Abstract
Introduction
Methods to induce T cell responses to protein vaccines have not been optimized. The immunostimulant AS15 has been administered with the recombinant MAGE-A3 protein (recMAGE-A3) i.m. but not i.d. or s.c. This study tests hypotheses that the i.d./s.c. route is safe and will increase CD4+ and CD8+ T cell responses to MAGE-A3.
Patients and methods
Twenty-five patients with resected stage IIB-IV MAGE-A3+ melanoma were randomized to immunization with recMAGE-A3 combined with AS15 immunostimulant (MAGE-A3 immunotherapeutic) either i.m. (group A, n = 13) or i.d./s.c. (group B, n = 12). Adverse events were recorded. Ab responses to MAGE-A3 were measured by ELISA. T cell responses to overlapping MAGE-A3 peptides were assessed in PBMC and a sentinel immunized node (SIN) after 1 in vitro stimulation with recMAGE-A3, by IFN-γ ELISPOT assay and by flow cytometry for multifunctional (TNF-α/IFN-γ) responses.
Results
Both routes of immunization were well tolerated without treatment-related grade 3 adverse events. All patients had durable Ab responses. For all 25 patients, the T cell response rate by ELISPOT assay was 30 % in SIN (7/23) but only 4 % (1/25) in PBMC. By flow cytometry, multifunctional CD8+ T cell responses were identified in one patient in each group; multifunctional CD4+ T cell response rates for groups A and B, respectively, were 31 and 64 % in SIN and 31 and 50 % in PBMC.
Conclusion
The MAGE-A3 immunotherapeutic was well tolerated after i.d./s.c. administration, with trends to higher CD4+ T cell response rates than with i.m. administration. This study supports further study of AS15 by i.d./s.c. administration.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1770-9) contains supplementary material, which is available to authorized users.
Keywords: Vaccine adjuvants, Cancer vaccines, Melanoma/im, T lymphocytes, TLR agonists, Human
Introduction
Traditional vaccines against infectious pathogens often employ all or part of the target pathogen, which can directly activate TLRs and other pattern recognition receptors. However, purified or synthetic Ag preparations alone lack those immune-stimulatory components and require addition of agents to activate DC and to signal through pattern recognition receptors. Such immunostimulants function as vaccine adjuvants, which are required to augment T cell responses to purified cancer Ags in vaccines [1–3]. The cellular and molecular events induced by such adjuvants are incompletely understood, and optimal vaccine adjuvants remain to be defined [4]. A general concern with protein vaccines has been the low rate of CD8+ T cell responses, often with low magnitude CD4+ T cell responses [5, 6], but effective induction of Ab. Even the FDA-approved prostate cancer vaccine sipuleucel-T, which encodes the full-length protein prostatic acid phosphatase (PAP), has not induced convincing T cell responses to that target [7]. Thus, better approaches to improve T cell responses to protein vaccines are needed.
TLR agonists have demonstrated value as vaccine adjuvants [8–13]. Monophosphoryl lipid A (MPL) activates TLR4 in DC in a TRIF-biased manner, induces a Th1 microenvironment, and augments induction of protective cytotoxic memory CD8+ T cells [12, 14]. Also the TLR9 agonist CpG 7909 added to incomplete Freund’s adjuvant (IFA) increased immunogenicity of a peptide vaccine for induction of CD8+ T cells about 10-fold [8]. AS15 is an immunostimulant formulation that incorporates these agonists for TLR4 and TLR9 plus the saponin adjuvant QS-21 Stimulon® (Quillaja saponaria Molina, fraction 21; licensed by Glaxo Smith Kline (GSK) from Antigenics Inc., a subsidiary of Agenus Inc., Lexington, MA, USA). QS-21 has been a potent adjuvant for promoting CD8+ T cell responses to subunit Ag vaccines [15] and to a long (69mer) malarial peptide [16]. QS-21 was superior to IFA as an adjuvant for vaccines using class I-MHC-restricted melanoma peptides from tyrosinase and gp100 [17]. Adjuvants combining MPL and QS-21 can induce potent CD4+ and CD8+ T cell responses to a protein vaccine, with multifunctional Th1-dominant CD4+ responses, inducing IFN-γ and IL-2 production [18]. As an adjuvant for a recMAGE-A3 vaccine, the combination immunostimulant AS15 induced favorable clinical outcome compared to another MPL/QS-21 combination, AS02B [5]. AS15 is a strong immunostimulant and, when administered to mice, monkeys and humans with recombinant protein, can support induction of Th1 responses and protection against tumor challenge [5, 19–21].
The MAGE gene family was the first discovered Ag recognized by human T lymphocytes in an MHC-restricted manner and is a cancer-testis Ag [22]. Vaccination with recMAGE-A3 showed promise for clinical benefit in phase II trials, melanoma and non-small cell lung cancer [5]. However, in recent reports, phase III trials with the MAGE-A3 immunotherapeutic have not met primary endpoints for improving OS [23–25]. Thus, the clinical impact of this and other protein vaccines has not yet met expectation. Nonetheless, experience with recMAGE-A3 immunotherapeutic provides a good model for studying adjuvant effects of the AS15 immunostimulant.
In prior preclinical studies and human trials, the MAGE-A3 immunotherapeutic has been administered i.m. However, other immunization routes may also be effective. Ag can be presented effectively to T cells by skin DC, which include at least three populations: CD1a+CD207+ Langerhans cells arising in the epidermis, CD1a+CD207negCD14neg dermal DC and CD14+ DC [26]. Langerhans cells are immature DC that are optimal for uptake of protein and subsequent presentation of Ag to T cells. When activated by innate immune signals in the skin, Langerhans cells mature and migrate to draining nodes [26, 27]. Dermal DC can also be very effective at activating CD8+ T cells [26]. Thus, there is rationale for i.d. or s.c. administration of Ag and immunostimulants. However, i.d./s.c. administration had not previously been studied, in the absence of safety data by that route. The present study was initiated to evaluate the safety of i.d./s.c. administration, as compared to i.m., and to obtain pilot data on the magnitude of Ab and CD4+ and CD8+ T cell responses to MAGE-A3 epitopes by the i.d./s.c. and i.m. routes of immunization with MAGE-A3 immunotherapeutic in patients with high-risk melanoma. We hypothesized that the i.d./s.c. route would be safe and would increase the CD4+ and CD8+ T cell responses to MAGE-A3. The trial incorporated biopsies of a vaccine-draining node to test whether immune monitoring in the vaccine-draining node is a more sensitive approach than monitoring T cell responses in the blood.
