Abstract
Background
Total body photography may aid in melanoma screening, but is not widely applied due to time and cost. We hypothesized that a near-simultaneous automated skin photo-acquisition system would be acceptable to patients and could rapidly obtain total body photographic images that enable visualization of pigmented skin lesions.
Methods
From 2/09–5/09, a study of 20 volunteers was performed at the University of Virginia to test a prototype 16-camera imaging booth built by the research team and to guide development of special purpose software. For each participant, images were obtained before and after marking ten lesions (5 “easy” and 5 “difficult”), and images were evaluated to estimate visualization rates. Imaging logistical challenges were scored by the operator, and participant opinion was assessed by questionnaire.
Results
Average time for image capture was 3 minutes (range 2–5). All 55 “easy” lesions were visualized (sensitivity 100%, 90%CI 95–100%) and 54/55 “difficult” lesions were visualized (sensitivity 98%, 90%CI 92–100%). Operators and patients graded the imaging process favorably, with challenges identified regarding lighting and positioning.
Conclusions
Rapid-acquisition automated skin photography is feasible with a low-cost system, with excellent lesion visualization and participant acceptance. These data provide a basis for employing this method in clinical melanoma screening.
Keywords: Skin cancer screening, melanoma, photography, cancer screening
Introduction
Early detection of melanoma is critical to maximizing a chance for cure. Since the vast majority of melanomas are visible on the skin, screening for melanoma through routine skin exams detects the majority of suspicious lesions. However, the sensitivity and specificity of a skin exam is likely altered by the expertise and the memory of the practitioner and patient (Slue et al., 1988;Helfand et al., 2001;Malvehy and Puig, 2002a;Risser et al., 2007). Many lesions are located in areas not visible to the patient (Slue et al., 1988), and this further limits existing approaches.
Primary care physicians are responsible for age-appropriate screening of their patients. In the era of decreasing reimbursement and shorter time for patient visits, skin exams have been marginalized. Primary care physicians generally consider digital rectal exam, manual breast exam, and Pap smear as more important uses of their time than skin exams(Altman et al., 2000). Dermatologists are a logical place to look to fill the gap in skin cancer screening. Unfortunately, there is already a shortage of dermatologists in the United States (Suneja et al., 2001;Tsang and Resneck, Jr., 2006), and further contraction in the effective supply of dermatologists is projected (Suneja et al., 2001;Jacobson et al., 2004). This shortage is exacerbated in underserved medical areas (Suneja et al., 2001). In a recent study, researchers posing as new patients called 851 dermatologists to request an appointment for a “changing mole” -- a possible sign of skin cancer: the average wait was more than a month(Tsang and Resneck, Jr., 2006). Many physicians have turned to physician extenders to meet the demand for skin cancer screening(Clark et al., 2000;Tsang and Resneck, Jr., 2006), but this still requires training and is subject to all the issues related to physical exam discussed above. If a convenient, reliable and affordable method were available for skin cancer screening, it is likely there would be increased access and stronger recommendations for annual skin cancer screening.
Various technologies have been employed to aid in skin cancer screening. Digital surface microscopy (dermoscopy) is useful in diagnosis and surveillance of skin lesions (Menzies et al., 2001;Terushkin et al., 2010), but it requires formal training and specialized equipment and is very operator-dependent (Bafounta et al., 2001;Malvehy and Puig, 2002a) Digital photography is used more and more often to document a baseline skin exam (Slue et al., 1988;Slue, Jr., 1992;Feit et al., 2004;Risser et al., 2007) or for lesion surveillance with serial imaging (Shriner et al., 1992;Shriner and Wagner, Jr., 1992). Select centers create a body map of these images to facilitate the tracking of lesions over time. (Malvehy and Puig, 2002) Photography as an adjunct to traditional clinician screening exams can improve accuracy and early detection of skin cancer(Slue et al., 1988; Rigel et al., 1989; Shriner et al., 1992; MacKie et al., 1993; Kelly et al., 1997; Rhodes, 1998; Hanrahan et al., 1998; Oliveria et al., 2004). While physicians and patients are interested in photography as an aid to melanoma screening (Hanrahan et al., 2000), adaptation of this technology as a routine population screening mechanism is limited by financial and logistical constraints (Terushkin et al., 2010). Most centers use a handheld camera and photographer (professional photographer, physician or nurse) to acquire an average of 24 images (range 4–50) (Slue et al., 1988;Rhodes, 1998;Nehal et al., 2002;Phelan et al., 2005;Terushkin et al., 2010).
