Summary
The zebrafish has emerged as an important model organism for understanding the cellular and molecular mechanisms of tissue regeneration. Adult zebrafish efficiently replace cardiac muscle after partial resection of their ventricle, or after transgenic ablation of cardiomyocytes. Here, we describe methodology for inducing these injuries and assaying indicators of regeneration.
Keywords: zebrafish, heart, surgery, ablation, cardiomyocyte, regeneration
1. Introduction
The adult mammalian heart has an extremely limited ability to repair itself through regeneration of new cardiomyocytes (CMs), and instead heals tissue damage by scarring. Nearly 4 decades ago, it was reported that newts survive removal of up to 25% of the cardiac ventricle, and CMs near the amputation plane initiate a proliferative response that involves DNA synthesis and mitosis (1, 2). The lack of molecular genetic tools for salamanders in the following years severely limited investigation of the underlying mechanisms. In 2002, Poss and colleagues reported that zebrafish fully regenerate cardiac muscle after a similar partial ventricular resection, through a mechanism in which new muscle creation occurs more efficiently than scarring. More recently it has been demonstrated that zebrafish efficiently regenerate muscle removed by severe cryoinjury (3–5), or even after extreme injuries that genetically ablate 60% or more of the animal’s total CMs (6).
The zebrafish is a central vertebrate model system used by several hundred laboratories, and there is a growing molecular toolbox. Studies from multiple groups over the past decade have revealed that zebrafish heart regeneration involves two key components: 1) proliferation of existing cardiomyocytes as the primary muscle source (7, 8); and 2) an environment, aided by non-muscle epicardial and endocardial cells, that stimulates regeneration by these cardiomyocytes (9–12).
This work has altered the way mammalian cardiac repair is considered. Most notably, increased attention has been paid to CM division in adult mice and humans, and multiple studies have now observed that adult mammalian CMs undergo low but measurable turnover. Additionally, recent evidence indicates that CM proliferation is stimulated at low levels after injury to the adult mouse heart (13–15). Studies of the epicardium in adult mice have similarly paired with findings in zebrafish, and epicardial cells are now being implicated in mammalian cardiac repair mechanisms. In vertebrates, epicardial cells can act as modulators of vascularization, muscle survival or regeneration, inflammation, and extracellular matrix regulation (10, 12, 16–22). Thus, evidence indicates that the analogous regenerative machinery present in zebrafish also exists in the mammalian heart, but is not activated to the same extent for significant regeneration. This fundamental concept supports the continued use of zebrafish as a platform to reveal potential methods to gauge and stimulate human heart regeneration.
Here, we describe procedures for cardiac surgery and genetic CM ablation in adult zebrafish. Additionally, we describe key assays for CM proliferation, a hallmark of heart regeneration, using nuclear markers of CMs and proliferation indicators.
2. Materials
Zebrafish mating tank (1L)
Stainless steel straight microscissors (Fig. 1A)
Stainless steel curved microscissors (Fig. 1A)
Stainless steel microforceps (Fig. 1A)
Sponge (1.5 x 5 x 3 cm) with groove cut in center using scissors (0.5 x 2.5 cm; Fig. 1A)
Plastic spoon
Plastic transfer pipette
Laboratory tissue wipers
Dissection microscope (Fig. 1A)
Glass bowl (90 x 50 mm; Fig. 1A)
Razor blade or scalpel
SuperFrost Plus Microscope Slides
Cover glasses
Hydrophobic pen
Coplin jar for slide staining
White tape
Thermal Cycler
Taq and 10x Buffer
Deoxynucleotide (dNTP) Solution Mix
4-Hydroxytamoxifen (4-HT). 4-HT powder is dissolved in 100% ethanol to a 1 mM stock solution that can be stored at 20°C for 2 weeks. The stock solution can be diluted in aquarium water for different working concentrations. Handle 4-HT with care.
Tricaine solution. For tricaine stock solution (4 mg/ml = 0.4%), dissolve Ethyl 3-aminobenzoate powder in 900 ml of water, then adjust pH to 7.4 with 1M Tris, pH 9.5 solution. Add water to 1000 ml. Aliquot in 50 ml tubes, label, and store frozen (keep working stock at 4 degrees).
-
Fish Fix. Combine all the following reagents except paraformaldehyde. Move solution to hood and warm, slowly adding the PFA; stir until dissolved completely. Adjust to pH 7.4 and filter sterilize. Will keep at 4°C for 1 week, or freeze in 10 ml aliquots.
