Abstract
Objective
Evaluate the performance of glycated albumin (GA) monitoring by comparing it to other measures of glycemic control during intensification of antidiabetic therapy.
Methods
This 12-week, prospective, multicenter study compared the diagnostic clinical performance of GA to glycated hemoglobin A1C (A1C), fructosamine corrected for albumin (FRA), fasting plasma glucose (FPG), and mean blood glucose (MBG) estimated from self-monitoring of blood glucose (SMBG) and continuous glucose monitoring (CGM) in 30 patients with suboptimally controlled type 1 or 2 diabetes.
Results
Mean A1C decreased from 9.5% to 8.1%. Mean SMBG correlated closely with CGM (Pearson r = 0.783 for daily estimates and r = 0.746 for weekly estimates, P<.0001). Both GA and FRA levels significantly correlated with changes from baseline in A1C and mean weekly SMBG (P<.001).The lowest observed median GA occurred at 4 weeks, followed by a small increase and then a slight reduction, mirroring changes in overall mean SMBG values. The median A1C fell throughout the treatment period, failing to reflect short-term changes in SMBG. A ≥1% reduction in GA at 4 weeks was significantly associated with a ≥0.5% change in A1C at 12 weeks (odds ratio [OR] = 19.0, 95% confidence interval [CI]: 1.4, 944, P = .018).
Conclusion
In patients receiving glucose-lowering therapy, changes in GA at 4 weeks were concordant with changes in A1C at 12 weeks, and both GA and FRA more accurately reflected short-term blood glucose fluctuations than A1C.
INTRODUCTION
Glycated hemoglobin A1C (A1C) and self-monitoring of blood glucose (SMBG) are critical to guide diabetes management but leave gaps in the data necessary for clinical decision making. As a reflection of glycemia over 2 to 3 months, A1C cannot be used to detect daily glycemic excursions; may not capture short-term responses to therapy; and may be affected by variant hemoglobins, anemia, and other medical conditions affecting erythrocyte survival (1,2). SMBG is limited by patients’ willingness to test frequently, as well as day-to-day variability. The cost and inconvenience of continuous glucose monitoring (CGM) limit wider application of this technology (1,3,4), while fructosamine is not yet widely used (1,5).
Glycated albumin (GA) measures the glycation of serum albumin, a protein with a half-life of approximately 14 days, representing an intermediate measure between A1C and SMBG (1). The Lucica® GA-L test is an enzymatic assay in which endogenous glycated amino acids and peroxide are eliminated by a ketoamine oxidase and peroxidase reaction. The GA is then hydrolyzed to amino acids or peptides by an albumin-specific proteinase and quantitatively measured. GA concentrations are presented as a percentage of total albumin measured in the same serum sample, minimizing effects of variations among individuals and in albumin concentrations (6,7).
Practicing clinicians often wait up to 3 months for changes in A1C or rely on SMBG alone to make treatment decisions. Physicians might find a measure of glycemic control with an intermediate time frame a useful tool for earlier evaluation of new treatment strategies. This rigorous study of the Lucica® GA-L assay was designed with this goal in mind. In a study population undergoing diabetes treatment modification to improve glycemic control, we compared the glucose assessment performance of GA to that of A1C, mean blood glucose (MBG), CGM, and fructosamine to evaluate the potential clinical utility of GA monitoring.
METHODS
Study Design
This was a prospective, multicenter, comparative study of diagnostic clinical performance sponsored by Asahi Kasei Pharma (Tokyo, Japan). It was conducted by an independent contract research organization (Medpace, Cincinnati, OH) under the direction of study investigators. Enrolled participants were seen at 1 of 3 study centers (Tulane University Hospital; Dallas Diabetes and Endocrine Center; University of Nebraska Medical Center, Division of Diabetes, Endocrinology, and Metabolism) for 15 weeks (1-3 weeks for the screening period plus 12 weeks for the main study period). All study participants provided informed consent, and this low-risk study conformed with institutional review board requirements. Conventional practices used at study sites for the routine monitoring of blood glucose levels using SMBG were followed by enrollees and investigators during the study. All GA measurements were evaluated in a central laboratory and were not available to study investigators until after completion of the trial. Study management, data collection, and statistical analyses were performed by an independent contract research organization (Medpace).
