of SNPs that are jointly associated with the odds of PA [20]. The number of selected variables was guided by a penalty parameter: the larger the parameter, the smaller the selected subset. A 20-fold cross-validation approach was performed to select the penalty parameter and the value yielding the smallest prediction error was used. A group penalty approach was also used to account for the membership in a gene [21]. Furthermore, we considered a bi-level selection approach that uses a composite minimax concave penalty [22] to select candidate genes associated with PA as well as relevant SNPs within those genes. These penalized regression methods do not accommodate missing values; hence we used the BEAGLE software version 3.3.2 [23] to impute missing genotypes.
Weighted genetic risk scores (wGRS) were computed by multiplying the number of risk alleles for each locus by its associated effect size. Once the wGRS were obtained for all individuals, the subjects were categorized into four groups defined by the quartiles in the control. We fitted a logistic regression model to derive odds ratios (OR) and 95% confidence intervals (95%CI) for the odds of PA using the lowest wGRS quartile as reference group. In multivariable analyses, we evaluated linear trends in risk by treating wGRS as ordinal variables after assigning a score (i.e., 1, 2 3, and 4) to each quartile.
We also explored the possibility of a nonlinear relation of the odds of PA in relation to increasing wGRS by fitting a generalized additive model (GAM). We further examined the extent to which the association of PA with wGRS is modified by maternal preeclampsia status. For these analyses, we specified women without preeclampsia and with a wGRS in the lowest quartile as the reference group. The global test for effect modification was evaluated using a likelihood ratio test. Statistical analyses were conducted using the following software: gPLINK (version 2.050), R (version i386 3.1.2) and STATA (Version 13). As for penalized logistic regression models, the R package “grpreg” was used [22].
3. Results
Characteristics of PA cases and controls are summarized in Table 1. Table 2 presents the top 20 individual SNPs (from genes in the circadian rhythm candidate pathway) associated with PA in univariate logistic regression analyses. For example, a common SNP (minor allele frequency=23%) in the RORA gene (rs2899663) was found to be associated with a 21% reduced risk of PA (P<0.05). Using penalized logistic regression procedures, we identified 65 SNPs from among the 119 SNPs included in the circadian rhythm candidate genes pathway that were associated with PA (Table 3). In multiple logistic regression analysis that included these 65 SNPs, 10 SNPs were associated with PA and had empirical P<0.05 (Table 3).
Table 1.
Maternal Characteristics of the Placental Abruption Cases and Controls, Lima, Peru
| Characteristics | Study Groups
|
P-value2 | |||
|---|---|---|---|---|---|
| Placental Abruption (N=470) | Control Group (N=473) | ||||
| n | % | n | % | ||
| Maternal age at delivery (years)1 | 27.7 ± 6.7 | 27.8 ± 6.6 | 0.95 | ||
| Maternal age at delivery (years) | |||||
| <20 | 52 | 11.1 | 51 | 10.8 | 0.99 |
| 20–29 | 240 | 51.1 | 238 | 50.3 | |
| 30–34 | 96 | 20.4 | 98 | 20.7 | |
| ≥35 | 82 | 17.4 | 85 | 18.0 | |
| Nulliparous | 189 | 40.2 | 189 | 40.0 | 0.99 |
| Maternal education ≤high school | 339 | 72.1 | 334 | 70.6 | 0.69 |
| Single marital status | 79 | 16.8 | 63 | 13.3 | 0.15 |
| Employed during pregnancy | 207 | 44.0 | 213 | 45.0 | 0.48 |
| Planned pregnancy | 183 | 38.9 | 194 | 41.0 | 0.80 |
| No prenatal care | 66 | 14.0 | 37 | 7.8 | 0.002 |
| No prenatal vitamins | 144 | 30.6 | 141 | 29.8 | 0.95 |
| Smoked during pregnancy | 17 | 3.6 | 7 | 1.5 | 0.04 |
| Alcohol consumption during pregnancy | 26 | 5.5 | 21 | 4.4 | 0.44 |
| Preeclampsia | 129 | 27.5 | 33 | 7.0 | <0.001 |
| Pre-pregnancy body mass index (kg/m2)1 | 23.8 ± 3.8 | 23.7 ± 3.8 | 0.65 | ||
| Pre-pregnancy body mass index (kg/m2) | |||||
| lean (<18.5) | 50 | 10.6 | 56 | 11.8 | 0.11 |
| normal (18.5–24.9) | 250 | 53.2 | 267 | 56.5 | |
| overweight (25–29.9) | 104 | 22.1 | 94 | 19.9 | |
| obese (≥ 30.0) | 25 | 5.3 | 33 | 7.0 | |
| unknown | 41 | 8.7 | 23 | 4.9 | |
| Gestational age at delivery (weeks) | 35.4 ± 4.0 | 38.4 ± 2.7 | 0.001 | ||
| Stillborn delivery | 95 | 20.2 | 3 | 0.6 | <0.001 |
| Live born Infant birthweight (grams) | 2490 ± 850 | 3181 ± 655 | <0.001 | ||
p-value are from Chi-square test/Fisher’s Exact test for categorical variables and Student t test for continuous variables
Table 2.
