Abstract
Background
There is no consensus about the optimal extent of surgery for patients with melanoma metastases to inguinal nodes, and this is further complicated by variations in terminology for these dissections. In patients without clinical evidence of iliac metastases, we routinely perform a superficial groin dissection (SGD), which clears node-bearing tissue superficial to the fascia lata. We hypothesized that SGD provides regional tumor control comparable to published experience with deep groin dissection (DGD) and iliac and obturator dissection (IOD), but with less morbidity.
Methods
A retrospective review of a prospectively collected database evaluated patients undergoing SGD April 1994 through May 2008. Patients with clinical evidence of iliac metastases were excluded. Clinical and pathologic data regarding recurrence and survival were evaluated.
Results
We identified 53 primary SGD: 27 for clinically palpable disease, and 25 for microscopic disease. Number and percentage of positive nodes were similar between groups. Median followup was 39 months, and two patients had primary recurrence in the groin (one in each group). Two additional patients had concurrent groin and systemic recurrence. Five-year Kaplan-Meier estimates for ipsilateral groin recurrence prior to systemic disease, were similar at 9.7% (SE 6.5) and 7.7% (SE 7.4), for microscopic and palpable disease respectively. Similarly, survival was comparable between groups (73% and 82%). Toxicities were comparable to previously published data.
Conclusion
SGD provides regional control rates similar to DGD and IOD, for lymph node metastases clinically limited to the groin, whether occult or clinically evident.
Introduction
Melanoma is a leading cause of skin cancer deaths. Patients with intermediate (1–4mm) and thick (>4mm) melanoma may benefit from a sentinel lymph node (SLN) biopsy.1,2 In the case of a positive SLN, therapeutic completion lymph node dissection (CLND) is typically performed. Other indications for a complete dissection are palpable nodal disease and positive findings on imaging studies, confirmed by cytology. There is reasonable consensus about the appropriate extent of CLND for melanoma metastases to cervical or axillary nodes; however, there is no consensus on the optimal extent of surgery for groin metastases.
There are three levels of dissection in the groin. Superficial groin dissection (SGD), captures all inguinal node-bearing tissue between the superficial fascia and the fascia lata, in a triangular area bound by the adductor longus medially, and the Sartorius laterally, and the inguinal ligament superiorly. The fascia lata is continuous with the fascia overlying the Sartorius and adductors, an easily identifiable plane defining the deep border of SGD and the roof of the femoral canal3. This area superficial to the fascia lata has the greatest number of inguinal nodes, which drains most of the cutaneous portion of the lower extremity.3,4 It also includes node-bearing tissue superficial to the inguinal ligament. A deep groin dissection (DGD) includes the same areas as the SGD, but also encompasses the tissue within the femoral sheath, deep to the fascia lata. This commonly contains one or two deep inguinal nodes, as well as several lymphatic channels.3 This requires skeletonization of the femoral vessels, and increased associated morbidity.5 Both SDG and DGD commonly include excision of Cloquet’s node when it can be identified, at the superior end of that dissection along the femoral canal. An iliac and obturator dissection (IOD) often accompanies a groin dissection (SGD or DGD), and involves dissection of obturator nodes and nodes along the external iliac vessels from the inguinal ligament to the origin of the internal iliac artery. IOD requires skeletonization of the external iliac vessels. In common usage, the term “deep dissection” often refers to an isolated iliac/obturator dissection (IOD) or the ilioinguinal dissection (combined SGD or DGD with IOD), but we suggest using terminology that more precisely defines the relevant inguinal and iliac anatomy.
Groin dissections are associated with significant morbidity. Overall morbidity rates have been reported between 17 and 90%, with incidence of wound infection of 13–33%.6,7 Other morbidity includes seroma formation, skin flap necrosis, and lymphedema.
Preoperative screening for patients with melanoma metastatic to inguinal node metastases includes full history and physical, preoperative laboratory analysis, chest X-ray, and pelvic CT scan. SGD plus excision of Cloquet’s node is our standard practice for patients with a positive groin sentinel node or limited palpable groin metastases, and without iliac disease on preoperative workup. We limit use of DGD to cases with extensive disease involving the fascia lata, and IOD when CT or clinical findings identify disease in the iliac chain, or if there is a positive Cloquet’s node at groin dissection.8 Others have recommended DGD, even combined with IOD, for all patients with inguinal nodal metastases.9 However, these approaches skeletonize the femoral vessels over a long distance, and the high rate of wound breakdown raises concerns for the risks of vascular injury in the postoperative period. To prevent this, a rotation flap of the Sartorius muscle has been used to cover the exposed femoral vessels.5,10 Though this is well-tolerated, it has not been shown to reduce complications associated with groin dissection and may actually increase rate of seroma.10,11
Our purpose in this study was to evaluate regional control and postoperative morbidity after SGD without skeletonization of the femoral vessels. We present our regional control rates in the context of reported experience where appropriate.6,7,12–17 We hypothesized that regional control would be better for patients with micrometastases (found on SLN biopsy) than for macroscopic metastases (found on clinical exam as palpable lymphadenopathy). We believed that SGD would have a morbidity equivalent to or better than that seen for DGD with or without IOD.