Paraformaldehyde 8 g Sucrose 8 g 1M CaCl2 24 μl 0.2 M Na2HPO4 77 ml 0.2 M NaH2PO4 23 ml dH2O up to 200 ml -
Fish Fix Buffer. Combine the following reagents with stir bar, adjust pH to 7.4 if necessary (work without heat). Filter sterilize and freeze in 50 ml tubes.
Sucrose 8 g 1M CaCl2 24 μl 0.2 M Na2HPO4 77 ml 0.2 M NaH2PO4 23 ml dH2O up to 200 ml Fin digestion solution: 10 mM Tris-Cl pH 7.5; 50 mM KCl; 0.3% Tween-20; 1 mM EDTA pH 8.0; 200 μg/mL Proteinase K.
Citrate Buffer (10 mM Citric Acid, 0.05% Tween 20, pH 6.0). Mix 1.92 g Anhydrous citric acid in 1 L dH2O. Adjust pH to 6.0 with 10N NaOH. Add 0.5 ml of Tween-20 and mix well. Store this solution at room temperature for 3 months or at 4° C for longer storage.
30% sucrose solution
TBS Tissue Freezing Medium
PBS solution
PBST: 1X PBS + 0.1% Tween-20.
Horse Serum
NCS-PBT: 10% NCS (heat inactivated newborn calf serum) + 89% PBST + 1% DMSO.
Vectashield HardSet Mounting Medium with DAPI
F59 anti-myosin antibody (Developmental Studies Hybridoma Bank, cat. no. F59-c)
Mouse anti-PCNA antibody (Sigma-Aldrich, cat. no. P88250)
Rabbit anti-Mef2 antibody (Santa Cruz Biotechnology, cat. no. sc-313)
Alexa Fluor 594 anti-mouse antibody (Life Technologies, cat. no. A11032)
Alexa Fluor 594 anti-rabbit antibody (Life Technologies, cat. no. A11037)
Alexa Fluor 488 anti-mouse antibody (Life Technologies, cat. no. A11029)
Zebrafish care and use. Outbred EK, AB, or EK/AB mixed background zebrafish (4 – 12 months of age) are used for ventricular resection surgeries or for CM ablation, whereas transgenic animals are used for CM ablation (6). Animal density is maintained at approximately 4 to 5 fish per liter.
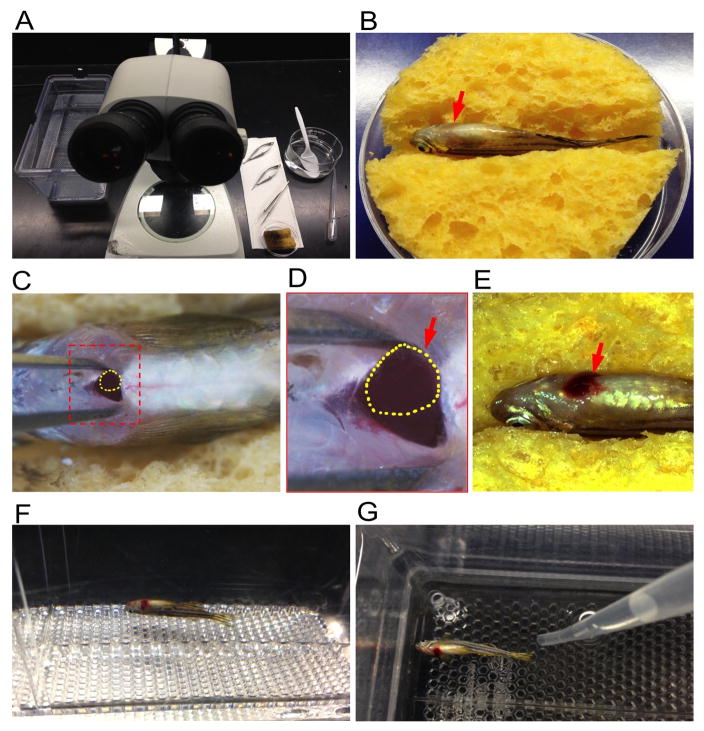
Fig. 1.
Resection of the ventricular apex in adult zebrafish. (A) Required equipment, including mating tank with aquarium water, dissecting microscope, microscissors, microforceps, pipette, sponge and glass bowl. (B) An anesthetized zebrafish placed ventral side up into a moist, slotted sponge. Arrow indicates the heart area. (C) View of the heart after puncturing the skin and pericardial sac. Yellow dashed line indicates the exposed ventricular apex. (D) High magnification of boxed area in (C). Yellow dashed line indicates the exposed ventricular apex (red arrow). (E) View of the blood clot near the injured heart after surgery. (F) A freshly injured fish remains on the bottom of the tank after it is returned to fish water. (G) Stimulate fish to gill by vigorously squirting water with a pipette.