Top 20 SNPs in univariate analyses of circadian rhythm candidate genes in relation to risk of placental abruption
| Genes | Chromosome | SNPs | Minor Allele | MAF | OR (95% CI) | Empirical p-value |
|---|---|---|---|---|---|---|
| RORA | 15 | rs341397 | A | 0.006 | 3.06 (1.21–7.74) | 0.013 |
| CRY2 | 11 | chr11:45829415 | A | 0.037 | 1.62 (1.05–2.50) | 0.028 |
| RORA | 15 | rs2899663 | G | 0.235 | 0.78 (0.63–0.98) | 0.030 |
| NPAS2 | 2 | rs17655180 | C | 0.054 | 0.64 (0.41–0.99) | 0.048 |
| CRY2 | 11 | chr11:45839844 | G | 0.063 | 0.69 (0.46–1.04) | 0.072 |
| CRY2 | 11 | chr11:45851212 | C | 0.067 | 0.70 (0.48–1.05) | 0.080 |
| CRY2 | 11 | chr11:45854227 | C | 0.103 | 0.76 (0.55–1.04) | 0.086 |
| CRY2 | 11 | chr11:45830840 | G | 0.062 | 0.70 (0.47–1.06) | 0.088 |
| CRY2 | 11 | chr11:45853653 | G | 0.073 | 0.73 (0.50–1.06) | 0.097 |
| CRY2 | 11 | chr11:45856367 | C | 0.068 | 0.73 (0.49–1.07) | 0.103 |
| ARNTL | 11 | rs12795264 | C | 0.017 | 0.50 (0.21–1.17) | 0.104 |
| CRY2 | 11 | chr11:45857667 | A | 0.060 | 0.71 (0.47–1.07) | 0.104 |
| CRY2 | 11 | chr11:45838368 | A | 0.060 | 0.71 (0.47–1.07) | 0.104 |
| CRY2 | 11 | chr11:45827319 | G | 0.060 | 0.71 (0.47–1.07) | 0.104 |
| CRY2 | 11 | chr11:45836686 | G | 0.061 | 0.72 (0.48–1.08) | 0.107 |
| RORB | 9 | rs10869412 | A | 0.325 | 1.16 (0.96–1.41) | 0.120 |
| CRY2 | 11 | chr11:45840879 | A | 0.018 | 1.62 (0.87–2.99) | 0.122 |
| RORA | 15 | rs1680446 | A | 0.019 | 0.55 (0.25–1.21) | 0.132 |
| RORA | 15 | rs340008 | A | 0.038 | 1.39 (0.90–2.16) | 0.141 |
| RORA | 15 | rs1370433 | A | 0.432 | 1.14 (0.95–1.37) | 0.155 |
MAF = Minor Allele Frequency in controls
Table 3.
Multiple logistic regression based on SNPs selected from candidate circadian rhythm genes using a bi-level selection approach
| Genes | Chromosome | SNPs | Minor Allele | MAF | OR | 95%CI | Empirical p-value |
|---|---|---|---|---|---|---|---|
| RORA | 15 | rs341397 | A | 0.006 | 7.22 | 2.39–21.73 | 0.000 |
| RORA | 15 | rs341392 | A | 0.441 | 1.31 | 1.05–1.63 | 0.017 |
| RORA | 15 | rs9788699 | G | 0.404 | 0.70 | 0.51–0.94 | 0.019 |
| RORA | 15 | rs3784610 | A | 0.377 | 1.49 | 1.07–2.08 | 0.020 |
| RORB | 9 | rs7869849 | A | 0.321 | 3.30 | 1.21–8.99 | 0.020 |
| CRY2 | 11 | chr11:45829415 | A | 0.037 | 1.72 | 1.06–2.78 | 0.028 |
| RORA | 15 | rs1680446 | A | 0.019 | 0.34 | 0.13–0.89 | 0.028 |
| NPAS2 | 2 | rs17655180 | C | 0.054 | 0.59 | 0.36–0.97 | 0.039 |
| DEC1 | 9 | rs7851481 | A | 0.037 | 2.76 | 1.04–7.33 | 0.042 |
| RORB | 9 | rs1407845 | G | 0.321 | 0.35 | 0.13–0.97 | 0.043 |
| RORA | 15 | rs11639241 | C | 0.179 | 1.60 | 0.96–2.69 | 0.072 |
| RORA | 15 | rs6494217 | A | 0.042 | 1.61 | 0.95–2.72 | 0.077 |
| RORA | 15 | rs340025 | A | 0.401 | 1.30 | 0.97–1.75 | 0.083 |
| RORA | 15 | rs7168782 | A | 0.080 | 0.68 | 0.44–1.06 | 0.087 |
| RORA | 15 | rs2899663 | G | 0.235 | 0.80 | 0.61–1.04 | 0.088 |
| RORB | 9 | rs10781238 | A | 0.287 | 1.28 | 0.96–1.71 | 0.096 |