Methods
Study approval was obtained from the University of Virginia Institutional Review Board. We reviewed a prospectively collected database, and identified 64 groin dissections performed between April 1994 and May 2008 and followed until May 2009. All patients had a preoperatively negative CT scan of the chest, abdomen, and pelvis if suspicion for distant disease was high. CT was occasionally deferred for patients with positive sentinel nodes with a small tumor burden, as well as pediatric patients. Patients who were found to have iliac nodal disease on CT or a positive Cloquet’s node at initial groin dissection were offered IOD and were excluded from this study. Fifty-three SGD were identified in 52 patients.
Superficial groin dissection was performed through a vertical incision from 1994 to 2000, and was performed through an oblique skin incision beginning in 2001. En bloc removal of the femoral triangle contents, ligation of the saphenous vein, and removal of Cloquet’s node, when identified, was standard. Selected patients had coverage of the femoral vessels with an adjacent fascial flap. Other studies have demonstrated that leaving fascia intact whenever possible has been shown to reduce morbidity.15 The Sartorius was not repositioned.
Clinical data and pathologic information were collected, including patient age, sex, breslow thickness and ulceration of the primary tumor (when known), pathologic status of nodal basin including total number of nodes, number of positive nodes, and the presence and positivity of Cloquet’s node. Chart review of physician notes was used to determine recurrence, survival, and morbidity such as wound infection, seroma, flap necrosis and lymphedema. Recurrence was defined as discovery of melanoma during followup clinical examination or imaging. Regional recurrence was identified as metastatic lymph node tissue found in a previous dissection basin (including inguinal, iliac and/or pelvic nodes). This was determined to be melanoma by fine needle aspiration (FNA) prior to any treatment decision. Nodal basins outside the original basin of dissection were classified as distant for this study. Patterns of recurrence were broken into local, in-transit, regional/nodal, or distant. In-transit metastasis is defined by subcutaneous or dermal involvement with tumor between the primary site and the draining lymph basin.
Wound infection was defined by the use of antibiotics for cellulitis or presence of purulent discharge postoperatively. Prolonged drain use was defined as >30days postoperatively. Seroma was defined as a palpable subcutaneous fluid collection requiring percutaneous drainage. Lymphocele was only found in a small number of patients, and was grouped with seroma for statistical purposes. Wound necrosis and breakdown is defined as prolonged separation of the wound lasting longer than 2 weeks. Lymphedema was defined as such if there was documented leg swelling no less than 3 months following groin dissection.
Statistical analysis was performed using SPSS 17. Statistical comparisons were done using chi2 test for categorical data and Fisher’s exact test for continuous endpoints, and a log-rank test for survival and recurrence endpoints. P values <=0.05 were considered significant.
Results
Patient characteristics are in table 1. Among the 52 patients, 22 were male and 30 female, with a mean age of 55 [range 0.2–85]. Three patients were under 14 years of age at time of SGD (1 month, 6 years, and 12 years of age respectively).18 One patient underwent bilateral groin dissections. Average Breslow depth of the primary melanomas was 3.93 mm. A total of 25 underwent dissection after metastatic melanoma was identified in one or more SLN, and 27 patients underwent dissection after groin adenopathy was noted on physical exam. These patients included 8 (30%) with palpable adenopathy at the time of primary melanoma diagnosis, 4 (15%) with palpable metastases from an unknown primary, and 15 (55%) who had a palpable recurrence following a previous wide local excision (WLE) or previous negative SLN biopsy. Also, 44 (83%) patients underwent preoperative CT to exclude distant metastases: 26 (96%) in the palpable group and 18 (72%) in the nonpalpable group.
Table 1.
Clinical and pathologic characteristics of patients undergoing SGD
| Characteristics | |
|---|---|
| Sex, n(%) | |
| Male | 22 (42) |
| Female | 30 (58) |
|
| |
| Age (yr) | |
| Mean | 55.8 |
| Median | 55.8 |
| Range | 0.2–85.2 |
|
| |
| Breslow depth (mm)* | |
| Mean | 3.93 |
| Median | 2.58 |
| Range | 0.55–13 |
|
| |
| Localization of primary | |
| Trunk | 10 |
| Lower extremity | 36 |
| Unknown | 5 |
|
| |
| Clinical examination of groin | |
| Nonpalpable | 25 |
| Palpable | 27 |
|
| |
| Followup (months) | |
| Median | 39 |
| Range | 5–160 |
|
| |
| No. positive nodes, n(%) ** | |
| 1 | 29 (57) |
| 2–3 | 16 (31) |
| 4+ | 6 (12) |
Three patients with unknown depth are excluded from these data
Number of positive nodes is for the entire basin, including sentinel nodes excised prior to SGD.
