Abstract
Objective
While several clinical studies have compared the prophylactic efficacy of oxytocin and misoprostol for prevention of postpartum hemorrhage (PPH), no studies have examined these interventions at the community level. This cost-effectiveness analysis is the first to do so.
Methods
This cost-effectiveness study accompanied a randomized trial comparing the prophylactic effectiveness of misoprostol with that of oxytocin conducted in rural Senegal from June to September 2013 of consenting women delivering in maternity huts. We compared the two interventions, with PPH referrals to a higher level facility being the outcome measure. We calculated costs and effects for two hypothetical cohorts of women delivering during a one-year period, each receiving one of the interventions. A third cohort simulated current standard of care (SOC). A sensitivity analysis was performed to estimate the impact of variation in model assumptions.
Results
The incremental cost-effectiveness ratios (ICER) for the misoprostol intervention was USD 40 per PPH case averted and USD 120 for oxytocin. In all scenarios, the misoprostol intervention dominated except in the worst-case scenario, where the oxytocin intervention was slightly more cost-effective.
Conclusion
Our findings suggest that use of misoprostol for PPH prevention would be cost-effective in countries with inadequate maternal health care.
Keywords: cost-effectiveness, CEA, misoprostol, oxytocin, Senegal, postpartum hemorrhage, PPH
I. Introduction
Among the major causes of death to women during pregnancy and delivery is postpartum hemorrhage (PPH). The World Health Organization (WHO) estimates that 27% of all maternal mortality is due to PPH [1]. Maternal mortality is overwhelmingly concentrated in low and middle-income countries—the WHO estimates that out of 289,000 maternal deaths that occurred worldwide in 2013, 286,000 were in these countries. In this respect, Senegal’s maternal mortality ratio (320 deaths per 100,000 live births) is fairly typical of Sub-Saharan Africa [2]. Tragically, while PPH is a manageable condition in high-income countries, it may be life-threatening and often fatal in countries like Senegal where access to adequate obstetric care and blood transfusions is limited.
The prophylactic administration of either misoprostol or oxytocin immediately after childbirth has been shown to be effective in preventing PPH [3, 4]. Both have been recommended by WHO for prevention and treatment of PPH although oxytocin remains the drug of choice [5–8]. Oxytocin, however, requires a cold chain logistical system since it degrades at room temperatures or higher and, furthermore, must be administered parenterally. Both these requirements make oxytocin more difficult to use in situations where trained practitioners and medical infrastructure are relatively scarce. Misoprostol, on the other hand, is thermostable and available in tablet form, making it easy to transport, store and administer.
While several clinical studies have demonstrated a higher level of efficacy of oxytocin vis-à-vis misoprostol in the prevention of PPH, [9] no studies have examined the relative merits of these two drugs in community-level studies, under sub-optimal conditions where many births take place, i.e., either at home or at sub-centers with only traditional birth attendants to assist in deliveries [10–13]. The cost-effectiveness analysis presented here focuses on comparing interventions using these two drugs for prevention of PPH in a community-based setting.