Materials and methods
Study design
This was an open-label randomized single-institution pilot study to evaluate the safety and immunologic response to MAGE-A3 immunotherapeutic administered by either of two injection routes (i.m. or i.d./s.c.). Patients were studied following IRB approval (IRB #15398) and documentation of informed consent. The trial was registered in clinicaltrials.gov (NCT01425749) and was performed at the University of Virginia. A schema is shown in Fig. 1.
Fig. 1.
Clinical trial schema
MAGE-A3 immunotherapeutic (0.5 ml) was administered five times (weeks 0, 3, 6, 9 and 12) in extremities uninvolved with melanoma. Vaccines 1 and 3 were administered at the same site: Other vaccine sites were rotated among available extremities. Subjects were randomized 1:1, within each stratum (AJCC stage II/III or IV), to i.m. (group A) or i.d./s.c. (group B) administration. The randomization code was generated by the study statistician using varying block sizes of 2–4. For group B patients, half of the dose was injected s.c.; then, the needle was withdrawn to the dermis, then advanced intradermally from that same puncture site, and the remaining half dose was injected i.d. Immune responses were evaluated in a SIN and PBMC.
Primary endpoints included (1) safety of cutaneous administration (i.d./s.c.) of MAGE-A3 immunotherapeutic, (2) CD4+ and CD8+ T cell reactivity to MAGE-A3 epitopes in the SIN (IFN-gamma ELISPOT, tetramer, proliferation). Secondary endpoints included CD4+ and CD8+ T cell reactivity to MAGE-A3 epitopes in peripheral blood and circulating Ab to MAGE-A3.
Lymph node biopsy
The SIN, which drained the first and third injection sites, was identified by lymphoscintigraphy using technetium 99m-sulfur colloid (Tc99m-sc) [28] to be injected i.m. (group A) or i.d. (group B). The node was sectioned immediately after excision and divided as follows: (a) 30 % for viable cell suspensions, (b) 20 % formalin-fixed and paraffin-embedded (FFPE), (c) 25 % in RNAlater (Ambion, Life Technologies, Grand Island, NY, USA) for gene expression profiling and (d) 25 % bisected, with each half quick-frozen in liquid nitrogen.
Skin biopsies
For patients in group B, skin at the MAGE-A3 immunotherapeutic injection sites was biopsied with three 4-mm punch biopsies, 1 week after dose 1 and 3 (days 8 and 50). Data on inflammatory changes in the vaccine site biopsies are reported separately (Pinczewski et al., manuscript in preparation).
Sample size considerations
We hypothesized that both immunization routes would be safe. However, there was concern that i.d./s.c. administration may induce local tissue necrosis and skin ulceration in some patients. Thus, monitoring dose-limiting toxicities guided decisions about accrual. To assess safety and to enable estimation of immune response rates in the SIN and PBMC, with 90 % confidence that the estimates would be accurate ±26 %, target enrollment was 12 eligible subjects per group. Limited additional enrollment was allowed for patients in screening at the time target enrollment was reached. Twelve patients per group also allowed 80 % power to test for an 87 % immune response rate in the SIN versus a null immune response rate of 56 % with a one-sided 5 % level test. The historical rate of 56 % was based on prior experience with detection of T cell responses in the SIN after vaccination with multiple peptides in IFA [29]. We expected both immunization routes to be immunogenic, but if the immune response rate for one study group was low, (e.g., 2, 5 or 10 %), we would have approximately 87, 83 and 79 % power, respectively, to detect a difference in immune response rates of 50 % between study groups.
Eligibility criteria
Patients were eligible if they had histologically or cytologically proven stage IIB-IV melanoma (seventh edition AJCC, at original presentation or at recurrence) rendered clinically free of disease within 6 months. Patients with up to three small treated brain metastases were eligible. Participants were required to have: age 18 years or above; Eastern Cooperative Oncology Group performance status 0–1; adequate hematologic, liver and renal function; negative serology for HIV and HCV; and ability to give informed consent. Patients with diabetes were required to have adequate glucose control (HgbA1c < 7.5 %). Multiple primary melanomas were allowed, as were cutaneous, mucosal or unknown primary sites. Patient tumors were screened for MAGE-A3 mRNA expression on FFPE tissue based on quantitative RT-PCR (performed by GSK [30]).
Adverse events were recorded using Common Terminology Criteria for Adverse Events v4.
Ab responses to MAGE-A3 were assessed in serum by ELISA, as described [31]. Seroconversion was defined as a detectable Ab response by ELISA (>20 EU/ml).
Evaluation of T cell responses to MAGE-A3 T cell responses to overlapping 15-mer MAGE-A3 peptides was measured after one in vitro sensitization. Lymphocytes (5–10 × 106) from PBMC or SIN were stimulated 2 h (37 °C, 5 % CO2) with MAGE-A3 Ag/Ab complexes (25 mcg/ml recMAGE-A3 plus pooled MAGE-A3-reactive serum (1:200) from several patients) in 1 ml complete medium [RPMI-1640 + pen/strept + 10 % human AB serum (Gemini, West Sacramento, CA, USA)]. IL-2 (10 U/ml) and IL-7 (20 ng/ml) were added in complete medium, for a final volume of 2 ml. Cells were incubated 14 days. Cytokines were replenished day 7, and proliferating wells were split as indicated.
ELISPOT assay
The cells were assayed day 12–14 for IFN-γ production by ELISPOT assay. Autologous Epstein–Barr virus-transformed B cells (EBV-B) were pulsed 2 h with (a) overlapping MAGE-A3 15-mer peptides (1 mcg/ml each, GSK, Rixensart, Belgium), (b) overlapping PRAME 15-mer peptides (1 mcg/ml each, GSK) as negative control or (c) complete medium only, and plated. The T cells were then washed and plated in quadruplicate wells at 25,000 and 12,500 cells per well and assayed as reported [29, 32]. HIV gag 9-mer peptide (4 mcg/ml) served as a negative control, and PMA (Sigma-Aldrich, St. Louis, MO, USA; 50 ng/mL) plus ionomycin (Sigma-Aldrich, 1 mcM) provided a positive control.