3. Methods
Carry out cardiac injury procedures at either room temperature or 25–29°C (the temperature of aquarium water).
3.1 Zebrafish heart surgery
Anesthetize zebrafish (4 months to 1 year old) in 0.02% Tricaine in a glass bowl for one to two minutes. Anesthesia is monitored by watching for slowing of gill movements and by checking for a response to tail pinch; wait for fish to stop moving, but be careful not to anesthetize any longer than necessary.
Use a plastic spoon to transfer anesthetized zebrafish; put fish ventral side up on a moist, slotted sponge (Fig. 1B). Visually locate the beating heart (Fig. 1B).
While using microforceps pressed to the left and right of the heart to pull the skin taut, use the straight microscissors to puncture the skin just posterior to the heart, being careful not to puncture the underlying atrium or ventricle. Cut carefully towards the anterior to make a small incision (~1 mm) that penetrates the skin and muscles above the posterior medial margin of the heart (Fig. 1C and D). The ventricle is exposed by opening the incision with microforceps and applying gentle abdominal pressure. Take care to expose the ventricle (pink muscular tissue) and not the atrium (dark red tissue).
Use microforceps inserted carefully into the incision, to the left and right of the heart, to open the incision while applying gentle pressure on the abdomen with the blunt edge of the scissors. Use the curved microscissors (tips pointing up) to quickly remove 10–20% of the ventricular apex (Fig. 1E), and then allow the heart to slip back inside the body. Ensure the resected heart tissue is completely and visibly removed.
Use a tissue to blot the incision and to prevent blood from entering the gills. When bleeding has stopped, return the fish to fresh fish water (Fig. 1F). Stimulate the fish to move its gills by vigorously squirting water over the gills with a pipette (Fig. 1G). The fish will begin gill movements after several seconds and will then try to swim; continue squirting water over the gills until regular swimming movements are observed.
3.2 Zebrafish cardiomyocyte ablation
Inducible Cre recombinase-based approaches have been established in adult zebrafish, typically employing cell type-specific promoters to facilitate lineage tracing (7). To ablate CMs with temporal precision, a dual transgene system was developed in which the first transgene has a CM-specific cmlc2 promoter driving a 4-hydroxytamoxifen (4-HT)-inducible Cre recombinase (CreER). The second transgene has a constitutive β-actin2 promoter driving a loxp-flanked transcriptional stop sequence followed by partially disabled Diphtheria toxin A-chain (DTA) (6). In adult cmlc2:CreER; bactin2:loxp-mCherry-STOP-loxp-DTA (bact2:RSD) double transgenic fish, activation of the Cre recombinase by 4-HT treatment allows recombination in CMs to release expression of the toxin DTA, thereby killing CMs.
Heterozygous cmlc2:CreER fish are mated with heterozygous bact2:RSD fish. The embryos are screened for mCherry red fluorescence with strong and ubiquitous expression throughout whole body by 4 days post fertilization.
-
Screen for cmlc2:CreER positive fish at 2 months of age by PCR amplification. Use 0.02% tricaine to anesthetize fish as described above, and then use a plastic spoon to place it on a piece of tape adhered to the lab bench. Using a razor blade or scalpel, cut the caudal fin tip (~ 2 mm) and put the fin tissue into a tube with 50 μl Fin digestion solution using tweezers. Fin digest protocol: incubate fins at 55°C for 60 minutes, then incubate fins at 95°C for 15 minutes. Take 2 μl of each fin digest solution in a PCR tube or plate for PCR (total volume: 25 μl per reaction). Run the PCR product on a 1.5% to 2% agarose gel. Cre-positive fish will show two bands: the cre PCR product (size is 200 bp) and the βactin2 PCR product (size is 400 bp).
PCR primers: PCR program: Cre forward: CGTACTGACGGTGGGAGAAT (1) 94°C 2 minutes Cre reverse: GTGGCAGATGGCGCGGCAACA (2) 94°C 30 seconds βactin forward: TGATGAGGCTCAGAGCAAGA (3) 61.5°C 30 seconds βactin reverse: CACAATCCACACTTCCATGC (4) 72°C 30 seconds Mix these primers together to make a primer mix containing 10 μM of each primer (2 – 4) X 45 (5) 72°C 5 minutes (6) 4°C hold Myocyte ablation. Four month-old cmlc2:CreER; bact2:RSD animals are treated for 12–16 hours in 0.1 μM 4-HT in fish water in a mating tank. Use 20 to 50 ml of diluted 4-HT per fish. 0.1% ethanol can be used as a vehicle control. The fish should be kept in a dark environment during incubation.