II. Materials and Methods
A cluster randomized trial at the community level was conducted in three predominantly rural districts of Senegal from June 2013 to September 2013 to compare the prophylactic effectiveness of misoprostol—600 mcg orally administered—with that of oxytocin—10 IU administered intramuscularly via the Uniject system (Instituto Biologico Argentina S.A.I.C., Buenos Aires, Argentina)—during the third stage of labor.[14] The Uniject system is a single-use injection device with a TTI (time temperature indicator) that signals when the medication contained in the device has been heat compromised. Auxiliary midwifes (matrones) conducted the trial in 28 village “health huts” (maternity huts with a delivery table but no instruments or medicines), with 14 huts in each of the two arms of the study. All consenting women delivering at health huts were included in the trial. The primary outcome measure was the change in hemoglobin level (g/dl) measured at a prenatal visit before delivery and again within 48 hours of delivery. Referral to health centers or hospitals for PPH treatment was recorded in the study as a secondary outcome measure, as was a drop in hemoglobin of 2 g/dl or more. In all, 1,445 women were recruited into the trial: 912 women in the misoprostol arm and 533 women in the oxytocin arm. The difference in the number of women in the two arms arose from inaccurate information on village characteristics used at the time of sample selection. A later inspection of health hut records showed that villages randomly selected for the oxytocin arm actually had fewer deliveries per year than in the other arm. Refusal rates to participate in the study were similar in the two arms
The key results of the main study’s randomized trial comparing the efficacy of the two drugs are presented elsewhere, but are summarized here.[14] There was no statistically significant difference between the two arms of the study in the primary outcome measure, change in hemoglobin level. The mean decrease in hemoglobin count pre- and post-intervention was 0.3 g/dl (±1.6) for misoprostol and 0.2 g/dl (±1.7) for oxytocin, a non-significant difference (p=0.17). There were no PPH referrals in the misoprostol arm and only one in the oxytocin arm. The referral rates, therefore, were 0.0% for misoprostol (95% confidence interval 0% – 1.2%) and 0.2% for oxytocin (95% confidence interval 0.0% – 2.0%). There were no deaths attributable to PPH in the trial. There were no serious side effects in either arm, although shivering was more common after having received misoprostol.
Utilizing the data and findings from the randomized trial, we conducted a cost-effectiveness analysis comparing the two strategies to prevent PPH at the community level: administering misoprostol versus administering oxytocin (Uniject®). The principal outcome measure that we used was the referral of PPH cases to a health center or hospital. This measure was a proxy variable for PPH, as the main study did not directly measure PPH (i.e., postpartum blood loss of 500+ ml).
We calculated costs and effects to two hypothetical cohorts of 150,000 women delivering during a one-year period, each receiving one intervention or the other. This number was chosen to approximate the annual number of non-institutional births presently occurring in Senegal [15, 16]. A third cohort of the same size simulated current standard of care (SOC) practices.
Costs were calculated for the year 2013, using US dollars. Since we adopted a health-system perspective, we ignored costs borne by the individual woman, her family or society, including losses in productivity and income, or other social, psychological and intergenerational costs.
For each intervention, the total cost per case (i.e., per delivery) was the sum of the cost of the commodity (misoprostol or oxytocin), the cost of training matrones to administer the drug, the cost of distribution and administration of the drug, the cold-chain cost, and the wastage cost (Table 1). The per-case commodity cost of oxytocin in Uniject (USD 1.44) was derived directly from the invoices of the main study and included shipping and insurance fees as well as a handling fee for refrigeration en route. The commodity cost for misoprostol (USD 0.42) was obtained from local organizations based on the costs of recent purchases.
Table 1.
Components of the Cost per Intervention to Prevent PPH, Senegal, 2013
| Cost Component | Cost in USD (2013)
|
|||
|---|---|---|---|---|
| Prevention Using Misoprostol | Prevention Using Oxytocin | |||
| Matrone training cost | $ 1.68 | $ 1.86 | ||
| Commodity cost | $ 0.42 | $ 1.44 | ||
| Cost of commodity wastage | $ 0.02 | $ 0.17 | ||
| Cost of cold chain | $ - | $ 0.84 | ||
| Distribution/use cost | $ 0.09 | $ 0.06 | ||
|
| ||||
| TOTAL COST PER PPH INTERVENTION | $ 2.21 | $ 4.38 | ||
The time taken to train matrones for the main study to be able to competently administer either misoprostol or oxytocin was used in calculating the training cost (computational details can be found in the online addendum). The per-case training costs on the two interventions were USD 1.86 (oxytocin) and USD 1.68 (misoprostol).
We estimated that the cost of distributing and using the two drugs contributed only a small proportion to the total per-case cost: USD 0.06 (oxytocin) and USD 0.09 (misoprostol). The computations required various assumptions, but measurement errors that these assumptions might have introduced to the overall cost calculation were slight (computational details can be found in the online addendum).
We also calculated the cost of wastage in the logistics of supplying the two drugs. We were unable to find an estimate of the extent of wastage in the public drug supply system for misoprostol in tablet presentation. In the main study the wastage rate for misoprostol was less than 1%. As this rate was from a controlled study and may not be representative of typical wastage rates, we used a commonly used wastage rate (5%). For oxytocin, we used the experience in the main study where 12.1% of the Uniject devices were discarded due to breakage, being compromised by heat or having passed the expiration date. The estimated per-case cost of wastage was USD 0.17 for oxytocin and USD 0.02 for misoprostol.