| RORA | 15 | rs4638514 | A | 0.206 | 1.28 | 0.93–1.75 | 0.132 |
| NPAS2 | 2 | rs3820785 | G | 0.057 | 1.37 | 0.91–2.08 | 0.135 |
| RORA | 15 | rs1533940 | G | 0.177 | 1.25 | 0.91–1.71 | 0.164 |
| RORA | 15 | rs1370431 | G | 0.412 | 1.25 | 0.81–1.71 | 0.176 |
| RORA | 15 | rs8033552 | A | 0.344 | 0.84 | 0.65–1.08 | 0.181 |
| PER2 | 2 | rs10462023 | A | 0.304 | 0.87 | 0.70–1.07 | 0.188 |
| RORB | 9 | rs10869412 | A | 0.325 | 1.30 | 0.87–1.96 | 0.202 |
| DEC1 | 9 | rs10120799 | A | 0.029 | 0.48 | 0.16–1.48 | 0.203 |
| PER2 | 2 | rs2304673 | C | 0.108 | 0.82 | 0.58–1.14 | 0.233 |
| CLOCK | 4 | rs3805148 | A | 0.408 | 1.14 | 0.92–1.40 | 0.235 |
| RORA | 15 | rs8036966 | C | 0.178 | 1.26 | 0.86–1.85 | 0.240 |
| CSNK1E | 22 | rs1534891 | A | 0.018 | 1.50 | 0.75–2.97 | 0.248 |
| PER3 | 1 | rs228641 | A | 0.014 | 0.57 | 0.20–1.59 | 0.281 |
| RORA | 15 | rs17270188 | G | 0.362 | 0.88 | 0.68–1.12 | 0.295 |
| ARNTL | 11 | rs12795264 | C | 0.017 | 0.61 | 0.24–1.55 | 0.299 |
| RORA | 15 | rs12908671 | A | 0.023 | 1.39 | 0.71–2.75 | 0.341 |
| CRY2 | 11 | chr11:45854227 | C | 0.103 | 0.82 | 0.55–1.23 | 0.342 |
| Genes | Chromosome | SNPs | Minor Allele | MAF | OR | 95%CI | p-value |
|---|---|---|---|---|---|---|---|
| RORA | 15 | rs17303530 | C | 0.297 | 0.80 | 0.50–1.28 | 0.344 |
| CRY2 | 11 | chr11:45840879 | A | 0.018 | 1.46 | 0.66–3.22 | 0.350 |
| ARNTL | 11 | rs2290037 | G | 0.050 | 1.22 | 0.80–1.87 | 0.359 |
| RORA | 15 | rs8042228 | A | 0.430 | 0.91 | 0.74–1.12 | 0.385 |
| RORA | 15 | rs8041381 | G | 0.190 | 0.86 | 0.58–1.26 | 0.425 |
| RORA | 15 | rs12594188 | A | 0.362 | 1.10 | 0.87–1.39 | 0.443 |
| NPAS2 | 2 | rs356651 | G | 0.022 | 1.28 | 0.68–2.41 | 0.450 |
| ARNTL2 | 12 | rs1562048 | A | 0.084 | 0.85 | 0.55–1.30 | 0.453 |
| CRY1 | 12 | rs12368868 | G | 0.456 | 0.93 | 0.76–1.14 | 0.471 |
| CRY1 | 12 | rs8192440 | A | 0.084 | 0.87 | 0.60–1.27 | 0.474 |
| RORA | 15 | rs340008 | A | 0.038 | 1.23 | 0.69–2.18 | 0.480 |
| RORA | 15 | rs341408 | A | 0.423 | 1.07 | 0.88–1.32 | 0.494 |
| CRY2 | 11 | chr11:45831968 | T | 0.427 | 1.10 | 0.82–1.46 | 0.523 |
| RORA | 15 | rs7163680 | C | 0.222 | 0.83 | 0.46–1.50 | 0.530 |
| RORC | 1 | rs3790515 | A | 0.078 | 0.89 | 0.62–1.28 | 0.533 |
| PER3 | 1 | rs875994 | G | 0.267 | 1.07 | 0.86–1.34 | 0.554 |
| PER3 | 1 | rs228654 | A | 0.039 | 1.15 | 0.70–1.91 | 0.581 |
| RORA | 15 | rs782958 | A | 0.220 | 0.93 | 0.72–1.21 | 0.596 |
| RORA | 15 | rs12903172 | G | 0.293 | 1.07 | 0.84–1.36 | 0.600 |
| RORB | 9 | rs4098048 | A | 0.439 | 1.09 | 0.78–1.51 | 0.630 |
| ARNTL2 | 12 | rs16931937 | G | 0.020 | 0.82 | 0.35–1.88 | 0.634 |
| RORA | 15 | rs17237810 | G | 0.093 | 1.08 | 0.76–1.53 | 0.660 |
| RORA | 15 | rs7176774 | A | 0.371 | 0.94 | 0.72–1.24 | 0.676 |
| CRY2 | 11 | chr11:45849748 | G | 0.024 | 0.83 | 0.33–2.12 | 0.701 |
| RORA | 15 | rs17270362 | A | 0.070 | 0.92 | 0.59–1.42 | 0.701 |
| CRY2 | 11 | rs12281674 | G | 0.244 | 1.06 | 0.78–1.45 | 0.703 |