An automated photography system to streamline and standardize this screeining practice has been evaluated in the private practice setting and found to be effective at diagnosing thin melanoma (Drugge et al., 2009). Patient satisfaction and feasibility of their imaging method were not reported. Success with this described system is promising and supports further research of rapid-photography systems in additional melanoma centers. We developed a novel, near-simultaneous multi-camera automated skin photo acquisition system. We hypothesized that this system could rapidly capture complete skin photographs with patients in just 2 positions and could be acceptable to patients.
Methods
Skin cancer screening survey
Prior to initiating the clinical trial, we initiated a study at our melanoma clinic investigating patient’s opinions and adherence to skin cancer screening. After receiving IRB approval (IRB HSR#13534), we contacted seventy patients through our melanoma clinic. Patients were selected for survey if they had previously agreed to be contacted by email for this purpose and if they had an active email address. The survey gathered information on the patient’s skin cancer history, screening regimen and their opinion about various screening modalities and the time they would be willing to spend for screening. The original survey was distributed electronically in February 2008 with two subsequent email reminders in February and March 2008. The survey was completed anonymously. All responses were received by April 2008.
Prototype development
Our team initiated development of a prototype device which includes a framed booth measuring 6ft by 6ft by 6ft, mounted with 16 digital cameras (Canon Powershot A520 4MP and Canon PowerShot A80 4MP). As shown schematically in Fig. 1, the cameras are fixed in a four vertical arrays of four cameras each, located in each corner of the square booth. To aid in background segmentation and to minimize specular reflection, the prototype system walls are draped in a blue fabric of relatively consistent color. The cameras are capable of simultaneous capture of 16 high-resolution images of patient skin in a single pose; 24-bit color images of size 1704×2272 pixels are obtained from each camera. A complete dataset contains multiple acquisitions of patient skin in two poses, ensuring complete skin coverage. The cameras are connected via USB extenders into a standard personal computer (PC). The PC is equipped with 2GB of memory and an Intel 2 Core Duo processor. A basic interface to obtain images and automated photo acquisition have been developed in the C-sharp programming environment.
Figure 1.
Diagram of prototype imaging system, Dermagram. Here, each green sphere represents a single camera focused on a different portion of patient skin.
The program currently uses Canon SDK version 7.3, which supports a number of Canon cameras, including the A520 and the A80. In order to allow for simultaneous shutter release, the program calls a process for each camera and uses TCP sockets to issue commands. Various parameters and modes of the camera can be controlled through the program interface. The program also allows the computer user to preview the subject using the viewfinders of the cameras to aid in calibration if necessary. After the shutter release is activated in the graphics user interface (GUI) and the cameras take their images, they send them back to the computer where they are stored in a folder. using 16 cameras, operated by software. To capture near-complete skin imaging with this device, patients were imaged in two unique positions, one standing and one kneeling on a stool.
Pilot clinical study
From 2/09–5/09, a pilot study of 20 volunteers was performed using this prototype device. The study was designed to allow for optimization of the imaging system during the first cohort of patients. Nine participants were imaged during this development phase and adjustments to patient positioning and the operating system were made.