Median followup time was 39 months. The five-year Kaplan-Meier survival estimate was 73% (SEM 11%). Primary isolated groin recurrence was found in two patients (3.7%, Figure 1). One recurrence was after SGD for palpable nodal disease fourteen years after WLE, and one recurrence was after SGD for micrometastatic disease found on SLN biopsy. For both patients with isolated primary recurrence in the groin, re-resection rendered them disease free, and they are alive without evidence of disease 7.5 years and 1.5 years after re-excision. Groin recurrence with concurrent systemic recurrence was identified in 2 additional patients (3.7%). For all SGD patients, median time to recurrence was 19 months.
Figure 1.
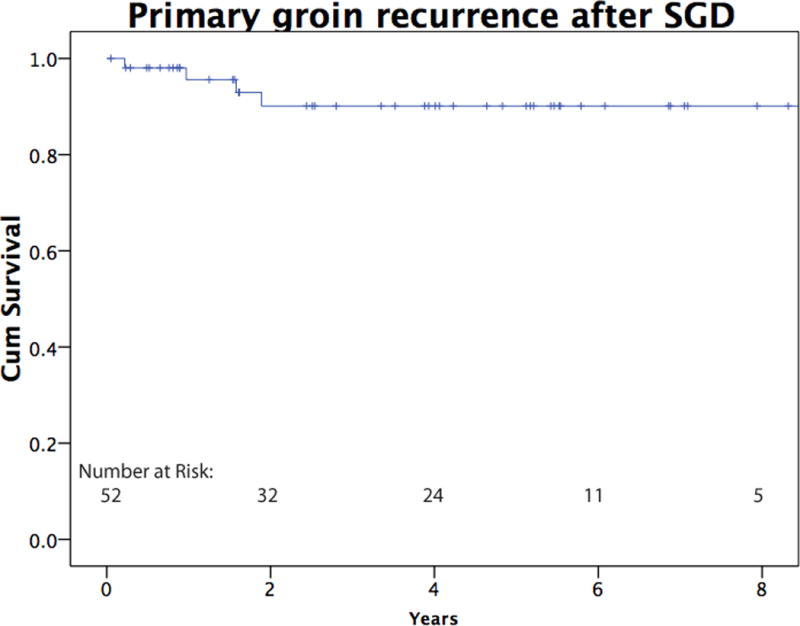
Kaplan Meier curve indicating primary recurrence in the groin following SGD for melanoma. This graph includes patients with isolated primary recurrence as well as primary recurrence concurrent with systemic recurrence.
When comparing patients with SGD for palpable disease to those having nonpalpable (micrometastatic) disease, there was no difference in the number of total lymph nodes resected (median 11 in the palpable group, 10 in the nonpalpable). Additionally, there was no significant difference between the number of positive nodes in the groin (mean 1.7 in the palpable group, 1.8 in the nonpalpable). The number of nodes in the basin included all nodes removed from the basin, including sentinel nodes and excisional biopsies when applicable.
Several factors were independently evaluated to determine whether they had a bearing on groin recurrence. Bulk of disease, number of positive lymph nodes, and extranodal extension had no impact on recurrence (p=0.26, 0.62 and 0.42, data not shown). The bulk of disease in the groin (palpable vs. micrometastatic disease) was not associated with any difference in overall survival (Figure 2a). The presence of extranodal extension approached significance as a negative prognostic feature (p=0.08, data not shown), and the presence of two or more positive nodes was associated with decreased survival (p=0.02, Figure 2b). Patients with palpable disease were more likely to have distant metastases as a first site of recurrence after groin dissection (p=0.018), while patients with micrometastatic disease were more likely to have in transit disease as the first site of recurrence (p=0.008, Table 2). Of the six patients with micrometastatic disease that developed in-transit recurrences, 4 of these (67%) went on to subsequently develop distant metastatic disease. Two patients (25%) who did not undergo preoperative CT experienced in transit recurrence, which is consistent with rates of patients who underwent preoperative CT scanning. None of the 3 pediatric patients had a recurrence after SGD.
Figure 2.