| RORA | 15 | rs1370433 | A | 0.432 | 1.10 | 0.67–1.79 | 0.704 |
| RORB | 9 | rs10781235 | A | 0.266 | 0.94 | 0.65–1.36 | 0.733 |
| DEC1 | 9 | rs1414141 | G | 0.467 | 1.06 | 0.75–1.51 | 0.737 |
| RORA | 15 | rs930359 | G | 0.049 | 0.91 | 0.52–1.60 | 0.738 |
| CRY2 | 11 | chr11:45835169 | A | 0.030 | 0.90 | 0.41–1.94 | 0.781 |
| DEC1 | 9 | rs2183700 | A | 0.388 | 1.03 | 0.72–1.47 | 0.883 |
MAF = Minor Allele Frequency in controls
We next computed circadian rhythm candidate genes weighted genetic risk scores (herein after referred to as wGRS) using the 65 SNPs. Median wGRS were higher for PA cases compared to controls (P<0.001). As shown in Table 4, the odds of PA increased across each successive quartile of wGRS (Ptrend <0.001). Multivariable-adjusted ORs for PA were 1.00, 1.83, 2.81 and 5.13 across successive quartiles of wGRS. Furthermore, we noted that women with very high wGRS (i.e., those with a wGRS ≥25.10, the upper decile) had a 6.97-fold (95%CI 4.10–11.85) increased risk of PA as compared with women who had a wGRS <23.80 (i.e., the lowest quartile). We also explored the possibility of a nonlinear relation of wGRS with PA using regression procedures based on a generalized additive model (GAM). The results (Figure 1) indicate increasing odds of PA with increasing wGRS. When we modeled the log odds of PA in relation to each increasing unit of the wGRS (expressed as a continuous variable in a logistic regression model), we noted that a 1-unit increase in the wGRS was associated with a 2.17-fold increased odds of PA (OR=2.17; 95%CI 1.75–2.69) after adjusting for maternal age, smoking during pregnancy, preeclampsia status and gestational age at delivery.
Table 4.
Odds ratio (OR) and 95% confidence interval (CI) for placental abruption in relation to categories of weighted genetic risk score (wGRS) computed from candidate circadian rhythm genes SNPs selected in multivariable analyses
| Circadian Rhythm Genes Weighted Genetic Risk Score (wGRS) | Median of WGRS | Placental Abruption (N=470) | Controls (N=473) | Unadjusted OR (95%CI) | Adjusted OR* (95%CI) |
|---|---|---|---|---|---|
|
| |||||
| n (%) | n (%) | ||||
| Quartile 1 (<23.80) | 23.4 | 45 (9.6) | 118 (25.0) | 1.00 (referent) | 1.00 (referent) |
| Quartile 2 (23.81–24.29) | 24.1 | 84 (17.9) | 119 (25.2) | 1.85 (1.19–2.88) | 1.83 (1.10–3.06) |
| Quartile 3 (24.30–24.74) | 24.5 | 116 (24.7) | 117 (24.7) | 2.60 (1.69–3.99) | 2.81 (1.71–4.60) |
| Quartile 4 (≥24.75) | 25.1 | 225 (47.9) | 119 (25.2) | 4.96 (3.29–7.46) | 5.13 (3.21–8.21) |
| P-value for linear trend | <0.001 | <0.001 | |||
| Quartile 1 (<23.80) | 23.4 | 45 (9.6) | 118 (25.0) | 1.00 (referent) | 1.00 (referent) |
| ≥90% decile (≥25.10) | 25.4 | 132 (28.1) | 49 (10.4) | 7.06 (4.39–11.36) | 6.97 (4.10–11.85) |
Adjusted for maternal age, smoking during pregnancy, preeclampsia status and gestational age at delivery
Figure 1.