The final 11 participants were imaged under the definitive design plan for data analysis. Participants were instructed on the correct positioning for the imaging prior to entering the Dermagram booth. A primary imaging session was completed, imaging patients in Positions 1 and 2. After initial imaging, a complete skin exam was performed by a physician, and 10 pigmented skin lesions were identified to be evaluated with the imaging system. These included 5 lesions in difficult locations (“difficult” lesions), defined as lesions in a skin fold, in hair-bearing areas, along hair lines, in the umbilicus, behind the ears, between the fingers or toes, and under the chin. They also included 5 “easy” lesions, defined as lesions on the arms and legs, trunk, buttocks, face and not in areas defined as difficult. Skin markers were placed next to each of these lesions, and a second series of photographs were obtained in the same manner described above, to document the location of these lesions. The maximum diameter of each lesion was recorded. At the completion of the trial, the images from all eleven participants were reviewed by a physician. Marked lesions were scored as identified or not identified on patient images.
Imaging logistical challenges were scored by the operator, and participant opinion was assessed by questionnaire.
Results
Skin cancer screening survey
The response rate on the patient survey about skin cancer screening was 73% (51/70). Even in this high-risk patient population (98% of responders report a history of melanoma), 10% of responders had never had a complete skin exam, and only 26% had ever had photographs taken as part of a skin exam. However, 70% wished to have complete skin photography, and 80% were interested in having it performed via an automated photo booth. The majority (70%) of participants was willing to spend any amount of time needed to obtain complete skin photography and the remaining participants reported the following time restrictions: less than 2 min (2%), 10 minutes (4%), 20 minutes (10%), 30 minutes (8%).
Pilot clinical trial lesion detection
Eleven participants were imaged under the definitive design for data analysis: 9 males and 2 females. Nine had a personal history of melanoma. The median time since their last skin exam by a health care professional was three months (range 0–12 months). Average time for image capture was 3 minutes (range 2–5). The 55 “easy” lesions had a mean lesion size of 4.7 mm (1–22 mm) and included 14 lesions on the extremities, 37 on the trunk, 2 on the neck and 2 on the face. All 55 “easy” lesions were visualized (sensitivity 100%, 90%CI 95–100%) The 55 “difficult” lesions had a mean lesion size of 3.9 mm (2–11 mm) and included 11 lesions on the extremities, 34 on the trunk, 5 on the neck and 5 on the face. Fifty-four of 55 “difficult” lesions were visualized (sensitivity 98%, 90%CI 92–100%). The lesion that was not detected measured 2mm and was on the participant’s right lateral arm, just distal to the elbow, in a dense hair-bearing area.
Operator and participant assessment
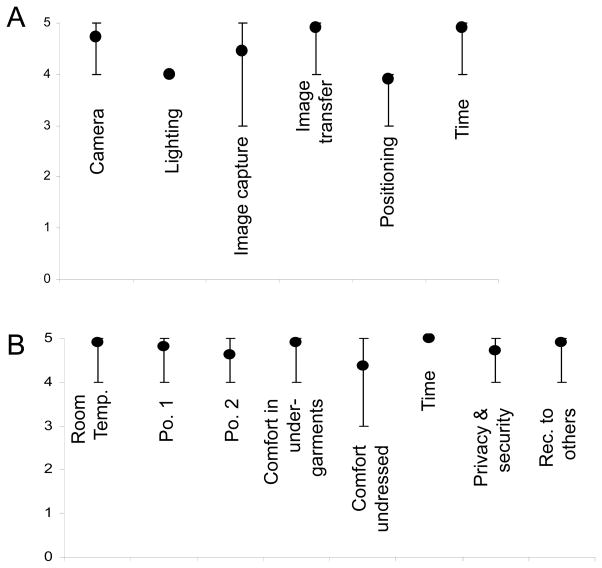
No adverse events occurred. Operator and participants graded the imaging process on a 5 point scale, with 5 being best and 1 being worst. The mean operator score for the feasibility and logistics of the system was 4.6. Positioning of the subjects and lighting were the biggest challenges with mean scores of 3.9 and 4, respectively (Figure 2A). The operator scored the time required favorably, with a mean score of 4.9.
Figure 2.
Images acquired with the prototype Dermagram imaging device with original image (left) and then zooming to 3X (center) and then (10X), demonstrating image resolution of lesions 1–2mm in diameter (right).