Flow cytometry
CD4+ and CD8+ T cells also were assayed for production of IFN-γ and TNF-α by flow cytometry. Cultured lymphocytes (≤100,000) were combined, in round-bottom polypropylene 96-well cluster dishes (CoStar), with an equal number of autologous EBV-B pulsed with test and control Ags in 200 mcL AIM V medium (Life Technologies, Grand Island, NY, USA) plus 5 % human AB serum. Brefeldin A (BD Biosciences, San Jose, CA, USA; 20 mcl of a 1:100 dilution) was added 2 h later. After 5 h more incubation, cells were washed and stained with the live/dead indicator Aqua (Life Technologies) and with Abs (BD Biosciences unless specified otherwise) to CD3 (v450, clone UCHT1), CD19 (PE, clone HIB19) and CD8 (APC, clone RPA-T8) for 30 min followed by washing.
The cells were fixed and permeabilized (Cytofix/Cytoperm, BD Biosciences), followed by incubation 45 min at 4 °C with Abs for the intracellular detection of IFN-γ (FITC, Clone B27), TNF-α (PE-Cy7, clone MAb11) and CD4 (PerCP-Cy5.5, clone OKT4; BioLegend, San Diego, CA, USA). Cells were evaluated on a Canto II flow cytometer (BD Biosciences), and data were acquired with DIVA software and analyzed with TreeStar FlowJo software using custom analysis templates to warrant reproducible gating strategy and accuracy of gating with curated checkpoints.
The analysis determined the proportion of CD4+ (and/or CD8+) T cells producing IFN-γ or TNF-α, or both, in response to MAGE-A3 peptide pools (with irrelevant peptide as negative control). T cell response was defined when T cells producing both IFN-γ and TNF-α in response to MAGE-A3 peptides exceeded (a) twice the maximum of two negative controls (PRAME peptides, media only), corrected for pre-existing response; and (b) exceeded the negative controls by at least 0.2 % of the T cell population. These criteria also were used to define immunogenicity by ELISPOT (IFN-γ only) and are similar to criteria used in prior trials [29, 32]. High T cell responses were also defined as a fivefold increase and at least a 1 % difference. If the negative control values for a given sample were zero, a meaningful fold increase could not be calculated; so, in those cases, we used the minimum detectable value among all similar assays (0.06 %) as the negative control value for that sample.
Enumeration of immune subsets in SIN
FFPE sections of SIN were assessed by IHC using mouse Abs to CD4 (1:120, Vector Labs, Burlingame, CA, USA), CD8 (1:200, Dako, Carpinteria, CA, USA), CD1a (1:50, Dako), CD83 (1:20, Leica Biosystems, Buffalo Grove, IL, USA), GATA3 (1:200, Biocare Medical, Concord, CA, USA), T-bet (1:40, Santa Cruz, Dallas, TX, USA), RORγt (1:300, EMD Millipore, Billerica, MA, USA) and rat Ab to FOXp3 (1:125, eBioscience, San Diego, CA, USA). Stained slides were imaged at 20× using the Leica SCN400 slide scanner. For all but CD1a counts, stained cells were enumerated per mm2, using Digital Image Hub Tissue IA software and the stained cell algorithm (Leica Biosystems, Buffalo Grove, IL, USA). Annotations were drawn on large areas of the SIN, excluding tissue folds. Automated cell counts were audited and verified manually. CD1a cell data were obtained by manual counting by two coauthors (ISM, CMR), with mean values reported.
Statistics
Point estimates and 90 % confidence intervals (CIs) were estimated to summarize immune response rates. For the analysis of Ab responses to MAGE-A3, a repeated-measure model [33] was fit to log10-transformed data to assess change in slope in weeks 0–7 compared to weeks 7–13 and weeks 7–13 compared to >13, and to determine whether there was a difference over time by group. F tests based upon the repeated-measure model were used to assess statistical significance.
Results
At final analysis, total enrollment was completed with 25 participants. Enrollment spanned 18 months (July 2011–January 2013). The two groups were well matched for disease stage and other clinical parameters (Table 1). All had been rendered clinically free of disease by surgery. None had received prior cytotoxic chemotherapy or checkpoint blockade therapy. Prior therapies included IFN (3A, 1B), vaccine (1A) and radiation therapy (5A, 4B).
Table 1.
Clinical characteristics
| Arm A (IM) | Arm B (ID/SQ) | Total | |
|---|---|---|---|
| N | 13 | 12 | 25 |
| Gender N male (%) | 5 (38 %) | 7 (58 %) | 12 (48 %) |
| Age: mean (range) | 56 (39–82) | 54 (40–69) | 55 (39–82) |
| Race/ethnicity | |||
| White, non-Hispanic | 13 | 11 | 24 |
| Native American | 0 | 1 | 1 |
| Stage at registration | |||
| IIIB | 3 | 5 | 8 |
| IIIC | 5 | 3 | 8 |
| IV | 5 | 4 | 9 |
| ECOG performance status | |||
| 0 | 12 (92 %) | 10 (83 %) | 22 (88 %) |
| 1 | 1 (8 %) | 2 (17 %) | 3 (12 %) |
| Reason off-treatment | |||
| Treatment completed | 13 | 11 | 24 |
| Disease progression | 0 | 1 | 1 |
Adverse events
Treatment-related adverse events were limited to grades 1–2 (Table 2; Supplemental Table 1), including local injection site reactions, without skin ulceration, that resolved almost completely within a week, plus transient systemic constitutional symptoms, usually observed for 1–2 days after each vaccine. Three patients experienced elevations in circulating anti-nuclear Ab, but all were asymptomatic (grade 1), listed as autoimmune disorders. Grade 2 injection site reactions occurred in 10 patients in group A (77 %, 90 % CI 51, 93 %) and seven patients in group B (58 %, 90 % CI 32, 82 %).
Table 2.