Relation between weighted genetic risk score (wGRS) computed from selected SNPS in candidate circadian rhythm genes and risk of placental abruption (solid line) with 95% confidence interval (shaded area). Model included the following covariates: maternal age, smoking status, preeclampsia status and gestational age at delivery.
When we stratified PA cases according to preterm (N=250) and term (N=220) delivery, we observed similar patterns of increasing odds of PA with successive quartiles of wGRS for pregnancies ending at term (1.00, 1.80, 2.56, and 4.74) (Ptrend <0.001) and for those pregnancies ending preterm (1.00, 2.13, 3.79, and 6.17) (Ptrend <0.001) (data not shown).
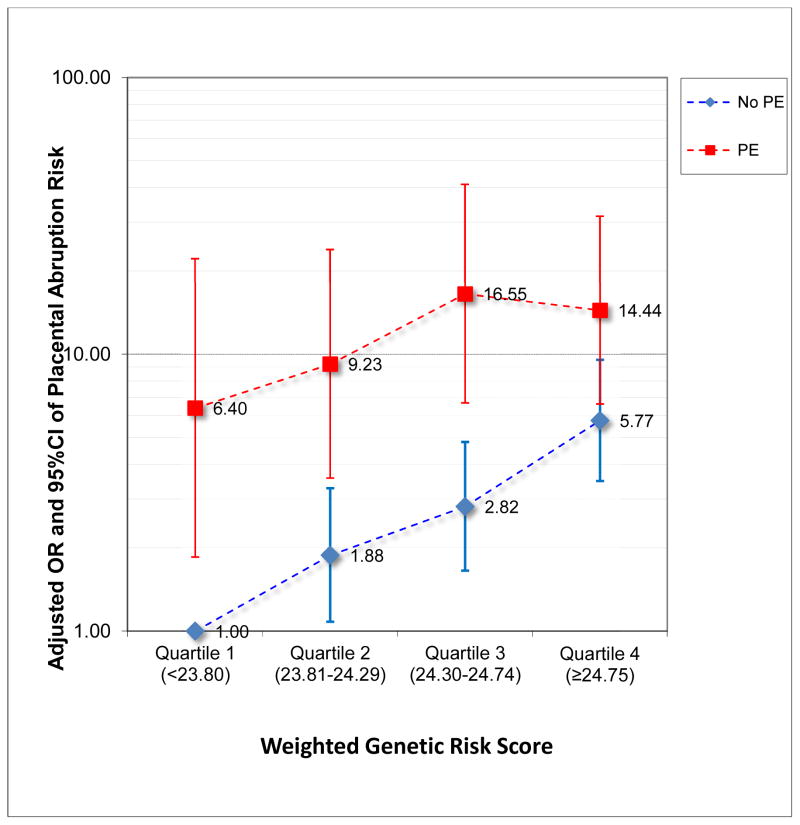
Given that preeclampsia is associated with an increased risk of PA, we also explored the extent to which the association between wGRS and PA was modified by preeclampsia (Figure 2). In this exploratory analysis, the reference group was defined as women without preeclampsia and with a wGRS in the lowest quartile. Relative to the reference group, preeclamptics with a wGRS in the first quartile had a 6.40-fold increased odds of PA (95%CI 1.85–22.13). Among preeclamptics, the odds of PA were 9.23, 16.55 and 14.44 for successive quartiles of WGRS when compared with normotensive women in the lowest quartile of the wGRS. However, our exploratory analysis yielded no evidence of statistical significance. Specifically, the p-value for the global test for effect modification by preeclampsia was 0.376.
Figure 2.
Odds ratios (OR) and 95% confidence intervals (95%CI) for placental abruption risk in relation to quartiles of weighted genetic risk score (wGRS) computed from selected SNPS in candidate circadian rhythm genes and according to preeclampsia (PE) status. Normotensive women with WGRS in the lowest quartile served as the single common reference group.
4. Discussion
Pregnancy is a complex well-regulated temporal event in which several metabolic and developmental milestones, including implantation, decidualization, placentation, and parturition, are finely orchestrated [24]. Alterations in these finely orchestrated events have been shown to have serious effects on fetal and maternal health. For example, alterations in maternal circadian rhythm secondary to engagement in shiftwork have been associated with increased risks of preterm delivery, intrauterine growth restriction and preeclampsia [25]. Furthermore, accumulating evidence indicate that the placenta may have a functional circadian system and that clock genes including BAMl1, PER1–2 and CRY1–2 are expressed in the feto-placental compartment [26]. Using the largest available GWAS dataset of PA cases and controls, we observed that a common SNP in the RORA gene (rs2899663) was associated with a 21% reduced odds of PA (P<0.05). By combining risk variants at 13 genes (65 SNPs) into a single wGRS, we documented the cumulative association of circadian rhythm related gene variants on the susceptibility of PA.