The mean overall participant opinion score was 4.8, with the most favorable scoring given to the time required (mean 5) and the least favorable score given to being fully naked for imaging (mean 4) and the comfort of position 2 (kneeling, 4) (Figure 2B). The participants were given the option to wear undergarments, and 9/11 participants elected to wear them. The two participants who chose not to wear undergarments each scored their comfort with this as 5. Overall, the participants would recommend this imaging to others strongly, mean score 4.9.
Discussion
In patients with atypical moles, skin photography improved detection of new or changing lesions (Barnes and Nordlund, 1987; Halpern et al., 1993) and earlier detection of melanoma (Rivers et al., 1990). As seen with our survey in this study and by previous reports (Hanrahan et al., 2000), patients would like to participate in photographic screening programs. The use of complete skin photography has increased in the past decade and with a CPT code introduced in 2007 (AMA CPT 2007), some support for this screening modality is available. However, access to complete skin photography continues to be limited by logistical and financial constraints on the part of health care providers. Access to a rapid-acquisition system with automated image processing and analysis would improve the cost: benefit ratio and would facilitate penetration of this type of screening tool into population-based screening programs.
The goal of our total body imaging system is not to diagnose individual skin lesions but to screen for high-risk lesions that require follow-up examination or biopsy by a health care practitioner. Broad adoption of skin photography as a routine screening mechanism would allow for monitoring of changes over time in repeated imaging sessions. Documented changes in skin lesions would have advantages over relying on the memory of the patient or physician to identify change in skin lesions.
The present study supports the feasibility of rapid-acquisition automated skin photography, with excellent visualization of small lesions and high participant acceptance. Low-cost population-based screening will be facilitated by the ability to acquire images without skilled staff and in less than 5 minutes. Participants scored this system very favorably. There was a selection bias for high-risk individuals, with 9 of the 11 final participants having a personal history of melanoma. This limits application of the participant scoring to the population at large; however, these participants have significant experience with clinician skin exams and therefore are familiar with alternate screening tools, adding strength to their evaluation. Additionally, this higher-risk population would be the most important target audience for skin cancer screening, and their acceptance of an automated imaging-system is critical to broad adoption of the screening tool.
Despite the encouraging findings, this trial also highlights challenges that need to be addressed before broad adoption of this method. The use of standard flash photography, with the use of multiple cameras with individual flashes, results in competing flashes and light detection with an imaging booth like this one, where cameras and lights are positioned on opposite sides of the imaging booth. The use of background lighting alone, however, did not provide optimal illumination of the entire body surface, especially in areas such as the axilla or beneath the chin. Future work is needed to develop a lighting source which is consistent over the body surface and over time.
One category the participants scored poorly was the suggestion of being (almost) nude for imaging sessions. Confounding factors in this data include that the trial was conducted in a research space and was not specifically for clinical decision-making. The participants also found the kneeling position (position #2) uncomfortable. We had designed this position as it would allow us to capture the axilla, bottoms of the feet and inner legs in one position. Given that the participants were happy with the time it took and that 70% of our survey respondents were willing to invest any amount of time required, future designs may benefit from using easier patient-positioning at the expense of a marginal increase in time required.
Despite some challenges with this prototype system, lesion detection was excellent, and 109/110 lesions (99%) marked in this pilot study were visualized on the images obtained. Clinical application of this screening tool would be facilitated by systems for easy image viewing and decision support software to guide a clinician’s use of these images. Overall, these data provide a basis for optimizing this method for use in clinical melanoma screening, with future plans to add automated lesion detection and longitudinal lesion tracking.
Figure 3.
Imaging sessions scored on a scale of 1–5, with 5 being the best and 1 being the worst. Circles represent mean values and error bars represent the maximum and minimum values assigned. A) Operator opinion. B) Participant opinion.
Acknowledgments
This research was funded by The Thelma R. Swortzel Collaborative Research Award and the Commonwealth Foundation for Cancer Research. Further funding for research team members was provided by a Cardiovascular Surgery Training Grant - 2T32 HL007849.