MEL 55 maximum grade treatment-related adverse events
| Adverse event category | Adverse event description | Study groups | Total study | |
|---|---|---|---|---|
| Arm A IM N = 13 |
Arm B ID/SC N = 12 |
Both arms N = 25 |
||
| Gr 2 | Gr 2 | Gr 2 | ||
| General disorders and administration site conditions | Chills | 1 | 1 | |
| Fatigue | 4 | 2 | 6 | |
| Fever | 1 | 1 | ||
| Flu-like symptoms | 1 | 2 | 3 | |
| Injection site reaction | 10 | 7 | 17 | |
| Injury, poisoning and procedural complications | Wound complication | 1 | 1 | |
| Investigations | Lymphocyte count DECR | 1 | 1 | |
| Total | 18 | 12 | 30 | |
Ab responses to MAGE-A3
One of 25 patients (4 %) had detectable Ab to MAGE-A3 (46 EU/ml, group A) in prestudy blood. By week 1, six of 25 (24 %) had seroconverted (less than 100 EU/ml Ab, Fig. 2). Ab increased markedly by week 7, exceeding 250 EU/ml for all evaluable samples (Supplemental Table 2). In both groups, the rate of change from week 0–7 was approximately 0.3 log per week, with flattening of the rate of change to week 13 (slope 0.1), a significant change (p < 0.001), followed by a slight but significant decrease (p < 0.01) after week 13 (Supplemental Figure 1). The average rate and level of response do not differ between the two study groups (Supplemental Table 3) though Ab responses may have been slightly earlier in group A, with seroconversion by week 1 in 33 % (90 % CI 12, 61 %) versus 8 % in group B (90 % CI 0.5, 34 %; Supplemental Table 2).
Fig. 2.
Titers of serum Ab to recMAGE-A3 through week 26 are shown for groups A (a, c) and B (b, d), without (a, b) and with (c, d) log transformation. Each line represents a different patient. Vertical dashed lines indicate the dates of serum collection. Vaccinations were completed by week 12
Identification and biopsy of SIN
SIN was identified and biopsied for 24 of 25 patients (96 %). One patient did not undergo SIN biopsy to enable workup of reported adverse events. Lymphatic channels from the injection site to the SIN were evident from the i.m. site (Supplemental Figure 2a) or the i.d. site (Supplemental Figure 2b). Overall, the lymphoscintigraphy signal in the SIN appeared slightly less intense after i.m. injection than after i.d. injection. Logistical and technical challenges arose because lymphatic mapping from i.m. sites is not otherwise routine. For three patients in group A, Tc99m-sc was not injected i.m., but was injected i.d. in skin overlying the i.m. immunization site, and SIN was identified and removed. In eight patients, Tc99m-sc was injected i.m., and a SIN was identified in 7 (VMM1079, 1080, 1091, 1100, 1103, 1107, 1109); in the one patient injected i.m. for whom a SIN was not identified by lymphoscintigraphic imaging, additional Tc99m-sc was injected i.d. directly overlying the i.m. immunization site, and a node was identified and removed. In two patients, Tc99m-sc was injected both i.d. and i.m., and a single node was highlighted (Supplemental Figure 2c), suggesting that nodal drainage patterns may be comparable from skin and muscle in the same site.
T cell responses to MAGE-A3
Initially, we tested for T cell responses to MAGE-A3 peptides by ex vivo ELISPOT assay; however, responses were not detected for the first three patients tested (not shown). Thus, we developed a more sensitive assay by which lymphocytes were stimulated with MAGE-A3 protein and then cultured 14 days prior to testing by ELISPOT assay and flow cytometry, for response to autologous EBV-B pulsed with a pool of MAGE-A3 peptides, compared to unpulsed EBV-B or EBV-B pulsed with PRAME peptides.
T cell responses by ELISPOT assay
IFN-γ responses were detected by ELISPOT assay in seven patients (28 %, 90 % CI 14, 46 %), with similar proportions in groups A (4/13 patients, 31 %) and B (3/12, 25 %), shown in Table 3. All responses, but one, were limited to the SIN. The hypothesis of improved immune response in the SIN compared to historical controls was rejected within both groups. In all seven patients with ELISPOT responses, CD4+ T cell responses were detected in the SIN by flow cytometry (both by measuring multifunctional T cells and by measuring all cytokine-secreting CD4 T cells).
Table 3.
Immune responses by ELISPOT and by flow cytometry
| Patient ID VMM | Study group | ELISPOT response | Multifunctional | CD4+ TNF-α+ and/or IFN-γ+ | CD8+ TNF-α+ and/or IFN-γ+ | |
|---|---|---|---|---|---|---|
| CD4+ TNF-α+IFN-γ+ | CD8+ TNF-α+IFN-γ+ | |||||
| 1075 | A | SIN | SIN | – | SIN | – |
| 1079 | A | – | – | – | SIN | – |
| 1080 | A | SIN | SIN | – | SIN | 13 |
| 1082 | A | – | 1 | – | – | – |
| 1087 | A | – | 7 | – | – | – |
| 1091 | A | – | 13 | – | – | – |