Circadian rhythms, known to be heritable [27–28], modulate human behavior, physiology and the timing of several neurological, medical and obstetric disorders including hemorrhagic and ischemic stroke [29], myocardial infarction [30], preeclampsia [31] preterm labor [15] and PA [17]. A conserved genetic network regulates circadian rhythms. At the core of this network is a transcription-translation feedback loop formed by the genes PER1–3, CRY1–2, BMAL1, and CLOCK that generates near 24-hour rhythmicity [32]. Mutations in these genes lead to alterations in or loss of circadian rhythmicity [32].
Variants in PER2 and CSNK1D, a PER2 kinase, have been implicated in select pedigrees with extreme circadian misalignment [33–34]. However, these are rare variants and their significance at the population level remains unclear. Notably, more common variants in PER1, PER3, and CLOCK have been associated with circadian preference [35–37]. To our knowledge, we are the first investigative team to document possible associations of variants in circadian rhythm-related genes with the occurrence of PA. The clinical implications of these findings, if confirmed are multifold. First, confirmation of our findings in independent dataset may serve to reinforce the role of sleep and rhythm disorders in the pathogenesis of PA and other reproductive disorders. Second, confirmation of our findings and the accumulation of findings from others [12, 25, 31–32] may motivate the development of behavioral and pharmacologic strategies to improve sleep and circadian organization of obstetric patients. Lastly, integration of genetic risk scores alone or in combination with other risk factors may improve clinical risk stratification and risk prediction efforts. However, the clinical utility of such risk prediction algorithms remain to be tested.
In our study the top 10 SNPs hits associated with PA risk were: rs341397 (RORA), rs341392 (RORA), rs9788699 (RORA), rs3784610 (RORA), rs7869849 (RORB), chr11: 45829415 (CRY2), rs1680446 (RORA), rs17655180 (NPAS2), rs7851481 (DEC1) and rs1407845 (RORB). The top 10 SNPs represented five known genes including RORA, CRY2, NPAS2, DEC1 and RORB. The RORA gene encodes RAR-related orphan receptor A, which is a member of the NR1 subfamily of nuclear hormone receptors. RORA has been identified as a novel candidate gene for autism spectrum disorders [38]. Two SNPs in RORA (rs1482057 and rs12914272) were found to be associated with breast cancer risk, possibly due to the interaction with reproductive hormones [39]. The CRY2 gene encodes cryptochrome circadian clock 2, a flavin adenine dinucleotide-binding protein that is a key component of the circadian core oscillator complex, which regulates the circadian clock. The CRY2 gene is up-regulated by CLOCK/ARNTL heterodimers but then represses this up-regulation in a feedback loop using PER/CRY heterodimers to interact with CLOCK/ARNTL. Of note, altered expression level of CRY2 gene is found in pregnancy complications including gestational diabetes mellitus [40]. Furthermore, based on the epidemiological literature documenting the co-occurrence of preeclampsia with PA, and evidence linking circadian misalignment with preeclampsia risk [31], we conducted exploratory analyses to determine the extent to which, if at all, the association of the wGRS with PA was modified by maternal preeclampsia status. Although we observed some evidence suggestive of possible effect modification the global test did not reach statistical significance; and may be attributable to insufficient statistical power.
The NPAS2 gene (a CLOCK homolog) encodes neuronal PAS domain protein 2, which is a member of the basic helix-loop-helix (bHLH)-PAS family of transcription factors. It is thought that the gene may function as a part of a molecular clock operative in the mammalian forebrain. Recent findings have shown that a polymorphism (rs11673746) in NPAS2 may be associated with decreased incidence of miscarriage [16]. Additionally, Frigato and colleagues documented circadian expression of the DEC1 gene in cells derived from human first-trimester trophoblast and suggested that circadian rhythm-regulating gene may orchestrate the functionality of several factors involved in the control of human trophoblast functions that are fundamental for pregnancy and parturition [41]. On balance, these studies and findings from animal studies which documented contributions of the uterine clock in the processes of implantation, fetal development and parturition [26, 42–43] underscore the biological plausibility of the cumulative association of circadian rhythm related gene variants on the susceptibility of PA in our epidemiological study.
Our study has some strength that deserves mention. First, we used multiple analytical approaches (candidate gene and genetic risk score) to assess the extent to which there is a genetic susceptibility for PA risk. Second, our analysis has drawn attention to genes and gene networks that control circadian rhythm which are increasingly recognized as being important regulators of human behavior, physiology and pathophysiology [44]. Third, our study was conducted among a high-risk population with relatively little population stratification [7].