Footnotes
Disclosures: Lynn Dengel, Scott Acton, and Craig Slingluff are partners in a limited liability corporation, Dermagram LLC, which was created for the purposes of attaining further funding through STTR grants from the NIH. These grants were not funded and Dermagram LLC currently holds no intellectual property with no outside investors or current revenue streams.
References
- 1.Altman JF, Oliveria SA, Christos PJ, Halpern AC. A survey of skin cancer screening in the primary care setting: a comparison with other cancer screenings. Arch Fam Med. 2000;9:1022–1027. doi: 10.1001/archfami.9.10.1022. [DOI] [PubMed] [Google Scholar]
- 2.Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137:1343–1350. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 3.Barnes LM, Nordlund JJ. The natural history of dysplastic nevi. A case history illustrating their evolution. Arch Dermatol. 1987;123:1059–1061. [PubMed] [Google Scholar]
- 4.Clark AR, Monroe JR, Feldman SR, Fleischer AB, Jr, Hauser DA, Hinds MA. The emerging role of physician assistants in the delivery of dermatologic health care. Dermatol Clin. 2000;18:297–302. doi: 10.1016/s0733-8635(05)70175-3. [DOI] [PubMed] [Google Scholar]
- 5.Drugge RJ, Nguyen C, Drugge ED, Gliga L, Broderick PA, McClain SA, Brown CC. Melanoma screening with serial whole body photographic change detection using Melanoscan technology. Dermatol Online J. 2009;15:1. [PubMed] [Google Scholar]
- 6.Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706–714. doi: 10.1111/j.0007-0963.2004.05892.x. [DOI] [PubMed] [Google Scholar]
- 7.Halpern AC, Guerry D, Elder DE, Trock B, Synnestvedt M, Humphreys T. Natural history of dysplastic nevi. J Am Acad Dermatol. 1993;29:51–57. doi: 10.1016/0190-9622(93)70151-i. [DOI] [PubMed] [Google Scholar]
- 8.Hanrahan PF, Hersey P, D’Este CA. Factors involved in presentation of older people with thick melanoma. Med J Aust. 1998;169:410–414. doi: 10.5694/j.1326-5377.1998.tb126830.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanrahan PF, Menzies SW, D’Este CA, Plummer T, Hersey P. Participation of older males in a study on photography as an aid to early detection of melanoma. Aust N Z J Public Health. 2000;24:615–618. doi: 10.1111/j.1467-842x.2000.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 10.Helfand M, Mahon SM, Eden KB, Frame PS, Orleans CT. Screening for skin cancer. Am J Prev Med. 2001;20:47–58. doi: 10.1016/s0749-3797(01)00258-6. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson CC, Resneck JS, Jr, Kimball AB. Generational differences in practice patterns of dermatologists in the United States: implications for workforce planning. Arch Dermatol. 2004;140:1477–1482. doi: 10.1001/archderm.140.12.1477. [DOI] [PubMed] [Google Scholar]
- 12.Kelly JW, Yeatman JM, Regalia C, Mason G, Henham AP. A high incidence of melanoma found in patients with multiple dysplastic naevi by photographic surveillance. Med J Aust. 1997;167:191–194. doi: 10.5694/j.1326-5377.1997.tb138843.x. [DOI] [PubMed] [Google Scholar]
- 13.MacKie RM, McHenry P, Hole D. Accelerated detection with prospective surveillance for cutaneous malignant melanoma in high-risk groups. Lancet. 1993;341:1618–1620. doi: 10.1016/0140-6736(93)90758-9. [DOI] [PubMed] [Google Scholar]
- 14.Malvehy J, Puig S. Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: a two-step method. Clin Dermatol. 2002b;20:297–304. doi: 10.1016/s0738-081x(02)00220-1. [DOI] [PubMed] [Google Scholar]
- 15.Malvehy J, Puig S. Follow-up of melanocytic skin lesions with digital total-body photography and digital dermoscopy: a two-step method. Clin Dermatol. 2002a;20:297–304. doi: 10.1016/s0738-081x(02)00220-1. [DOI] [PubMed] [Google Scholar]