Patients
Eligible participants were males or females ≥18 years of age with either type 1 or 2 diabetes. Patients were enrolled only if their A1C was ≥7.5% and their therapeutic regimen was being changed to improve glycemic control. Treatment could include oral antidiabetic agents, insulin, or noninsulin injectable antidiabetic medications given at the investigator’s discretion. Willingness to complete the protocol requirements including the use of SMBG and CGM devices and attendance at all scheduled study visits were required for inclusion. Finally, enrolled participants had to demonstrate their ability to complete home SMBG measurements during the study screening period.
Patients were excluded if they had any clinically significant disease that would interfere with study evaluations or ongoing treatment for other medical conditions, including chronic kidney or end-stage renal disease, liver cirrhosis, uncontrolled thyroid disease, anemia, a known hemoglobinopathy, or any other acute or chronic condition that might significantly influence albumin or glucose metabolism. Patients using steroid medications, who were pregnant or breast feeding, or who had had a blood transfusion within 6 months of study entry were also ineligible.
Assessments
During the study period, fasting blood samples drawn at weeks 1, 2, 3, 4, 6, 8, and 12 were tested for GA, fasting plasma glucose (FPG), A1C, and fructosamine. Throughout the study, participants conducted routine SMBG measurements using a blood glucose meter with memory capabilities (OneTouch® Ultra® 2 Blood Glucose Meter; LifeScan, Inc, Milpitas, CA). Meters used during the trial were supplied by the investigators and were cleared by the Food and Drug Administration. Participants were also instructed to use their SMBG device 6 to 7 times at least 1 day per week, collecting 3 preprandial, 3 postprandial, and 1 bedtime measurement. During weeks when a study visit was scheduled, patients were asked to conduct the 7-point SMBG measurements the day before the scheduled study visit. Patients unable to conduct SMBG on that day were asked to measure SMBG earlier in the week; those who failed to bring SMBG data to a scheduled visit were asked to conduct SMBG the following day. Participants also used a CGM device (Dexcom G4™ PLATINUM Continuous Glucose Monitoring System; Dexcom) beginning at enrollment (Visit 2) and continuing for at least 2 weeks, but with a target duration of 4 weeks (i.e., through Visit 6). CGM devices were initiated and replaced by healthcare providers at each study center. For the purposes of CGM device calibration during this period, participants were asked to use the SMBG device at least twice daily. Patients were masked to the CGM data during the 2- to 4-week period they wore the device, but at the next study visit they received a printout of the results.
Participants attended each study visit in the fasting state, and the following data were collected: vital signs (blood pressure, heart rate); whole blood, serum, and plasma for assaying FPG, fructosamine, GA, and A1C; SMBG data (downloaded from meter); and CGM data between visit 2 (week 0) and visit 6 (week 4). All samples were analyzed at a central laboratory. The GA value was measured using a Roche/Hitachi Modular P instrument (Roche, Basel, Switzerland; Hitachi, Tokyo, Japan) and determined using the Lucica® GA-L assay. FPG and fructosamine were determined using glucose and fructosamine reagents manufactured by Roche and analyzed on a Synchron® system (Beckman Coulter, Brea, CA). A1C was determined using the G7 and G8 HPLC Analyzers manufactured by Tosoh Bioscience Inc (Tokyo, Japan). The coefficients of variation for these reagents and instruments were <2%. The fructosamine value was analyzed as the absolute fructosamine value and fructosamine corrected for albumin (FRA) by dividing the total concentration by the albumin concentration according to standard methods. FRA values were used in the comparative analyses.
Medications and adverse events were also recorded at each visit. At the last visit on week 12 (visit 9), a full physical examination and patient survey were conducted, along with assessment of body mass index (BMI), complete blood count, comprehensive metabolic profile, urine microalbumin, and urine creatinine.
Statistical Analysis
The study was prospectively designed to collect data on 30 participants beginning a standard monitoring and treatment program to reduce blood glucose levels. Although endpoints were prespecified, statistical analyses were intended to be exploratory in nature and not powered to detect statistical significance. The safety population consisted of all study participants who enrolled in the study and had any measured GA values or other glycemic indices and/or safety data after enrollment (visit 2 [week 0]). All statistical summaries and analyses of the clinical data were based on the safety population.
Descriptive statistics were used to summarize all study results. Analyses of continuous variables are presented by sample size, mean, SD, median, minimum, and maximum. Analyses of categorical variables were summarized with counts and percentages. Individual participant data were tabulated within listings. Data not available because of withdrawals were considered missing and not imputed.
MBG was estimated by 2 methods based on SMBG data downloaded from each participant’s device: Method 1 was based on the average area under the curve (AUC) for data obtained through the multiple readings taken on the day before (or after) each scheduled study visit, and Method 2 was based on the weekly average of readings taken during successive 7-day intervals between study visits. MBG was also estimated from the measurements obtained by and downloaded from participants’ CGM devices.