Despite these strengths, our findings must be interpreted with some caution. First, we used the same dataset to estimate the wGRS and test their association with PA. The validity of our wGRS will have to be examined in an independent study population [45]. Second, although our study is the largest to date on the topic, we remain cautious that it may be underpowered to detect statistically significant associations between rare variants and PA risk. Third, we used a research operational definition of placental abruption which may have led to some misclassification. For instance, sub-clinical cases of placental abruption (i.e., those not presenting with abnormal vaginal bleeding) may be missed or misclassified among controls. Fourth, we did not collect information pertaining to maternal shiftwork and sleep traits in the current study; hence we were unable to evaluate possible effect modification by these important covariates. Absence of a replication study population and lack of follow-up functional studies are other limitations. Finally, the generalizability of our findings should be confirmed in studies that are conducted in other geographically and ethnically diverse study populations.
5. Conclusions
In conclusion, genetic variants in circadian rhythm genes may contribute to the pathogenesis of PA. Larger molecular epidemiology studies are needed to confirm these findings and to further elucidate the pathogenesis of this important clinical complication of pregnancy. Increased understanding of the role biological rhythms play in human reproduction and pregnancy may provide important opportunities for clinical risk stratification that may enhance the precision of clinical obstetric risk management and disease control and prevention protocols.
Highlights.
Genetic variations in circadian clock gene pathways are associated with risk of placental abruption (candidate gene and weighted genetic risk score).
The weighted genetic risk score (wGRS) did not appear to influence the timing of placental abruption, as associations of similar directions and magnitudes were observed for preterm and term abruptions.
There was some suggestive evidence of an effect modification between wGRS and preeclampsia such that the wGRS appeared to have a greater than additive effect size in preeclamptics. However, that the global test for effect modification did not reach statistical significance.
Acknowledgments
This study was supported by grants from the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD059827).
Footnotes
Author Contributions
Conceived and designed the study: CQ BG MAW. Analyzed the data: CQ MD. Contributed to the writing of the manuscript: CQ, BG, MD, MGT, MALF, DAG, CVA, SES and MAW.
Conflict of Interests
There are no known conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ananth CV, Vintzileos AM. Medically indicated preterm birth: recognizing the importance of the problem. Clin Perinatol. 2008;35:53–67. doi: 10.1016/j.clp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Toivonen S, Keski-Nisula L, Saarikoski S, Heinonen S. Risk of placental abruption in first-degree relatives of index patients. Clin Genet. 2004;66:244–246. doi: 10.1111/j.1399-0004.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- 3.Jaaskelainen E, Keski-Nisula L, Toivonen S, et al. Polymorphism of the interleukin 1 receptor antagonist (IL1Ra) gene and placental abruption. J Reprod Immunol. 2008;79:58–62. doi: 10.1016/j.jri.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Zdoukopoulos N, Zintzaras E. Genetic risk factors for placental abruption: a HuGE review and meta-analysis. Epidemiology. 2008;19:309–323. doi: 10.1097/EDE.0b013e3181635694. [DOI] [PubMed] [Google Scholar]
- 5.Moore A, Enquobahrie DA, Sanchez SE, et al. A genome-wide association study of variations in maternal cardiometabolic genes and risk of placental abruption. Int J Mol Epidemiol Genet. 2012;3:305–313. [PMC free article] [PubMed] [Google Scholar]
- 6.Workalemahu T, Enquobahrie DA, Moore A, et al. Genome-wide and candidate gene association studies of placental abruption. Int J Mol Epidemiol Genet. 2013;4:128–139. [PMC free article] [PubMed] [Google Scholar]
- 7.Denis M, Enquobahrie DA, Tadesse MG, et al. Placental genome and maternal-placental genetic interactions: a genome-wide andcandidate gene association study of placental abruption. PLoS One. 2014;9:e116346. doi: 10.1371/journal.pone.0116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen SI, Irgens LM. Occurrence of placental abruption in relatives. BJOG. 2009;116:693–699. doi: 10.1111/j.1471-0528.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- 9.Olcese J. Circadian aspects of mammalian parturition: a review. Mol Cell Endocrinol. 2012;349:62–71. doi: 10.1016/j.mce.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010:813764. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurminen T. Shift work and reproductive health. Scand J Work Environ Health. 1998;24(Suppl 3):28–34. [PubMed] [Google Scholar]
- 13.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Lawson CC, Whelan EA, Lividoti Hibert EN, Spiegelman D, Schernhammer ES, Rich-Edwards JW. Rotating shift work and menstrual cycle characteristics. Epidemiology. 2011;22:305–312. doi: 10.1097/EDE.0b013e3182130016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindow SW, Jha RR, Thompson JW. 24 hour rhythm to the onset of preterm labour. BJOG. 2000;107:1145, e8. doi: 10.1111/j.1471-0528.2000.tb11114.x. [DOI] [PubMed] [Google Scholar]