Finally, we estimated a per-case cost of maintaining a cold chain for oxytocin, keeping in mind that the cold chain reaches down only to the health center/rural hospital level (oxytocin in Uniject form was not kept refrigerated at the health-hut level). We obtained data from the ministry of health on annual outlays for the existing cold chain (computational details can be found in the online addendum). We estimated that the cold-chain component added USD 0.84 to the total per-case cost of oxytocin.
As discussed above, two effects measured in the main study were available for our cost-effectiveness analysis: (1) hemoglobin drops of ≥2 g/dl; and (2) cases referred due to PPH. The methodological difficulties in measuring PPH have been widely acknowledged [17] and the relationship between hemoglobin drop and blood loss is not well established, some finding a positive correlation while others find none [18–22]. In view of this uncertainty we opted to not use this measure of effectiveness, relying instead on the rate of PPH referrals.
We compared the effects of the two prophylactic interventions to the standard of care (SOC) as it exists in rural Senegal. In an environment where women deliver at home or in a health hut with no equipment or drugs for even basic emergency obstetric care and no trained professional to undertake such care, the standard of care consisted entirely of referral of PPH cases to a higher-level facility. The SOC rate of PPH referral should roughly equal the incidence of severe PPH (blood loss >1000 ml), assuming that all referrals reached higher-level facilities. We used a published estimate for rural populations of 3% of delveries as the rate of severe PPH [23] and hence, under SOC, a PPH referral rate of 3%.
Incremental costs were calculated as the difference between the cost of providing the misoprostol or oxytocin intervention to a cohort of 150,000 women giving birth versus the cost associated with SOC to the same cohort of women. The incremental outcomes were the differences between number of PPH referrals in the two intervention arms of the study and the same outcomes under SOC. Incremental costs and incremental outcomes were used to compute incremental cost-effectiveness ratios (ICERs). An ICER is the incremental change in costs after an intervention divided by the incremental change in the effect after the intervention. In general, statistical significance was taken to mean a p-value of 0.05 or less. Ordinary least squares regression was used in the main study to test significance. The significance, or lack of significance, found in the main study was assumed to carry over to the cost-effectiveness analysis.
We undertook univariate sensitivity analysis to examine how uncertainty in several of the parameters that fed into calculations of ICERs affected our findings and to see which parameters affected the results most when their values were altered. The main study’s findings were used to determine upper and lower limits for the outcome measure using its 95% confidence intervals. For other parameters we used ±25% of the central estimates. Table 2 summarizes the maximum and minimum values of the parameters.
Table 2.
Parameter Input Values
| Base Case | Low Case | High Case | Sources | |
|---|---|---|---|---|
|
| ||||
| Costs of misoprostol intervention (US$ 2013) | ||||
| Matrone training cost | $1.68 | $1.35 | $2.10 | Base: Main study; Low: −25% of base case; High: +25% of base case |
| Commodity cost | $0.42 | $0.34 | $0.53 | Same |
| Cost of commodity wastage | $0.02 | $0.02 | $0.03 | Same |
| Cost of cold chain | $0.00 | $0.00 | $0.00 | Base: Side study**; Low: −25% of base case; High: +25% of base case |
| Distribution/use cost | $0.09 | $0.07 | $0.11 | Same |
| Costs of oxytocin intervention (US$ 2013) | ||||
| Matrone training cost | $1.86 | $1.49 | $2.33 | Same |
| Commodity cost | $1.44 | $1.15 | $1.80 | Same |
| Cost of commodity wastage | $0.17 | $0.14 | $0.22 | Same |
| Cost of cold chain | $0.84 | $0.67 | $1.05 | Same |
| Distribution/use cost | $0.06 | $0.05 | $0.08 | Same |
| Cost of SOC (US$ 2013) | ||||
| Minimal inputs | $1.00 | $0.80 | $1.25 | We assume a cost of $1 for SOC (matrones time, incidental medicines such as analgesics) |
| Outcomes under misoprostol* | ||||
| Proportion of delivering women referred due to PPH | 0.000 | 0.000 | 0.012 | Base: Main study; Low and High: 95% confidence interval |
| Outcomes under oxytocin* | ||||
| Proportion of delivering women referred due to PPH | 0.002 | 0.000 | 0.020 | Base: Main study; Low and High: 95% confidence interval |
| Other rates | ||||
| Proportion of delivering women with severe PPH | 0.032 | 0.025 | 0.040 | Base: Several studies***; Low: −25% of base case; High: +25% of base case |
| Proportion of delivering women receiving misoprostol | 0.984 | 0.982 | 0.987 | Base: Main study; Low and High: 95% confidence interval |
| Proportion of delivering women receiving oxytocin | 0.958 | 0.954 | 0.961 | Base: Main study; Low and High: 95% confidence interval |
The differences between the outcomes of the misoprostol and oxytocin interventions were not statistically significant.