| 1094 | A | – | (13, 26) | – | – | – |
| 1100 | A | – | – | 1, (26) | – | – |
| 1103 | A | – | – | – | – | – |
| 1107 | A | – | – | – | – | – |
| 1109 | A | SIN | 7, SIN | – | SIN | – |
| 1110 | A | – | – | – | – | – |
| 1117 | A | SIN | SIN | – | SIN | – |
| 1073 | B | – | 1 | – | 1 (7) | – |
| 1076 | B | 1, SIN | SIN | SIN | – | – |
| 1077 | B | SIN | 1, (7, 13), SIN | – | (1, 13), SIN | – |
| 1078 | B | – | SIN | 1 | SIN | 1 |
| 1086 | B | – | (1) 7, 13, SIN | – | (1, 7, 13), SIN | – |
| 1089 | B | – | – | – | 26 | – |
| 1093 | B | – | – | – | – | – |
| 1095a | B | a | – | – | – | – |
| 1098 | B | – | SIN | 26 | SIN | 26 |
| 1106b | B | b | b; 13 | b | b | b |
| 1112 | B | SIN | 7, 13, SIN | – | SIN | – |
| 1113 | B | – | (13), 26, SIN | – | (13, 26), SIN | – |
| T cell response in SIN | A | 4/13 (31 %) | 4/13 (31 %) | 0/13 (0 %) | 5/13 (38 %) | 0/13 (0 %) |
| B | 3/10 (30 %) | 7/11 (64 %) | 1/11 (9 %) | 6/11 (55 %) | 0/11 (0 %) | |
| All | 7/23 (30 %) | 11/24 (46 %) | 1/24 (4 %) | 11/24 (46 %) | 0/24 (0 %) | |
| T cell response in PBMC | A | 0/13 (0 %) | 4/13 (31 %) | 1/13 (8 %) | 0/13 (0 %) | 1/13 (8 %) |
| B | 1/12 (8 %) | 6/12 (50 %) | 2/12 (17 %) | 2/12 (17 %) | 2/12 (17 %) | |
| All | 1/25 (4 %) | 10/25 (40 %) | 3/25 (12 %) | 2/25 (8 %) | 3/25 (12 %) | |
| T cell response in SIN and PBMC | A | 0/13 (0 %) | 1/13 (8 %) | 0/13 (0 %) | 0/13 (0 %) | 0/13 (0 %) |
| B | 1/10 (10 %) | 4/11 (36 %) | 0/11 (0 %) | 0/11 (0 %) | 0/11 (0 %) | |
| All | 1/23 (4 %) | 5/24 (21 %) | 0/24 (0 %) | 0/24 (0 %) | 0/24 (0 %) | |
| T cell response in SIN or PBMC | A | 4/13 (31 %) | 7/13 (54 %) | 1/13 (8 %) | 5/13 (38 %) | 1/13 (8 %) |
| B | 3/12 (25 %) | 9/12 (75 %) | 3/12 (25 %) | 8/12 (67 %) | 2/12 (17 %) | |
| All | 7/25 (28 %) | 16/25 (64 %) | 4/25 (16 %) | 13/25 (52 %) | 3/25 (12 %) | |
Responses are denoted by the week in which the response was observed from PBMC, or by the presence of a response in the SIN (e.g., 13, SIN = response in PBMC week 13, and in the SIN). Weeks marked in parentheses [e.g., (13)] represent weeks in which a fold increase of 1.5–2 when at least one other time point met criteria for positivity (2x)
aSIN ELISPOT not done for VMM1095; b SIN not done for VMM1106; NE not evaluable
T cell responses by flow cytometry
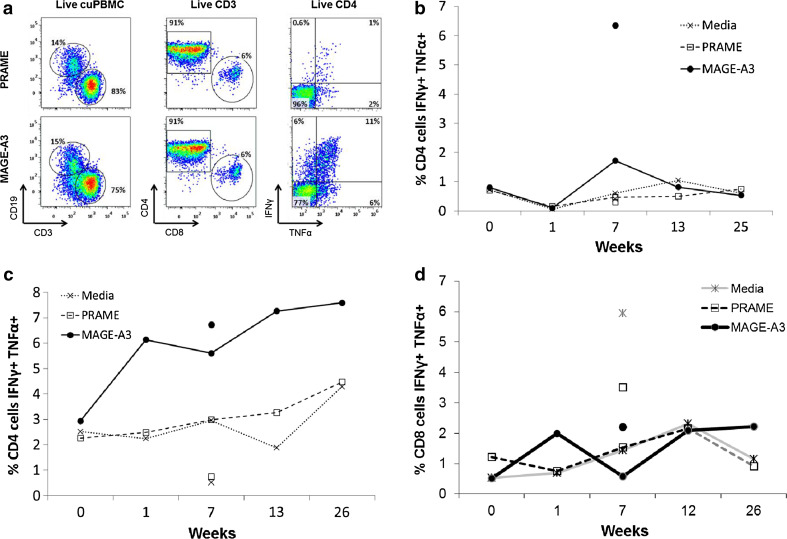
The primary analysis was based on the percent of multifunctional T cells producing both IFN-γ and TNF-α. Additional analyses included measures of T cells producing either of those cytokines alone. The gating strategy is illustrated in Fig. 3a, and examples of the data are shown in Fig. 3.
Fig. 3.
In vitro multifunctional T cell responses to MAGE-A3 after administration of MAGE-A3 immunotherapeutic. Lymphocytes from PBMC or SIN of vaccinated patients were stimulated in vitro with recMAGE-A3 for 14 days; then, intracellular cytokine levels were measured after an additional stimulation with a pool of overlapping MAGE-A3 peptides or negative controls (a pool of peptides from the negative control protein, PRAME and culture media only); corrected for pre-existing response; and ≥negative controls by 0.2 %. High responses were >5x and >1 % difference. a An example gating strategy is shown. Cultured PBMC was detected using forward and side scatter and the live/dead indicator, Aqua, from which live CD3 cells were identified, representative individual patient examples of CD4+ T cell responses include; b high T cell response in the SIN and response in PBMC week 7, patient VMM1109 (group A—i.m.); c high response in SIN and response in PBMC week 1, patient VMM1077 (group B—i.d./s.c.); and d a CD8+ T cell response is shown for patient VMM1100 (group A—i.m.)
CD4+ T cell responses by flow cytometry
In the SIN, multifunctional CD4+ T cell responses to MAGE-A3 were detected in 11 of 24 patients (46 %, 90 % CI 28, 64 %) and were more frequent for group B (7/11 patients, 64 %, 90 % CI 35, 86 %) than group A (4/13 patients, 31 %, 90 % CI 11, 57 %) (Table 3). Multifunctional high responders in the SIN were detected in 3 (31 %) and 4 (36 %) patients in groups A and B, respectively (Fig. 3b, c).
In PBMC, multifunctional CD4+ T cell responses were detected for 31 % in group A and 50 % in group B. None of the PBMC responses met criteria for high responses.
Considering all patients with either a PBMC or SIN response, multifunctional CD4+ T cell responses were detected in 64 % of patients (16/25), including 54 % [(90 % CI 29, 78 %) of group A (7/13) and 75 % (90 % CI 47, 93 %] of group B (9/12, Table 3). Responses in both PBMC and SIN were detected in just 1 patient (8 %) in group A and in 4 (36 %) in group B (examples in Fig. 3b, c).