- 16.Kovanen L, Saarikoski ST, Aromaa A, Lonnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One. 2010;5:e10007. doi: 10.1371/journal.pone.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luque-Fernandez MA, Ananth CV, Sanchez SE, et al. Absence of circadian rhythms of preterm premature rupture of membranes and preterm placental abruption. Ann Epidemiol. 2014;24:882–887. doi: 10.1016/j.annepidem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voight BF, Kang HM, Ding J, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet. 2012;8:e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing Under Dependency. Ann Statist. 2001;29:1165–1188. [Google Scholar]
- 20.Tibshirani R. Regression shrinkage and selection via the lasso. J Roy Stat Soc B. 1996;58:267–288. [Google Scholar]
- 21.Zhou H, Sehl ME, Sinsheimer JS, Lange K. Association screening of common and rare genetic variants by penalized regression. Bioinformatics. 2010;26:2375–2382. doi: 10.1093/bioinformatics/btq448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breheny P, Huang J. Penalized methods for bi-level variable selection. Statistics and its interface. 2009;2:369–380. doi: 10.4310/sii.2009.v2.n3.a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing data inference for whole genome association studies using localized haplotype clustering. Am J Hum Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha J, Dey SK. Cadence of procreation: orchestrating embryo-uterine interactions. Semin Cell Dev Biol. 2014;34:56–64. doi: 10.1016/j.semcdb.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: a systematic review with meta-analysis of currently available epidemiological studies. BJOG. 2011;118:1429–1437. doi: 10.1111/j.1471-0528.2011.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waddell BJ, Wharfe MD, Crew RC, Mark PJ. A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta. 2012;33:533–539. doi: 10.1016/j.placenta.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Evans DS, Snitker S, Wu SH, et al. Habitual sleep/wake patterns in the Old Order Amish: heritability and association with non-genetic factors. Sleep. 2011;34:661–669. doi: 10.1093/sleep/34.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barclay NL, Eley TC, Buysse DJ, et al. Diurnal preference and sleep quality: same genes? A study of young adult twins. Chronobiol Int. 2010;27:278–296. doi: 10.3109/07420521003663801. [DOI] [PubMed] [Google Scholar]
- 29.Casetta I, Granieri E, Portaluppi F, Manfredini R. Circadian variability in hemorrhagic stroke. JAMA. 2002;287:1266–1267. doi: 10.1001/jama.287.10.1266. [DOI] [PubMed] [Google Scholar]
- 30.Holmes DR, Jr, Aguirre FV, Aplin R, et al. Circadian rhythms in patients with ST-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2010;3:382–389. doi: 10.1161/CIRCOUTCOMES.109.913343. [DOI] [PubMed] [Google Scholar]
- 31.Ditisheim AJ, Dibner C, Philippe J, Pechere-Bertschi A. Biological rhythms and preeclampsia. Front Endocrinol (Lausanne) 2013;4:47. doi: 10.3389/fendo.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–765. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 34.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 35.Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 36.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 37.Carpen JD, Archer SN, Skene DJ, et al. A single-nucleotide polymorphism in the 5′-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005;14:293–297. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 38.Hu VW, Sarachana T, Kim KS, et al. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009;2:78–97. doi: 10.1002/aur.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truong T, Liquet B, Menegaux F, et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr Relat Cancer. 2014;21:629–638. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- 40.Pappa KI, Gazouli M, Anastasiou E, Iliodromiti Z, Antsaklis A, Anagnou NP. Circadian clock gene expression is impaired in gestational diabetes mellitus. Gynecol Endocrinol. 2013;29:331–335. doi: 10.3109/09513590.2012.743018. [DOI] [PubMed] [Google Scholar]
- 41.Frigato E, Lunghi L, Ferretti ME, Biondi C, Bertolucci C. Evidence for circadian rhythms in human trophoblast cell line that persist in hypoxia. Biochem Biophys Res Commun. 2009;378:108–111. doi: 10.1016/j.bbrc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Johnson MH, Lim A, Fernando D, Day ML. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod Biomed Online. 2002;4:140–145. doi: 10.1016/s1472-6483(10)61931-1. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura TJ, Sellix MT, Kudo T, et al. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids. 2010;75:203–212. doi: 10.1016/j.steroids.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim AS, Chang AM, Shulman JM, et al. A common polymorphism near PER1 and the timing of human behavioral rhythms. Ann Neurol. 2012;72:324–334. doi: 10.1002/ana.23636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomsen TF, McGee D, Davidsen M, Jorgensen T. A cross-validation of risk-scores for coronary heart disease mortality based on data from the Glostrup Population Studies and Framingham Heart Study. Int J Epidemiol. 2002;31:817–822. doi: 10.1093/ije/31.4.817. [DOI] [PubMed] [Google Scholar]