3.3 Histological analysis for cardiac muscle ablation and regeneration
To assess cardiomyocyte ablation and recovery, myofibers can be stained with an antibody directed against myosin heavy chain (MHC) (Fig. 2).
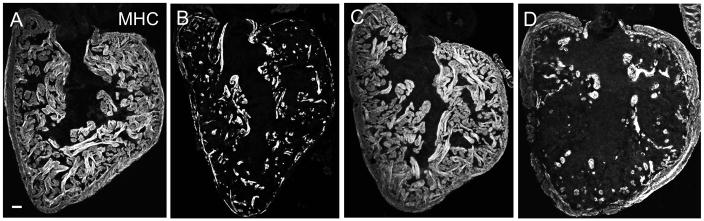
Fig. 2.
Regeneration of ventricular cardiomyocytes after ablation-induced injury. (A–D) Myosin heavy chain (MHC) staining of ventricular sections from cmlc2:CreER; bact2:RSD fish treated with vehicle (A), or 0.1 μM 4-HT, at 7 (B) or 30 (C) days post treatment. (C) One example of an ablated heart with successful recovery. (D) One example of an ablated heart with greater than 60% cardiomyocyte loss by 7 dpi that displays partial regeneration at 30 days post treatment. Scale bars, 50 μm.
Adult zebrafish hearts are extracted and fixed in fish fix solution for 1 hour at room temperature (RT). After fixation, hearts should be washed in fish fix buffer 3 x 5 minutes, then in 30% sucrose overnight at 4°C.
Hearts are embedded in TBS Tissue Freezing Medium, frozen on dry ice, cryosectioned at 10 μm thickness, and dried for 1 hour at RT or 30 minutes on a 37°C slide warmer.
Circle sections with a hydrophobic pen (Vector ImmEdge pen, e.g.) and allow to dry. Then, wash slides 4 x 5 minutes in PBST in a Coplin jar. All of the following incubations are done in NCS-PBT (with 300 μl of each solution added on top of sections).
Block with 2% horse serum in NCS-PBT, in a humidified chamber at 37°C for 30 minutes. Remove block (do not wash), then incubate with mouse anti-myosin heavy chain (F59, stored at 4°C) diluted 1:150 in NCS-PBT, in a humidified chamber at 37°C for 3 hours.
Wash 4 x 5 min in PBST in a Coplin jar. Incubate with secondary (fluorescent) antibody in a humidified chamber at 37°C for 1 hour (e.g. Alexa Fluor 594 goat anti-mouse diluted 1:200 in NCS-PBT).
Wash 3 x 5 minutes in PBST. Mount slides using one drop of Vectashield with DAPI, or stain with DAPI and cover slip slides with appropriate mounting medium. Store in the dark at 4°C, until ready for analysis by fluorescence microscopy. The ablation and regeneration of ventricular muscle are shown in representative sections in Figure 2.
3.4 Assays for cardiomyocyte proliferation during heart regeneration
Zebrafish fully regenerate cardiac muscle after heart surgery and ablation within one month, by cardiomyocyte hyperplasia (6, 7, 23). Cardiomyocyte proliferation can be evaluated by co-staining for Mef2, a nuclear marker of cardiomyocytes, and the marker Proliferating cell nuclear antigen (PCNA) (Fig. 3).
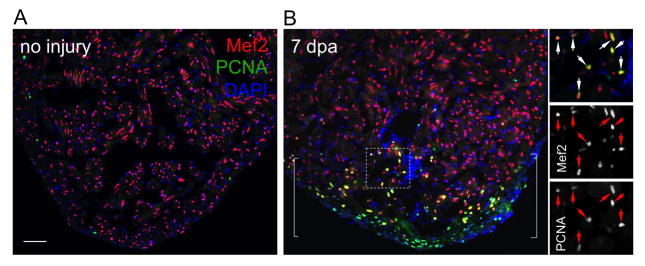
Fig. 3.
Ventricular cardiomyocyte proliferation after cardiac surgery, assessed by Mef2 and PCNA staining. (A) Uninjured hearts show rare Mef2+PCNA+ cardiomyocytes. (B) Injured ventricular apices at 7 days after resection injury (dpa) display many Mef2+PCNA+ cardiomyocytes in wound area. Bracket, injury site. Inset in (B), high magnification of boxed area. Arrows, proliferating cardiomyocytes. Scale bars, 50 μm.