CD8+ T cell responses by flow cytometry
CD8+ T cell responses met criteria for positivity in four patients [1 in group A (8 %); 3 in group B (25 %)] but were of low magnitude and isolated. For example, one patient in group A met criteria for response in PBMC at week 1 and approximated a response at week 26 (1.9x, >1 % difference), but other samples were negative; the pattern for that patient includes substantial sample-to-sample variability compared to background (Fig. 3d) and is not as convincing as the measured CD4 T cell responses (Fig. 3b, c).
Detection of immune responses in SIN as a function of route of Tc99m-sc injection
Among patients in group A, T cell responses were detected in the SIN in similar proportions of patients with Tc99m-sc injected i.d. (2/6) or only i.m. (2/7) both by ELISPOT and by flow cytometry.
Cellular composition of sentinel immunized nodes
We also evaluated for differences in the cellular composition of the SIN by IHC, depending on the route of vaccination (Supplemental Figure 3). There were no significant differences in the density of any of these cell populations between study groups. There is variability among patients in all of these measures. On average, numbers of CD4+ and CD8+ T cells were similar, and numbers of T cells greatly exceeded numbers of DC. T-bet+ cells were more common than those expressing GATA3 or RORγt. There were similar numbers of FOXp3+ cells between groups, and FOXp3+ cells were more numerous overall than T-bet+ cells. The numbers of FOXp3+ cells per mm2 did not predict immune response, though there was a weak (NS) trend toward more FOXp3+ cells in those with CD4+ T cell response to the vaccine (Supplemental Figure 3i).
Clinical outcome
Overall patient survival to 2 years has been about 90 % (Supplemental Figure 4).
Discussion
This was a pilot study to evaluate the safety and immunogenicity of administering MAGE-A3 immunotherapeutic in the skin (i.d./s.c.). Vaccine site reactions were limited to grades 1 and 2 and were transient, with grade 2 injection site reactions in 58 % after i.d./s.c. injection and 77 % after i.m. injection. Thus, there is much less toxicity locally than we have observed when vaccinating with peptides in IFA [32, 34]. There were only minor differences in the constitutional/systemic toxicities between groups. These data provide the first human experience with cutaneous (i.d./s.c.) administration of protein Ag plus AS15 and demonstrate that it is well tolerated. This supports the safety of future studies of cutaneous administration of AS15 or its components (QS-21, MPL, or CpG) with recMAGE-A3 and/or other Ags.
Protein vaccines have typically been effective at inducing Ab responses and CD4+ T cell responses, but have been less effective at inducing CD8+ T cell responses [35]. Our data are consistent with that prior experience. We found durable Ab responses in all patients. The role of these Abs is not known, but in other studies, Ab responses to vaccination with peptides or protein are associated with T cell responses to those antigens [36, 37]. We also detected multifunctional CD4+ T cell responses in 64 % of patients and weak CD8+ T cell responses in 16 %. The primary immunologic endpoint was T cell response in the SIN, which was numerically higher in group B for CD4+ T cell response rates [64 % (90 % CI 35, 86 %), vs. 31 % (90 % CI 11, 57 %)]. These are not convincingly different in this small pilot study, but may suggest a trend toward higher induction of CD4+ T cell responses with the i.d./s.c. route. Similar trends favored the i.d./s.c. route for alternative criteria for defining CD4+ T cell responses (Table 3). None of these rates reached the target of 87 % from the study design, but may be compared to the 54 % null rate, based on responses to MAGE-A3 peptides using a peptide vaccine [29]. The rate of CD8+ T cell responses falls well below the target for both study groups.
In prior work, we developed methods for measuring T cell responses in the SIN [28, 38–40]. This approach yields high numbers of T cells, and assessment of T cell responses in the SIN has been more sensitive than assessing those responses in PBMC [28]. There has been limited prior experience mapping lymphatic drainage from muscle [41, 42]. The present study is, to our knowledge, the first to test whether i.m. vaccines can be monitored by mapping SIN by i.m. injection of Tc99m-sc. We have found that i.m. injection does map a draining node, and that the resulting node reflects immune responses to the vaccine with greater sensitivity than the PBMC. Anecdotally, because a few patients were injected i.d. overlying the i.m. vaccine sites, it may be that accurate mapping from i.m. sites can also be obtained by i.d. injection, but i.m. injection is straightforward and may be less painful than i.d. injection. In prior experience with monitoring T cells in the SIN, we vaccinated three times in the same skin site, 1 week apart, and harvested a node a week later [28, 29, 43]. We adjusted the approach for this study to match the schedule used for MAGE-A3 immunotherapeutic in prior studies (every 3 weeks), with only two vaccines administered in the same site. This appears to have been effective for immune monitoring. Thus, the SIN biopsy approach may be adapted to different vaccine schedules and regimens.
Enumeration of T cell and DC populations in the SIN revealed no significant differences between study groups. Numbers of FOXp3+ cells in the SIN generally exceeded numbers of T-bet+ cells and were about half the number of CD4+ cells. This may reflect transient immune activation and/or a regulatory T cell response to vaccine-induced activation of other cells. The numbers of FOXp3+ cells did not differ significantly between groups or between immune responders and non-responders. In particular, in settings where the immunotherapy induces larger Ag-specific T cell populations, more significant differences in immune activation may be expected in the nodes. It would be appealing also to evaluate the Ag specificity, activation state and differentiation state of T cells responding to Ag, as we have done in vaccine sites and blood after peptide vaccination [44], and where peptide/MHC tetramers enabled identification of the reactive T cells. It is, however, less straightforward to identify T cells responding to protein in the setting of a wide range of MHC molecules and in a population of patients that was not selected for the expression of a particular HLA allele.