Since SMBG and CGM could provide direct, paired comparisons of estimated blood glucose levels, the observed differences in readings were summarized and statistically compared using paired Student’s t tests. The daily, weekly, and between-visit estimates of MBG levels also were summarized and compared between blood glucose monitoring methods using repeated measures analyses of variance (ANOVAs). MBG determined using Methods 1 (daily) and 2 (weekly) were compared to CGM separately. The ANOVA models for the MBG contained device (SMBG [daily or weekly, separately] or CGM), diabetes type (type 1 or 2), and visit (weeks 0 through 4) as factors.
Comparisons of GA to other glycemic indices were evaluated both at the individual patient level and for the total patient population. GA was compared with MBG (from both SMBG and CGM) to determine the correlations of changes from baseline. The correlations between changes in GA and changes in A1C, FPG, FRA, and MBG (determined by daily and weekly SMBG and CGM) were evaluated using Pearson correlation coefficients. Comparisons between parameters within individuals were evaluated using nonparametric Wilcoxon signed-rank tests.
The amount of change in GA values during the preceding 3-month period that would be required to predict a clinically meaningful change in A1C of 0.5% during the study was estimated using linear regression methods. A change in A1C of 0.5% is generally considered clinically meaningful by diabetes specialists and has been endorsed as a benchmark of efficacy by the National Institute for Clinical Excellence in the UK, while the U.S. Food and Drug Administration has suggested a change of 0.3% may be considered clinically meaningful (8-10). The concordance of a short-term change in GA from baseline of 1% at 4 weeks to the long-term change in A1C of 0.5% at 12 weeks was examined by a Fisher exact test and estimation of the associated odds ratio (OR).
RESULTS
A total of 31 patients with diabetes enrolled in the study, with 30 patients contributing blood glucose and assay data and 29 completing follow-up requirements. One participant withdrew consent, and the other was hospitalized for a serious adverse event unrelated to the study. Table 1 summarizes baseline demographic data. Patients were evenly divided between males and females; the majority were non-Hispanic whites with a mean age of 48 years. One-third of participants had type 1 diabetes, and 71% of patients took at least 1 form of insulin (long-, intermediate-, or rapid-acting or premixed) during the study period. Metformin was used by 55% of patients, and a thiazolidinedione, a glucagon-like peptide 1 receptor agonist, a dipeptidyl peptidase 4 inhibitor, and pramlintide were each taken by 1 patient during the study. Investigators adjusted each patient’s treatment regimen at their discretion. The mean A1C was 9.5% at baseline and decreased to 8.1% after 12 weeks.
Table 1. Patient Demographics at Baseline.
| Variable | Study population (n = 31) |
|---|---|
| Age (years), mean (range) | 48 (26-65) |
| Male, n (%) | 15 (48.4) |
| Race | |
| White, n (%) | 27 (87.1) |
| Black, n (%) | 3 (9.7) |
| Native American, n (%) | 1 (3.2) |
| Ethnicity | |
| Hispanic | 11 (35.5) |
| Not Hispanic | 20 (64.5) |
| Diabetes type | |
| Type 1 | 10 (32.3) |
| Type 2 | 21 (67.7) |
| Albumin ≥3.5 g/dL, n (%) | 29 (93.5) |
| Glycemic indices | |
| A1C (%), mean ± SD | 9.5 ± 1.5 |
| FPG (mg/dL), mean ± SD | 189.2 ± 68.53 |
| MBGa (mg/dL), mean ± SD | 203.7 ± 62.13 |
| Fructosamine, (mmol/L), mean ± SD | 347.5 ± 55.06 |
| GA (%), mean ± SD | 23.39 ± 4.42 |
| Weight (kg), mean ± SD | 90.2 ± 22.68 |
| BMI (kg/m2), mean ± SD | 31.0 ± 6.31 |
Abbreviations: A1C = hemoglobin A1C; BMI = body mass index; FPG = fasting plasma glucose; GA = glycated albumin; MBG = mean blood glucose.
Estimated based on the average area under the curve (AUC) for data obtained through multiple readings taken on the day before (or after) each scheduled study visit (Method 1).