Adult zebrafish hearts should be extracted, fixed, embedded, and cryostat-sectioned as described above. Circle sections with a hydrophobic pen and allow to dry. Then, perform treatment with citrate buffer to remove native fluorescence and denature proteins. (Perform these steps in a chemical fume hood.). If not using Citrate buffer, incubate slides in 10% SDS for 5 – 10 minutes as a pre-treatment.
Place citrate buffer in a Coplin jar. Place Coplin jar in a beaker with water reaching midway to the height of the Coplin jar. Place the Coplin jar lid on loosely, and cover the beaker with an ice bucket lid. Heat to 98°C (boiling – check with thermometer). Add slides and boil at 98°C for 20 minutes. Remove the Coplin jar from the beaker and allow to cool in the hood for 20 minutes.
Re-circle sections with a hydrophobic pen if necessary. Wash slides 4 x 5 minutes in PBST.
Block with 2% horse serum in NCS-PBT, in a humidified chamber at 37°C for 30 minutes. Remove block (do not wash) and then incubate with 1:75 rabbit anti-Mef2 (stored at 4°C) diluted 1:75, and mouse anti-PCNA (stored at −20°C) diluted 1:200 in NCS-PBT, in a humidified chamber at 37°C for 3 hours.
Wash 4 x 5 minutes in PBST. Incubate with secondary (fluorescent) antibodies in a humidified chamber at 37°C for 1 hour (e.g., Alexa Fluor 594 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse, both diluted 1:200 in NCS-PBT).
Wash 3 x 5 minutes in PBST. Mount slides using Vectashield with DAPI, or stain with DAPI and cover slip slides with appropriate mounting medium. Store in the dark at 4°C. Representative sections showing cardiomyocyte proliferation in normal and injured ventricles are shown in Figure 3.
4. Notes
All handling of zebrafish pre- and post- cardiac injury should follow institutional guidelines and should be approved by institutional animal care and use committees.
The age of zebrafish should be greater than 3 months old. Ninety percent of the fish should survive surgery, with all deaths occurring on the day of surgery. Removal of greater than 25% of the ventricle drastically reduces survival.
Do not puncture the heart with scissors during the ventral incision into the pericardial cavity, as extensive bleeding will occur.
Revival times following anesthetization may vary. Keep stimulating the amputated fish for at least 5 minutes by squirting water over the gills until regular gill movement is observed.
Tricaine concentration is critical for animal revival and survival after heart surgery. Ensure the fish are anesthetized such that they are unresponsive to a tail pinch, typically within 1 and 3 minutes. If animals go under too quickly, adjust tricaine concentration by adding aquarium water.
The wounds bleed profusely for 5–30 seconds before clotting. Body wall incisions are not sutured, and heal within 1–2 days.
Cardiomyocyte ablation may be variable among different clutches. To assess ablation within a given clutch, test the efficiency of myocyte ablation using different doses of 4-HT ranging from 0.1 μM to 5 μM, treated for 12–16 hours.
Acknowledgments
This work was funded by postdoctoral fellowships from the American Heart Association to J.W. and grants from NIH (HL081674) and American Federation for Aging Research to K.D.P. We thank Amy Dickson and Anne Knecht for reviewing and contributing to this manuscript.
References
- 1.Bader D, Oberpriller JO. Repair and reorganization of minced cardiac muscle in the adult newt. Journal of Morphology. 1978;155:349–357. doi: 10.1002/jmor.1051550307. [DOI] [PubMed] [Google Scholar]
- 2.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. The Journal of Experimental Zoology. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel K, Wu CC, Kurth T, Weidinger G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS One. 2011;6:e18503. doi: 10.1371/journal.pone.0018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 2011;138:1663–1674. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 5.Chablais F, Veit J, Rainer G, Jazwinska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138:3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Developmental Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, Poss KD. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138:2895–2902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Wu Q, Zhang Y, Wiens KM, Huang Y, Rubin N, Shimada H, Handin RI, Chao MY, Tuan TL, Starnes VA, Lien CL. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci U S A. 2010;107:17206–17210. doi: 10.1073/pnas.0915016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 13.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, Bassel-Duby R, Olson EN. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338:1599–1603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Karra R, Dickson AL, Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Developmental biology. 2013;382:427–435. doi: 10.1016/j.ydbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 21.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel–Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]