Study of cold chain of ministry of health conducted by an independent consultant using unpublished government reports and data.
Carroli et al. 2008: Bateman et al. 2010; Derman et al. 2006; Govt. of India 2013; Mehrabadi et al. 2012; Mobeen et al. 2011; Oyelese and Anath 2010; and Prata et al. 2007.
III. Results
In the baseline case—using the central estimates of all parameters—a cohort of 150,000 delivering women would experience 74 referrals for severe PPH under a prophylactic regime using misoprostol and 490 referrals if oxytocin in Uniject were used. Compared to SOC, using misoprostol would avert 4,666 PPH referrals, while using oxytocin would prevent 4,250 referrals (Table 3). The corresponding ICERs are USD 38.96 (misoprostol) per PPH case averted and USD 119.15 (oxytocin). The misoprostol intervention, therefore, dominates the oxytocin one.
Table 3.
Cost-effectiveness of Misoprostol and Oxytocin as Interventions to Prevent PPH Applied to 150,000 Deliveries
| Number of Referrals | Number of Referrals Averted | Total Cost (for 150,000 Births) | Change in Total Cost | ICER | |
|---|---|---|---|---|---|
| Outcome Measure: Referral to Higher-Level Facility | |||||
| Baseline | |||||
| Misoprostol | 74 | 4,666 | $331,758 | $181,758 | $38·96 |
| Oxytocin | 490 | 4,250 | $656,388 | $506,388 | $119·15 |
| SOC | 4,740 | 0 | $150,000 | ||
| Misoprostol Worst Case | |||||
| Misoprostol | 1,827 | 2,913 | $414,697 | $264,697 | $90·86 |
| Oxytocin | 186 | 4,554 | $525,110 | $375,110 | $82·37 |
| SOC | 4,740 | 0 | $150,000 | ||
| Misoprostol Best Case | |||||
| Misoprostol | 86 | 4,654 | $265,406 | $115,406 | $24·80 |
| Oxytocin | 3,129 | 1,611 | $820,485 | $670,485 | $416·29 |
| SOC | 4,740 | 0 | $150,000 | ||
We also calculated best-case and worst-case scenarios using the misoprostol arm of the study as the reference point. In the best case scenario the values of all parameters were given their low-case or high-case values, as shown in Table 2, depending on which would be more favorable to the misoprostol ICER. In the worst case scenario parameter values were set in exactly the opposite pattern so as to be least favorable to the misoprostol ICER (and hence most favorable to the oxytocin ICER). As shown in Table 3, the misoprostol intervention dominates the oxytocin one in both the baseline and the best-case scenarios. In the worst-case scenario the oxytocin intervention is slightly more cost-effective. We see, therefore, that the strategy of preventive use with misoprostol dominates the competing strategy of using oxytocin (in Uniject format) except under the unlikely assumption that all the underlying parameters take on values least favorable to misoprostol.