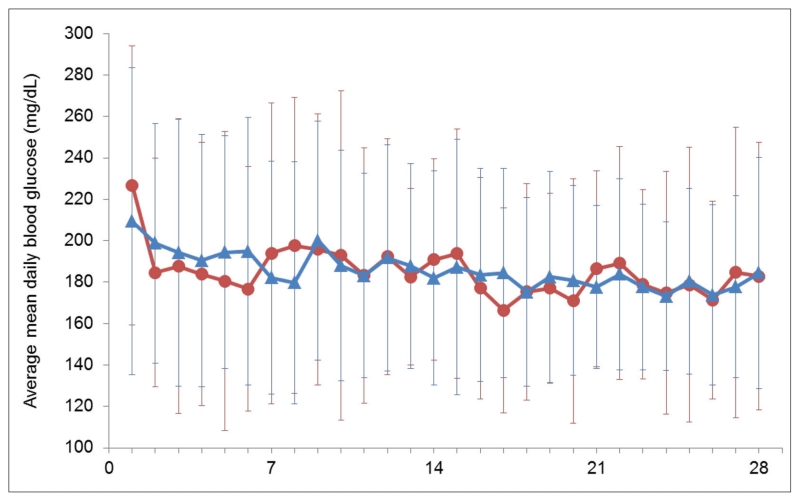
MBG values estimated using the daily and weekly SMBG methods and determined by CGM were highly correlated, with Pearson correlation coefficients of 0.783 (P<.0001) for CGM versus SMBG Method 1 (daily values) and 0.746 (P<.0001) for CGM versus SMBG Method 2 (weekly values). Figure 1 shows the MBG values across patients determined from daily SMBG and CGM data obtained over the first 28 days of the study. MBG values determined with CGM and daily SMBG (Method 1) were not significantly different at any visit except the one at week 3 (183.0 ± 45.9 vs. 191.7 ± 45.2 mg/dL [10.16 ± 2.55 vs. 10.64 ± 2.51 mmol/L]; P = .03). Mean glucose values estimated with CGM were not significantly different from the weekly SMBG means (Method 2) at any visit. Based on these findings, MBG determined from weekly SMBG (Method 2) was chosen as the primary comparator for other glycemic indices.
Fig. 1.
Plot of average mean ± SD daily blood glucose measured using SMBG (red circles) and CGM (blue triangles) over 4 weeks. CGM = continuous glucose monitoring; SMBG = self-monitoring of blood glucose.
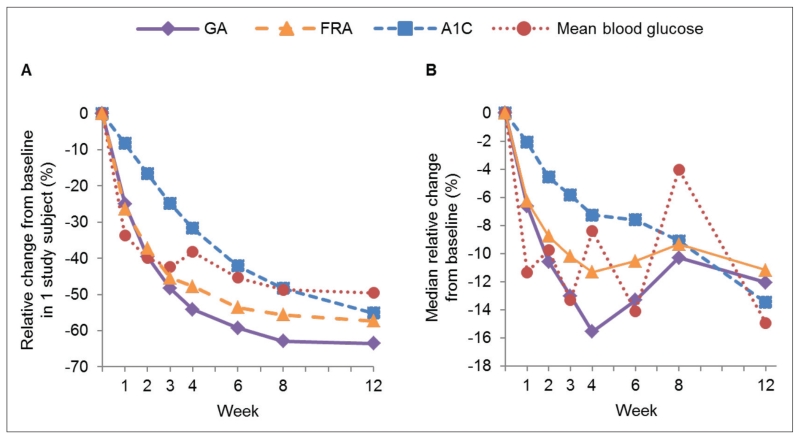
Although MBG levels decreased in the study population over the study period, the glucose levels of some individual patients did not decrease, and some increased. Figure 2 A illustrates the relative percentage changes from baseline in MBG, GA, FRA, and A1C values for a patient whose glucose decreased. Reductions in MBG levels were more closely reflected by GA and FRA changes than A1C values, with the period prior to 3 weeks showing the greatest decrease in MBG followed by a relative plateau out to 12 weeks. GA and FRA values decreased rapidly during the first 4 weeks with a slower decline thereafter, but A1C showed an uninterrupted decrease out to 12 weeks.
Fig. 2.
A, Relative percentage changes from baseline in GA, A1C, MBG, and FRA in an individual patient from site 3 over 12 weeks. B, Median relative percentage changes from baseline in GA, A1C, MBG, and FRA over 12 weeks in the entire study population. A1C = hemoglobin A1C; FRA = fructosamine corrected for albumin; GA = glycated hemoglobin; MBG = mean weekly blood glucose from self-monitoring.