Conclusion
Overall, the data in the present manuscript support i.d./s.c. immunization with MAGE-A3 immunotherapeutic, both as a safe approach and as one that is at least as immunogenic as i.m. injection. These data also demonstrate the feasibility of identifying and analyzing SIN draining i.m. injection sites. The rates of CD4+ T cell responses to MAGE-A3 were numerically greater with i.d./s.c. injection than with i.m. injection but were not significantly different, given the limited sample size for this pilot study. It is possible that CD8+ T cell responses may be more frequent than were detected in this study because overlapping 15-mer peptides may be less effective for reconstituting the MHC-class I-restricted T cell epitope than the minimal epitope (commonly a 9-mer). Randomized clinical trials of MAGE-A3 immunotherapeutic in melanoma and non-small cell lung cancer have not yet provided convincing clinical benefit with this strategy [24, 25]. However, AS15 continues to offer promise as a component of active specific immunotherapy, especially for induction of Ab and CD4+ T cell responses. Thus, the present manuscript supports administration of AS15 with other Ags, with or without other immune modulators. This study also provides preliminary data about the range of responses to i.d./s.c. versus i.m. vaccination that may help to design future randomized trials with adequate power to detect significant immunologic effects and whether these may lead to higher clinical benefit.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was funded by GlaxoSmithKline Biologicals SA. Support was also provided by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579: Clinical Trials Office, Biorepository and Tissue Research Facility, Flow Cytometry Core, and Biomolecular Core Facility), Rebecca Clary Harris Memorial fellowship (to Ileana Mauldin) and T32 CA009109 fellowship (to Ileana Mauldin). Additional philanthropic support was provided by George S. Suddock. The Beirne Carter Center for Immunology Research provided flow cytometry support. We appreciate the work of Patrice Neese, Carmel Nail and Kathleen Haden for administering vaccines and for recording and managing toxicities. Appreciation also goes to clinical research coordinators Emily Allred, and Chris Blackwell, and to Cheryl Murphy Chase for preparing Epstein–Barr virus-transformed B cell lines.
Abbreviations
- EBV-B
Epstein–Barr virus-transformed B cells
- FFPE
Formalin-fixed and paraffin-embedded
- GSK
Glaxo Smith Kline
- IFA
Incomplete Freund’s adjuvant
- MAGE-A3
Immunotherapeutic recombinant MAGE-A3 combined with AS15 immunostimulant
- MPL
Monophosphoryl lipid A
- recMAGE-A3
Recombinant MAGE-A3 protein
- SIN
Sentinel immunized node
- Tc99m-sc
Technetium 99m-sulfur colloid
- TLR
Toll-like receptor
Compliance with ethical standards
Conflict of interest
Dr. Slingluff has the following relationships directly related to this work: The recombinant MAGE-A3 protein, overlapping peptides and AS15 were provided to the University of Virginia for this trial by GlaxoSmithKline, and the trial was funded largely by a grant to the University of Virginia from GlaxoSmithKline. The remaining authors have no conflicts.
References
- 1.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105(5):1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burny W, Dantinne C, Amrani N, Vantomme V, Coulie P, Brichard V, Van Mechelen M. Measurement of the specific T cell response in melanoma patients immunized with a recombinant MAGE-A3 immunotherapeutic: development of a sensitive read-out for serial monitoring. J Immunother. 2006;29(6):659–660. [Google Scholar]
- 3.Pellegrino P, Clementi E, Radice S. On vaccine’s adjuvants and autoimmunity: current evidence and future perspectives. Autoimmun Rev. 2015;14(10):880–888. doi: 10.1016/j.autrev.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Olafsdottir T, Lindqvist M, Harandi AM. Molecular signatures of vaccine adjuvants. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.04.099. [DOI] [PubMed] [Google Scholar]
- 5.Kruit WH, Suciu S, Dreno B, Mortier L, Robert C, Chiarion-Sileni V, Maio M, Testori A, Dorval T, Grob JJ, Becker JC, Spatz A, Eggermont AM, Louahed J, Lehmann FF, Brichard VG, Keilholz U. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31(19):2413–2420. doi: 10.1200/JCO.2012.43.7111. [DOI] [PubMed] [Google Scholar]
- 6.Goepfert PA, Tomaras GD, Horton H, Montefiori D, Ferrari G, Deers M, Voss G, Koutsoukos M, Pedneault L, Vandepapeliere P, McElrath MJ, Spearman P, Fuchs JD, Koblin BA, Blattner WA, Frey S, Baden LR, Harro C, Evans T. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25(3):510–518. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Sheikh NA, Petrylak D, Kantoff PW, dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8 + T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miconnet I, Koenig S, Speiser D, Krieg A, Guillaume P, Cerottini JC, Romero P. CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol. 2002;168(3):1212–1218. doi: 10.4049/jimmunol.168.3.1212. [DOI] [PubMed] [Google Scholar]
- 10.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171(8):4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 11.den Boer AT, van Mierlo GJ, Fransen MF, Melief CJ, Offringa R, Toes RE. CD4+ T cells are able to promote tumor growth through inhibition of tumor-specific CD8+ T-cell responses in tumor-bearing hosts. Cancer Res. 2005;65(15):6984–6989. doi: 10.1158/0008-5472.CAN-04-3344. [DOI] [PubMed] [Google Scholar]
- 12.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25(41):7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macleod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, Marrack P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc Natl Acad Sci USA. 2011;108(19):7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kensil CR, Wu JY, Anderson CA, Wheeler DA, Amsden J. QS-21 and QS-7: purified saponin adjuvants. Dev Biol Stand. 1998;92:41–47. [PubMed] [Google Scholar]
- 16.Meraldi V, Romero JF, Kensil C, Corradin G. A strong CD8+ T cell response is elicited using the synthetic polypeptide from the C-terminus of the circumsporozoite protein of Plasmodium berghei together with the adjuvant QS-21: quantitative and phenotypic comparison with the vaccine model of irradiated sporozoites. Vaccine. 2005;23(21):2801–2812. doi: 10.1016/j.vaccine.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 17.Schaed SG, Klimek VM, Panageas KS, Musselli CM, Butterworth L, Hwu WJ, Livingston PO, Williams L, Lewis JJ, Houghton AN, Chapman PB. T-cell responses against tyrosinase 368–376 (370D) peptide in HLA*A0201+ melanoma patients: randomized trial comparing incomplete Freund’s adjuvant, granulocyte macrophage colony-stimulating factor, and QS-21 as immunological adjuvants. Clin Cancer Res. 2002;8(5):967–972. [PubMed] [Google Scholar]