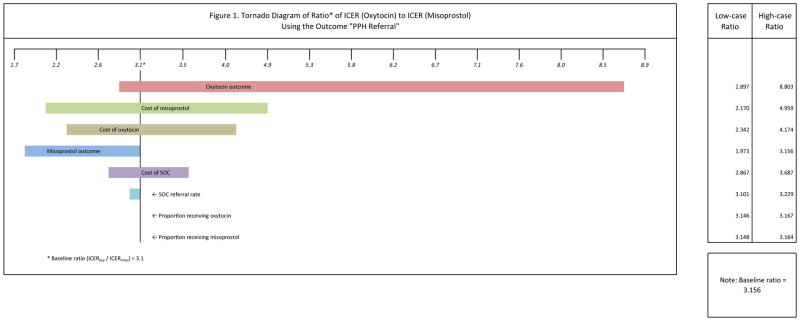
We performed a sensitivity analysis by replacing, in turn, the baseline value of each parameter with its corresponding low-case value and then with its high-case value (see Table 2). The results of the sensitivity analysis are shown in the tornado diagram in Figure 1. Since we are determining cost-effectiveness by comparing the ICERs of the two arms of the study, we examine the sensitivity of the ratio of the oxytocin ICER ($119.15) to the misoprostol ICER ($38.96) to changes in parameter values. In the baseline case this ratio was 3.1 (119.15/38.96), meaning that the oxytocin ICER was 3.1 times greater than the misoprostol. The size of the bar of a parameter shows how sensitive the relative effect is to changes in the value of the parameter.
Figure 1.
Tornado Diagram of Ratio* of ICER (Oxytocin) to ICER (Misoprostol) Using the Outcome “PPH Referral”
The relative cost-effectiveness of misoprostol compared to oxytocin was most sensitive to changes in the rate of PPH referrals after oxytocin prophylaxis. For example, if the rate of referrals was increased to the upper limit of the confidence interval, the cost of using oxytocin to prevent one PPH referral case would be 8.7 times greater than using misoprostol. Changes in the cost of misoprostol also affected relative cost-effectiveness, though to a lesser extent. Cost-effectiveness results were also moderately sensitive to changes in the cost of oxytocin, changes in the rate of referrals after misoprostol prophylaxis and the cost of SOC. The ratio of the two ICERs was virtually unaffected by changes to the proportions of delivering women receiving misoprostol or oxytocin. The overall finding of the sensitivity analysis was that the misoprostol intervention dominated no matter what changes were made to the relevant parameters.
IV. Discussion
In Senegal, where maternal mortality is a major health problem and where a significant proportion of deliveries take place at home or at rural health huts, finding cost-effective interventions that help prevent PPH is a health policy priority. This study shows that, although the preventive administration of misoprostol or oxytocin immediately after delivery may be equally effective in reducing PPH, misoprostol is more cost-effective in a health-hut, rural setting. The lower cost of the misoprostol intervention, vis-à-vis the oxytocin one, is the main factor behind this result. The finding is further bolstered if we recall that this study’s oxytocin intervention utilized the Uniject modality, which obviated the need for a cold chain reaching down to the health-hut level and also implied less training than oxytocin in its traditional format (ampoules, syringes, etc.). Without Uniject, the logistics of using oxytocin would have been substantially more expensive since the cold chain would have to continue down to the health hut level. [24]
A limitation of this study is that, although matrones were trained to recognize the signs of incipient PPH, some subjectivity remained in decisions to refer a postpartum woman. Another source of uncertainty was the incidence of PPH in the absence of an adequate standard of health care. We relied on findings in the literature to estimate the incidence rate of severe PPH. However, the sensitivity analysis showed that changes in this rate would have little effect on the study’s findings (see the sixth bar of Figure 1).