Similar overall patterns of relative GA, FRA, and A1C performances were observed in the study population. Figure 2 B shows the median relative percentage changes from baseline in GA, A1C, FRA, and MBG across study patients. From baseline to study end, GA decreased 2.8%, A1C by 1.2%, and MBG by 24.5 mg/dL (1.36 mmol/L). MBG reached a nadir at 3 and 6 weeks, rose at 8 weeks, and fell again. The lowest median GA occurred at 4 weeks, followed by a small increase and then a slight reduction, mirroring the group changes in MBG. A small increase of 0.1% in median A1C occurred at week 6; otherwise A1C fell steadily throughout the treatment period and did not reflect the variability in MBG.
Table 2 lists the Spearman correlations between GA and the other glycemic indices measured in the study. GA was well correlated with A1C, MBG (Methods 1 and 2), CGM, fructosamine, and FRA throughout the study (Spearman’s correlations ≥0.375 were statistically significant at P<.05). The highest correlations were between GA and the 2 fructosamine measures (correlations generally >0.9). The correlations between A1C and GA were highest at weeks 8 and 12, while the correlations between GA and weekly SMBG (Method 2) were higher than the correlations with daily SMBG values (Method 1). GA correlations with FPG were statistically significant only at weeks 2 and 12. Correlations between A1C and the other glycemic indices were all numerically lower than the GA correlations and did not reach statistical significance at any time point for SMBG, fructosamine, or FPG.
Table 2. Spearman’s Correlations Between GA and Other Glycemic Indices.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 6 | Week 8 | Week 12 | |
|---|---|---|---|---|---|---|---|
| GA vs. | |||||||
| FPG | 0.206 | 0.431a | 0.154 | 0.290 | 0.133 | 0.281 | 0.471a |
| FRA | 0.866a | 0.913a | 0.908a | 0.949a | 0.964a | 0.950a | 0.952a |
| FRA/ALB | 0.908a | 0.948a | 0.943a | 0.945a | 0.931a | 0.959a | 0.925a |
| A1C | 0.649a | 0.538a | 0.484a | 0.424a | 0.592a | 0.701a | 0.755a |
| SMBG Method 1 | 0.375a | 0.524a | 0.590a | 0.429a | 0.213 | 0.477a | 0.562a |
| SMBG Method 2 | 0.533a | 0.559a | 0.455a | 0.464a | 0.668a | 0.633a | 0.671a |
| CGM | 0.449a | 0.495a | 0.655a | 0.487a | |||
| A1C vs. | |||||||
| FPG | 0.062 | 0.272 | −0.006 | 0.120 | 0.229 | 0.301 | 0.175 |
| FRA | 0.414a | 0.335 | 0.262 | 0.247 | 0.486a | 0.585a | 0.641a |
| FRA/ALB | 0.560a | 0.510a | 0.405a | 0.422a | 0.577a | 0.756a | 0.712a |
| GA | 0.649a | 0.538a | 0.484a | 0.424a | 0.592a | 0.701a | 0.755a |
| SMBG Method 1 | 0.211 | 0.386a | 0.509a | 0.270 | 0.536a | 0.531a | 0.386 |
| SMBG Method 2 | 0.480a | 0.322 | 0.489a | 0.341 | 0.647a | 0.769a | 0.649a |
| CGM | 0.114 | 0.348 | 0.421a | 0.271 |
Abbreviations: A1C = hemoglobin A1C; CGM = continuous glucose monitoring; FPG = fasting plasma glucose; FRA = fructosamine; FRA/ALB = albumin-corrected fructosamine; GA = glycated albumin; SMBG = self-monitoring of blood glucose.
P<.05 for correlation.