- 18.Vandepapeliere P, Horsmans Y, Moris P, Van MM, Janssens M, Koutsoukos M, Van BP, Clement F, Hanon E, Wettendorff M, Garcon N, Leroux-Roels G. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26(10):1375–1386. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Garcon N, Silvano J, Kuper CF, Baudson N, Gerard C, Forster R, Segal L. Non-clinical safety evaluation of repeated intramuscular administration of the AS15 immunostimulant combined with various antigens in rabbits and cynomolgus monkeys. J Appl Toxicol. 2015 doi: 10.1002/jat.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Destexhe E, Stannard D, Wilby OK, Grosdidier E, Baudson N, Forster R, Gerard CM, Garcon N, Segal L. Nonclinical reproductive and developmental safety evaluation of the MAGE-A3 Cancer Immunotherapeutic, a therapeutic vaccine for cancer treatment. Reprod Toxicol. 2015;51:90–105. doi: 10.1016/j.reprotox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Destexhe E, Grosdidier E, Baudson N, Forster R, Gerard C, Garcon N, Segal L. Non-clinical safety evaluation of single and repeated intramuscular administrations of MAGE-A3 Cancer Immunotherapeutic in rabbits and cynomolgus monkeys. J Appl Toxicol. 2015;35(7):717–728. doi: 10.1002/jat.3025. [DOI] [PubMed] [Google Scholar]
- 22.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 23.Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clin Lung Cancer. 2009;10(5):371–374. doi: 10.3816/CLC.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 24.GlaxoSmithKline (2013) The investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoint in Phase III melanoma clinical trial. Retrieved from: http://www.gsk.com/en-gb/media/press-releases/2013/the-investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoint-in-phase-iii-melanoma-clinical-trial/. Accessed 10 Sept 2015
- 25.GlaxoSmithKline (2014) Investigational MAGE-A3 antigen-specific cancer immunotherapeutic does not meet first co-primary endpoints in MAGRIT, a phase III non-small cell lung cancer clinical trial [Press release]. Retrieved from http://www.gsk.com/en-gb/media/press-releases/2014/investigational-mage-a3-antigen-specific-cancer-immunotherapeutic-does-not-meet-first-co-primary-endpoints-in-magrit-a-phase-iii-non-small-cell-lung-cancer-clinical-trial/. Accessed 10 Sept 2015
- 26.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cumberbatch M, Dearman RJ, Griffiths CE, Kimber I. Epidermal Langerhans cell migration and sensitisation to chemical allergens. APMIS. 2003;111(7–8):797–804. doi: 10.1034/j.1600-0463.2003.11107811.x. [DOI] [PubMed] [Google Scholar]
- 28.Slingluff CL, Jr, Yamshchikov GV, Hogan KT, Hibbitts SC, Petroni GR, Bissonette EA, Patterson JW, Neese PY, Grosh WW, Chianese-Bullock KA, Czarkowski A, Rehm PK, Parekh J. Evaluation of the sentinel immunized node for immune monitoring of cancer vaccines. Ann Surg Oncol. 2008;15(12):3538–3549. doi: 10.1245/s10434-008-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, Patterson JW, Fink R, Rehm PK. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13(21):6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 30.Gruselle O, Coche T, Louahed J. Development of a quantitative real-time RT-PCR assay for the detection of MAGE-A3-positive tumors. J Mol Diagn. 2015;17(4):382–391. doi: 10.1016/j.jmoldx.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Vantomme V, Dantinne C, Amrani N, Permanne P, Gheysen D, Bruck C, Stoter G, Britten CM, Keilholz U, Lamers CH, Marchand M, Delire M, Gueguen M. Immunologic analysis of a phase I/II study of vaccination with MAGE-3 protein combined with the AS02B adjuvant in patients with MAGE-3-positive tumors. J Immunother. 2004;27(2):124–135. doi: 10.1097/00002371-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, Grosh WW, Boisvert ME, Kirkwood JM, Chianese-Bullock KA. Effect of GM-CSF on circulating CD8+ and CD4+ T cell responses to a multipeptide melanoma vaccine: Outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15(22):7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowder MJ, Hand DJ. Analysis of repeated measures. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- 34.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW. A randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29(21):2924–2932. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koup RA, Douek DC. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb Perspect Med. 2011;1(1):a007252. doi: 10.1101/cshperspect.a007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed CM, Cresce ND, Mauldin IS, Slingluff CL, Jr, Olson WC. Vaccination with melanoma helper peptides induces antibody responses associated with improved overall survival. Clin Cancer Res. 2015;21(17):3879–3887. doi: 10.1158/1078-0432.CCR-15-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, Hensley ML, Konner J, Tew W, Spriggs DR, Hoffman EW, Venhaus R, Pan L, Salazar AM, Diefenbach CM, Old LJ, Gnjatic S. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18(23):6497–6508. doi: 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]
- 38.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Hibbitts S, Grosh WW, Chianese-Bullock KA, Bissonette EA, Barnd DL, Deacon DH, Patterson JW, Parekh J, Neese PY, Woodson EM, Wiernasz CJ, Merrill P. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22(22):4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 39.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbitts S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EM, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21(21):4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Teates D, Neese P, Grosh WW, Petroni GR, Engelhard VH, Slingluff CL., Jr Evaluation of peptide vaccine immunogenicity in draining lymph nodes and blood of melanoma patients. Int J Cancer. 2001;92(5):703–711. doi: 10.1002/1097-0215(20010601)92:5<703::AID-IJC1250>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Stanton AW, Mellor RH, Cook GJ, Svensson WE, Peters AM, Levick JR, Mortimer PS. Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat Res Biol. 2003;1(2):121–132. doi: 10.1089/153968503321642615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brautigam P, Vanscheidt W, Foldi E, Krause T, Moser E. The importance of the subfascial lymphatics in the diagnosis of lower limb edema: investigations with semiquantitative lymphoscintigraphy. Angiology. 1993;44(6):464–470. doi: 10.1177/000331979304400606. [DOI] [PubMed] [Google Scholar]
- 43.Slingluff CL, Jr, Petroni GR, Olson W, Czarkowski AR, Grosh WW, Smolkin M, Chianese-Bullock KA, Neese PY, Deacon DH, Nail CJ, Merrill P, Fink R, Rehm PK. Helper T cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26(30):4973–4980. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL., Jr Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol Immunother. 2013;62(7):1149–1159. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.