A full scale-up of the preventive intervention using misoprostol would cost the Senegal health system around USD 332,000 annually (assuming that the intervention covered all deliveries taking place at home or in health huts) and would avert 4,666 referrals due to severe PPH. We can roughly compute what this would mean for maternal mortality in Senegal. Currently, around 1,740 maternal deaths occur each year in Senegal and, of these, perhaps 720 occur to women delivering at health huts. We assume that women delivering at health huts would have a higher maternal mortality ratio (MMR) than the general population. For this illustrative example, we estimate the MMR to be 50% higher than the national average (320 per 100,000 live births). [2] Using the WHO estimate that 27% of maternal deaths are due to PPH, around 195 deaths can be attributed to complications from PPH among women delivering in health huts. If we further assume that the “natural” incidence rate of severe PPH is around 3% of deliveries, then the PPH referral cases averted by this preventive intervention would translate to around 192 fewer maternal deaths annually. (Even though it is unlikely that any single intervention would almost entirely eliminate one component of maternal mortality, the trial results did show zero referrals from the misoprostol arm.) The estimated ICER for averting one maternal death due to PPH would then be about USD 1,700. This level of cost effectiveness may be compared to the WHO recommendation of what is “highly cost-effective” (USD 800) and “cost-effective” (USD 2,400) [25, 26].
Clinical studies in hospital settings have amply demonstrated that misoprostol administered immediately after delivery is effective in the prevention of PPH and that its level of effectiveness is broadly equivalent to that of oxytocin [9]. The side effects of misoprostol use have also been shown by these studies to be mild, short lasting, and generally acceptable to patients. In this study we demonstrate that, in a rural community setting with only minimal health care provided by matrones, use of misoprostol as a prophylactic is a more cost-effective strategy than is use of oxytocin. Furthermore, introducing this intervention nationally would reduce maternal deaths and maternal morbidity, and the health-care costs associated with treating referred PPH cases would be lowered. This would offset the cost of implementing this intervention nationwide and might even result in net savings. If the average cost to the health system per PPH case were greater that the ICER (USD 38.96) then introducing this preventive intervention would result in a net saving. Our findings suggest that in countries characterized by a substantial proportion of births taking place without the presence of skilled health providers, implementation of PPH prevention based on misoprostol would be cost-effective and help improve the health of mothers in low and middle-income countries.
Synopsis.
Prevention of postpartum hemorrhage using misoprostol was more cost-effective than oxytocin in a home-delivery context in Senegal.
Acknowledgments
The study was funded by Gynuity Health Projects through a grant from the Bill and Melinda Gates Foundation.
Footnotes
Declaration of interests
We declare no competing interests.
References
- 1.Say L, Chou D, Gemmill A, Tuncalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet. 2014;2(6):e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Trends in Maternal Mortality: 1990 to 2013. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 3.Prata N, Passano P, Bell S, Rowen T, Potts M. New hope: community-based misoprostol use to prevent postpartum haemorrhage. Health Policy and Planning. 2013;28(4):339–46. doi: 10.1093/heapol/czs068. [DOI] [PubMed] [Google Scholar]
- 4.Gulmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet. 2001;358(9283):689–95. doi: 10.1016/s0140-6736(01)05835-4. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO Statement Regarding the Use of Misoprostol for Postpartum Haemorrhage Prevention and Treatment. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 6.Tunçalp O, Souza JP, Gülmezoglu M. New WHO recommendations on prevention and treatment of postpartum hemorrhage. IJGO. 2013;123:254–56. doi: 10.1016/j.ijgo.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial. PLOS Medicine. 2013;10(10):e1001524. doi: 10.1371/journal.pmed.1001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gizzo S, Patrelli TS, Gangi SD, Carrozzini M, Saccardi C, Zambon A, et al. Which uterotonic is better to prevent the postpartum hemorrhage? Latest news in terms of clinical efficacy, side effects, and contraindications: a systematic review. Reproductive Sciences. 