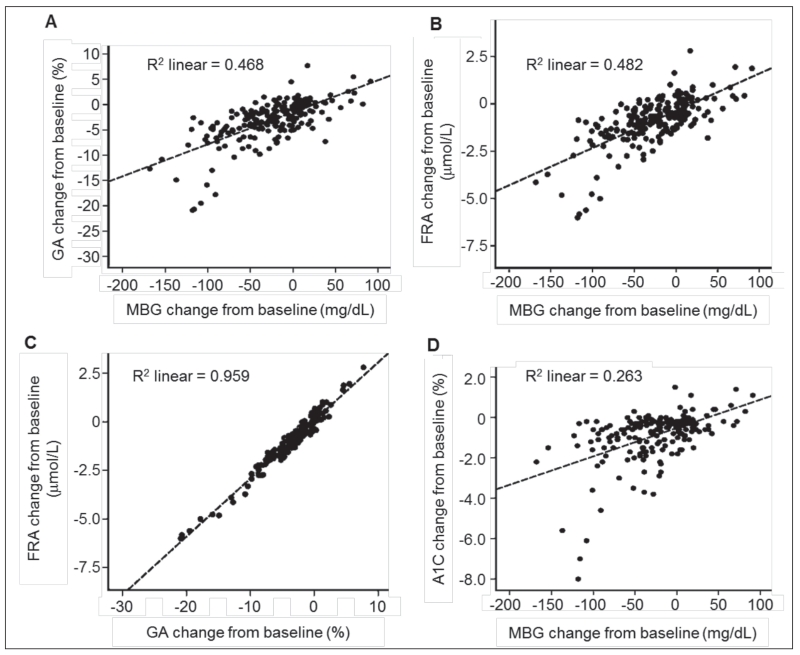
Figure 3 shows scatter plots between GA and MBG, FRA and MBG, FRA and GA, and A1C and MBG with the fitted regression lines and coefficients of determination (R2, square of the Pearson correlation coefficient), which provide estimates of the proportion of variation in data explained by the regression line. The estimated R2 values were 0.468 between GA and MBG, 0.482 between FRA and MBG, 0.959 between FRA and GA, and 0.263 between A1C and MBG. There was close agreement between the FRA and GA assay results in the presence of MBG variability.
Fig. 3.
Scatterplots of absolute change from baseline in GA, MBG, FRA, and A1C over study period. A, GA vs. MBG. B, FRA vs. MBG. C, FRA vs. GA. D, A1C vs. MBG. The R2 value represents the squared correlation coefficient between paired values. A1C = hemoglobin A1C; FRA = fructosamine corrected for albumin; GA = glycated hemoglobin; MBG = mean weekly blood glucose from self-monitoring.
Linear regression analysis was performed to determine whether GA at 4 weeks could be used to predict a 0.5% change in A1C. This was the prespecified threshold representing a clinically meaningful improvement. The change in A1C from week 0 to week 12 was the dependent variable, and change in GA from week 0 to week 4 was a covariate. The incremental OR predicted from the model was 1.2 (P = .206) per unit change of GA. The ORs for 4-week GA reductions of 1.8%, 2.0%, and 3.5% were all higher than this level, indicating that a patient achieving a GA reduction ≥1.8% at 4 weeks would be very likely to experience an A1C reduction of at least 0.5% at 12 weeks. An exploratory analysis further showed that a reduction in GA ≥1% at 4 weeks was significantly associated with an A1C reduction ≥0.5% at 12 weeks in a 2 × 2 classification table (OR 19.0, 95% confidence interval: 1.4, 944, P = .018). Additional post hoc analyses showed that the slope estimate for the linear regression was positive (0.375, P<.0001), and the Pearson correlation coefficient for this association was 0.806 (P<.0001).
Adverse events were reported by 26 (83.9%) of the 31 patients comprising the safety population. Adverse events occurred in 21 (67.7%) participants upon or after enrollment. Thirteen (41.9%) patients reported treatment-emergent, mild hypoglycemia. One patient had a serious cardiac adverse event and withdrew from the study upon hospitalization. No adverse events were related to the study procedure.
DISCUSSION
This study evaluated the performance of GA measured using the Lucica® GA-L assay relative to other glycemic indices in 30 patients with suboptimally controlled type 1 or 2 diabetes who were undergoing intensification of antidiabetic therapy. Two key findings emerged from the results. First, GA (the primary focus of the study) and FRA both accurately reflected changes in MBG values not detected by A1C. Second, GA at 4 weeks predicted A1C at 12 weeks in the population studied.
Diabetes treatment decisions are typically based primarily on patients’ A1C levels, with secondary consideration of SMBG results (3). A unique element of this study design was selection of a study population with changing, rather than stable, glycemia, which permitted the investigators to assess how the different glucose indices reflected patients’ response to therapy. This study highlighted the limitations of both A1C and SMBG and suggests the potential of an intermediate glycemic index to assess the early impact of diabetes management choices. In Figure 2 B, despite the modest 0.1% uptick at week 6, the overall trend in A1C is downward. In contrast, the downward trend of GA stops at week 4, reflecting the abrupt and dramatic increase in MBG values at that time point. As the MBG fluctuates over the following weeks, GA rises gradually and levels off with a slight downward trend between weeks 8 and 12. The study data also highlight the limitations of SMBG as a marker of glycemic control. MBG values determined from SMBG and CGM were well-correlated with each other (Fig. 1), but the broad day-to-day variation within patients shown by the SDs of both SMBG and CGM highlights the unreliability of blood glucose values for therapeutic decision making, except for individual patients who might have suspected hypoglycemia or excess postprandial glucose elevations or those who require SMBG results to determine bolus insulin doses.