- 16.Menzies SW, Gutenev A, Avramidis M, Batrac A, McCarthy WH. Short-term digital surface microscopic monitoring of atypical or changing melanocytic lesions. Arch Dermatol. 2001;137:1583–1589. doi: 10.1001/archderm.137.12.1583. [DOI] [PubMed] [Google Scholar]
- 17.Nehal KS, Oliveria SA, Marghoob AA, Christos PJ, Dusza S, Tromberg JS, Halpern AC. Use of and beliefs about baseline photography in the management of patients with pigmented lesions: a survey of dermatology residency programmes in the United States. Melanoma Res. 2002;12:161–167. doi: 10.1097/00008390-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Oliveria SA, Chau D, Christos PJ, Charles CA, Mushlin AI, Halpern AC. Diagnostic accuracy of patients in performing skin self-examination and the impact of photography. Arch Dermatol. 2004;140:57–62. doi: 10.1001/archderm.140.1.57. [DOI] [PubMed] [Google Scholar]
- 19.Phelan DL, Oliveria SA, Halpern AC. Patient experiences with photo books in monthly skin self-examinations. Dermatol Nurs. 2005;17:109–114. [PubMed] [Google Scholar]
- 20.Rhodes AR. Intervention strategy to prevent lethal cutaneous melanoma: use of dermatologic photography to aid surveillance of high-risk persons. J Am Acad Dermatol. 1998;39:262–267. doi: 10.1016/s0190-9622(98)70086-6. [DOI] [PubMed] [Google Scholar]
- 21.Rigel DS, Rivers JK, Kopf AW, Friedman RJ, Vinokur AF, Heilman ER, Levenstein M. Dysplastic nevi. Markers for increased risk for melanoma. Cancer. 1989;63:386–389. doi: 10.1002/1097-0142(19890115)63:2<386::aid-cncr2820630231>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Risser J, Pressley Z, Veledar E, Washington C, Chen SC. The impact of total body photography on biopsy rate in patients from a pigmented lesion clinic. J Am Acad Dermatol. 2007;57:428–434. doi: 10.1016/j.jaad.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Rivers JK, Kopf AW, Vinokur AF, Rigel DS, Friedman RJ, Heilman ER, Levenstein M. Clinical characteristics of malignant melanomas developing in persons with dysplastic nevi. Cancer. 1990;65:1232–1236. doi: 10.1002/1097-0142(19900301)65:5<1232::aid-cncr2820650533>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Shriner DL, Wagner RF., Jr Photographic utilization in dermatology clinics in the United States: a survey of university-based dermatology residency programs. J Am Acad Dermatol. 1992;27:565–567. doi: 10.1016/0190-9622(92)70223-3. [DOI] [PubMed] [Google Scholar]
- 25.Shriner DL, Wagner RF, Jr, Glowczwski JR. Photography for the early diagnosis of malignant melanoma in patients with atypical moles. Cutis. 1992;50:358–362. [PubMed] [Google Scholar]
- 26.Slue W, Kopf AW, Rivers JK. Total-body photographs of dysplastic nevi. Arch Dermatol. 1988;124:1239–1243. [PubMed] [Google Scholar]
- 27.Slue WE., Jr Total body photography for melanoma surveillance. N Y State J Med. 1992;92:494–495. [PubMed] [Google Scholar]
- 28.Suneja T, Smith ED, Chen GJ, Zipperstein KJ, Fleischer AB, Jr, Feldman SR. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303–1307. doi: 10.1001/archderm.137.10.1303. [DOI] [PubMed] [Google Scholar]
- 29.Terushkin V, Oliveria SA, Marghoob AA, Halpern AC. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: An update. J Am Acad Dermatol. 2010 doi: 10.1016/j.jaad.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Tsang MW, Resneck JS., Jr Even patients with changing moles face long dermatology appointment wait-times: a study of simulated patient calls to dermatologists. J Am Acad Dermatol. 2006;55:54–58. doi: 10.1016/j.jaad.2006.04.001. [DOI] [PubMed] [Google Scholar]