2013;20(9):1011–9. doi: 10.1177/1933719112468951. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeyr GJ, Gulmezoglu AM, Noviova N, Lawrie TA. Postpartum misoprostol for preventing maternal mortality and morbidity (review) The Cochrane Database of Systematic Reviews. 2013;7:CD008982. doi: 10.1002/14651858.CD008982.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Diadhiou M, Dieng T, Ortiz C, Mall I, Dione D, Sloan NL. Introduction of misoprostol for prevention of postpartum hemorrhage at the community level in Senegal. Int J Gynecol Obstet. 2011;115(3):251–5. doi: 10.1016/j.ijgo.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Oladapo OT. Misoprostol for preventing and treating postpartum hemorrhage in the community: A closer look at the evidence. Int J Gynecol Obstet. 2012;119(2):105–10. doi: 10.1016/j.ijgo.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Smith JM, Gubin R, Holston MM, Fullerton J, Prata N. Misoprostol for postpartum hemorrhage prevention at home birth: an integrative review of global implementation experience to date. BMC Pregnancy and Childbirth. 2013;13:44. doi: 10.1186/1471-2393-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley SE, Prata N, Young-Lin N, Bishai DM. Cost-effectiveness of misoprostol to control hemorrhage in low-resource settings. Int J Gynecol Obstet. 2007;97(1):52–6. doi: 10.1016/j.ijgo.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Diop A, Daff B, Sow M, Blum J, Diagne M, Sloan NL, et al. Oxytocin in Uniject versus misoprostol for prevention of postpartum hemorrhage at the community level: A cluster randomized controlled trial. Lancet Global Health. doi: 10.1016/S2214-109X(15)00219-3. in press. [DOI] [PubMed] [Google Scholar]
- 15.Senegal National Statistical and Demographic Agency. Demographic and Health Survey and Multiple Indicator Cluster Survey, 2010–2011, Final report. Dakar, Senegal: National Statistical and Demographic Agency; 2012. [Google Scholar]
- 16.United Nations, Population Division. [accessed June 1, 2014];World Population Prospects: The 2012 Revision. http://esa.un.org/unpd/wpp/unpp/panel_population.htm.
- 17.Rath W. Postpartum hemorrhage – update on problems of definitions and diagnosis. ACTA Obstetricia et Gynecologica. 2011;90(5):421–8. doi: 10.1111/j.1600-0412.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruns B, Lindsey M, Rowe K, Brown S, Minei JP, Gentilello LM, et al. Hemoglobin drops within minutes of injuries and predicts need for an intervention to stop hemorrhage. Journal of Trauma. 2007;63(2):312–5. doi: 10.1097/TA.0b013e31812389d6. [DOI] [PubMed] [Google Scholar]
- 19.Knottenbelt JD. Low initial hemoglobin levels in trauma patients: an important indicator of ongoing hemorrhage. Journal of Trauma. 1991;31(10):1396–9. doi: 10.1097/00005373-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Pazarli P, Yarkin T, Karakurt Z, Yetis Duman D, Salturk C, Celik B, et al. Follow-up hemoglobin concentrations in ICU: relationship between diagnostic blood loss and daily fluid balance. Tuberk Toraks. 2007;55(4):323–8. [PubMed] [Google Scholar]
- 21.Rajaei M, Karimi S, Shahboodaghi Z, Mahboobi H, Khorgoei T, Rajaei F. Safety and efficacy of misoprostol versus oxytocin for prevention of postpartum hemorrhage. Journal of Pregnancy. 2014;2014:713879. doi: 10.1155/2014/713879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelop C. Postpartum hemorrhage: becoming more evidence-based. Obstetrics and Gynecology. 2011;117(1):3–5. doi: 10.1097/AOG.0b013e318202ec9a. [DOI] [PubMed] [Google Scholar]
- 23.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practices & Clinical Obstetrics and Gynaecology. 2008;22(6):999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Pichon-Riviere A, Glujovsky D, Garay OU, Augustovski F, Ciapponi A, Serpa M, et al. Oxytocin in Uniject Disposable Auto-Disable Injection System versus Standard Use for the Prevention of Postpartum Hemorrhage in Latin America and the Caribbean: A Cost-Effectiveness Analysis. PLoS One. 2015 Jun 9;10(6):e0129044. doi: 10.1371/journal.pone.0129044. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO–Choosing Interventions that are Cost Effective (WHO-CHOICE) Cost effectiveness thresholds. Geneva: World Health Organization; 2010. [accessed May 29, 2010]. http://www.who.int/choice/costs/CER_levels/en/index.html. [Google Scholar]
- 26.Sutherland T, Bishai DM. Cost-effectiveness of misoprostol and prenatal iron supplementation as maternal mortality interventions in home births in rural India. Int J Gynecol Obstet. 2009;104(3):189–93. doi: 10.1016/j.ijgo.2008.10.011. [DOI] [PubMed] [Google Scholar]