The high correlation between the GA and FRA in this study further supports the ability of GA to predict short-term therapeutic responses and is consistent with other reports (11,12). Like GA, FRA reflects changes in glycemia over 2 to 3 weeks and can be useful for short-term monitoring of glycemic control. Fructosamine consists of ketoamines formed from albumin, as well as other glycated serum proteins such as lipoproteins and globulins. Fructosamine measurement may be influenced by albumin (for which it can be corrected, as done in our study) and other serum protein concentrations, as well as by urea and uric acid, which can fluctuate in various clinical conditions such as renal impairment (13-15). GA is specific to albumin and may be less influenced by variations in other molecules.
The Pearson correlation coefficients between GA and MBG and between FRA and MBG across study observations exceeded that between A1C and MBG (Fig. 3). A significant Pearson correlation coefficient of 0.806 (P<.0001) demonstrated the strength of the relationship between GA and future A1C levels. Furthermore, the significant and positive slope estimate of 0.375 (P<.0001) established that GA and A1C change in the same direction.
Recent studies have shown that short-term assessment of glycemia may be useful as a complement to A1C measurement, and that GA correlates well with both A1C and SMBG values (11,15-18). GA proved more useful than A1C in detecting early response to insulin therapy and also correlated better with FPG than A1C in patients with suboptimally controlled diabetes (19). The correlation between FPG and GA was also superior to the correlation between FPG and A1C in the Atherosclerosis Risk in Communities (ARIC) Study, and GA and FPG performed comparably as screening tools for diabetes (20). In a study involving 538 hemodialysis patients with diabetes, GA more accurately estimated glycemic control than A1C, because the use of erythropoietin led to high proportions of young erythrocytes in these patients (21). Similarly, in patients with stage 4 or 5 chronic kidney disease and diabetes, GA more accurately reflects glycemic control than fructosamine or A1C (14,22). The accuracy of GA is negatively affected by very low albumin levels, however (22).
The relationship between glucose elevations and diabetes complications is well established (23,24). Assessing GA as a complement to A1C may be useful for clinical decision making because like A1C, GA levels are strongly associated with diabetes complications. In 227 patients with diabetes participating in the ARIC Study, GA and A1C both had strong associations with albuminuria, kidney disease, and retinopathy (25). In another ARIC analysis involving 11,348 individuals without and 958 patients with diabetes, fructosamine and GA values were associated with retinopathy prevalence and the risks of chronic kidney disease and diabetes (12). Data from the Diabetes Control and Complications Trial also showed similar associations of GA and A1C with the risks of retinopathy and nephropathy (26). GA elevations were also associated with increased intima media thickness and high sensitivity C-reactive protein levels in an observational study of a mostly nondiabetic population in Japan (n = 1,575; 4.6% had diabetes) (26,27).
CONCLUSION
In summary, this study was the first to comprehensively examine the response to therapy using short-, intermediate-, and long-term measures of glycemia in patients undergoing changes to their diabetes treatment regimens. The results showed that GA results were consistent with FRA results and that GA was more sensitive to daily glycemic excursions than A1C while also predicting long-term changes in glycemia that are normally measured by A1C.
ACKNOWLEDGMENT
The authors thank Amanda Justice for editorial support and medical writing and the staff of Medpace for performing the statistical analyses. Asahi Kasei provided financial support for the trial and manuscript preparation. Dr. Fonseca and clinical research at Tulane are supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURE
Cyrus V. Desouza, MD, has received honoraria and consulting fees from Novo Nordisk. Julio Rosenstock, MD, has served on scientific advisory boards and received honoraria or consulting fees from Merck, Roche, Sanofi, Novo Nordisk, Eli Lilly, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Janssen, Novartis, Boehringer Ingelheim, Intarcia, and Lexicon. He has also received grants or research support from Merck, Pfizer, Sanofi, Novo Nordisk, Roche, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin, Janssen, Daiichi Sankyo, MannKind, Intarcia, Boehringer Ingelheim, and Lexicon. Rong Zhou, PhD, works for Medpace, which was paid to perform the statistical analyses. Richard G. Holcomb, PhD, has received consulting fees from Asahi Kasei. Vivian A. Fonseca, MD, has served on scientific advisory boards and received honoraria or consulting fees from Takeda, Sanofi, Novo Nordisk, Astra Zeneca, Intarcia, and Janssen and has received grants or research support from Eli Lilly, Abbott, Mesoblast, Asahi Kasei, and Bristol-Myers Squibb.
Abbreviations
- A1C
glycated hemoglobin A1C
- ARIC
Atherosclerosis Risk in Communities
- CGM
continuous glucose monitoring
- FPG
fasting plasma glucose
- FRA
fructosamine corrected for albumin
- GA
glycated albumin
- MBG
mean blood glucose
- OR
odds ratio
- SMBG
self-monitoring of blood glucose
REFERENCES
- 1.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 2.Bloomgarden ZT, Inzucchi SE, Karnieli E, LeRoith D. The proposed terminology ‘A(1c)-derived average glucose’ is inherently imprecise and should not be adopted. Diabetologia. 2008;51:1111–1114. doi: 10.1007/s00125-008-1027-7. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(Suppl 1):S1–S93. [PubMed] [Google Scholar]
- 4.Blevins TC, Bode BW, Garg SK, et al. Statement by the American Association of Clinical Endocrinologists Consensus Panel on continuous glucose monitoring. Endocr Pract. 2010;16:730–745. doi: 10.4158/EP.16.5.730. [DOI] [PubMed] [Google Scholar]
- 5.Cohen RM, Sacks DB. Comparing multiple measures of glycemia: how to transition from biomarker to diagnostic test? Clin Chem. 2012;58:1615–1617. doi: 10.1373/clinchem.2012.196139. [DOI] [PubMed] [Google Scholar]
- 6.Kohzuma T, Yamamoto T, Uematsu Y, Shihabi ZK, Freedman BI. Basic performance of an enzymatic method for glycated albumin and reference range determination. J Diabetes Sci Technol. 2011;5:1455–1462. doi: 10.1177/193229681100500619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther. 2010;14:49–51. doi: 10.1007/BF03256353. [DOI] [PubMed] [Google Scholar]
- 8.Little RR, Rohlfing CL, Sacks DB, National Glycohemoglobin Standardization Program (NGSP) Steering Committee Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57:205–214. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 9.Center for Drug Evaluation and Research . Guidance for Industry: Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention. Food and Drug Administration; Rockville, MD: 2008. [Google Scholar]
- 10.National Institute for Health and Care Excellence . NICE clinical guideline 87. Type 2 diabetes: the management of type 2 diabetes. National Institute for Health and Care Excellence; London, UK: 2014. [Google Scholar]
- 11.Beck R, Steffes M, Xing D, et al. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediatr Diabetes. 2011;12:690–695. doi: 10.1111/j.1399-5448.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279–288. doi: 10.1016/S2213-8587(13)70199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153–2163. [PubMed] [Google Scholar]
- 14.Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton) 2012;17:182–188. doi: 10.1111/j.1440-1797.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 15.Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9:169–176. doi: 10.1177/1932296814567227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14:548. doi: 10.1007/s11892-014-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga M, Suzuki S, Matsuo K, Tanahashi Y, Azuma H, Kasayama S. Calculation of HbA1c and glycated albumin from serially measured self-monitored blood glucose in patients with type 1 diabetes mellitus. Clin Chim Acta. 2013;425:188–191. doi: 10.1016/j.cca.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Jung CH, Hwang YC, Kim KJ, et al. Development of an HbA1c-based conversion equation for estimating glycated albumin in a Korean population with a wide range of glucose intolerance. PLoS One. 2014;9:e95729. doi: 10.1371/journal.pone.0095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paroni R, Ceriotti F, Galanello R, et al. Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem. 2007;40:1398–1405. doi: 10.1016/j.clinbiochem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58:1648–1655. doi: 10.1373/clinchem.2012.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 22.Harada K, Sumida K, Yamaguchi Y, Akai Y. Relationship between the accuracy of glycemic markers and the chronic kidney disease stage in patients with type 2 diabetes mellitus. Clin Nephrol. 2014;82:107–114. doi: 10.5414/CN108027. [DOI] [PubMed] [Google Scholar]
- 23.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 25.Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34:960–967. doi: 10.2337/dc10-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan DM, McGee P, Steffes MW, Lachin JM, DCCT. EDIC Research Group Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/ EDIC study. Diabetes. 2014;63:282–290. doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furusyo N, Koga T, Ai M, et al. Plasma glycated albumin level and atherosclerosis: results from the Kyushu and Okinawa Population Study (KOPS) Int J Cardiol. 2013;167:2066–2072. doi: 10.1016/j.ijcard.2012.05.045. [DOI] [PubMed] [Google Scholar]