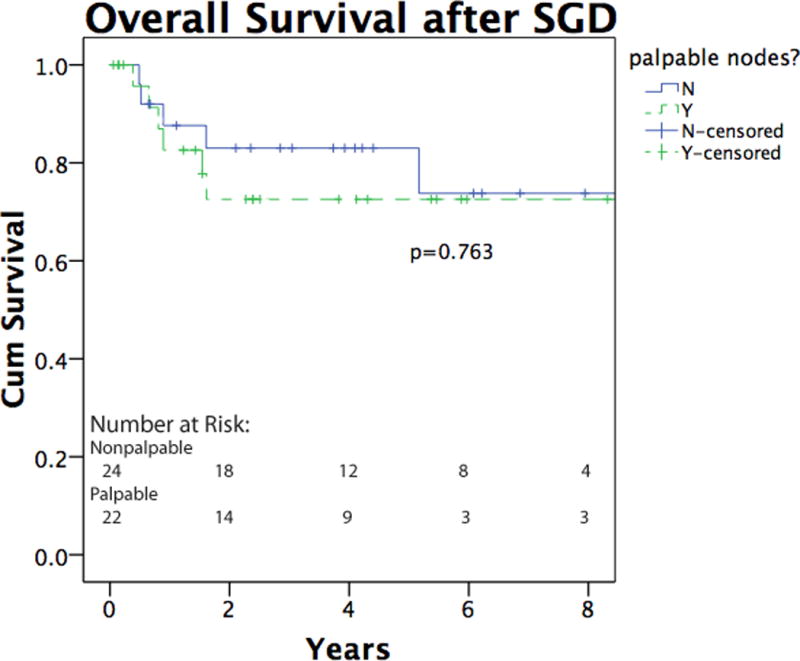
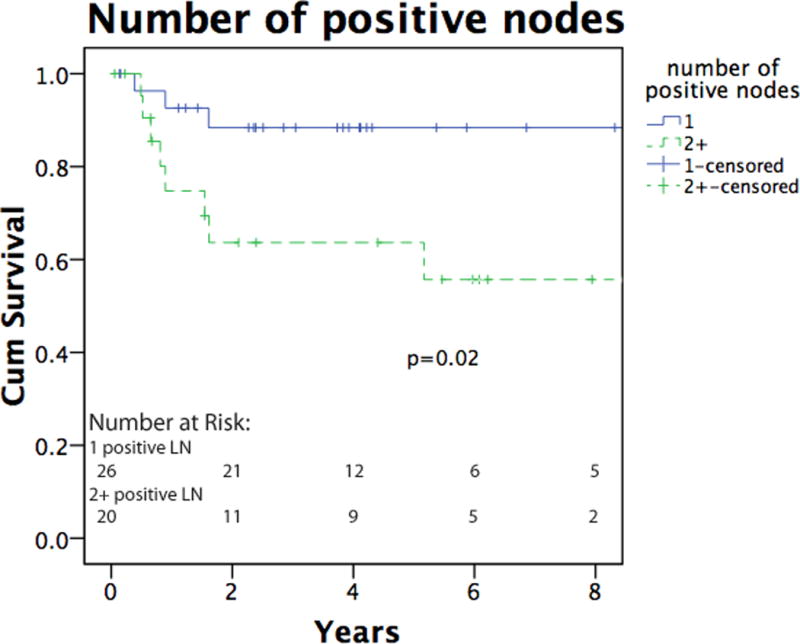
a. Overall survival of melanoma patients following SGD. The solid line indicates patients with micrometastatic, nonpalpable groin disease, and the dashed line indicates patients with clinically palpable groin disease. b. Overall survival as a function of lymph node positivity. Overall survival was significantly higher in patients with 1 total positive lymph node (solid line) as compared to 2 or more positive nodes (dashed line). Lymph node total includes previously positive sentinel node(s) when applicable.
Table 2.
Location of first documented recurrence after superficial groin dissection
| Site of recurrence | Palpable No.(%) |
Nonpalpable No.(%) |
P value |
|---|---|---|---|
| None | 16 (59) | 16 (64) | NS* |
| In-transit | 0 | 6 (24) | 0.008 |
| Regional/Nodal | 1 (4) | 1 (4) | NS* |
| Distant (inc. distant nodal) | 10 (37) | 2 (8) | 0.018 |
NS denotes p<0.05.
Overall, lymphedema and wound infection were the most common complications, occurring in 21 patients (40%) and 22 patients (42%), respectively (Table 3). There was no correlation between incidence of wound infection or location of primary melanoma and development of lymphedema (data not shown). Seroma occurred in 9 patients (17%), and an additional 19 patients (36%) had JP drains in place longer than 30 days. Oblique incision was associated with longer duration of JP drainage compared with vertical incision (p = 0.04). There was no difference between wound complication rates for patients with palpable and micrometastatic disease (data not shown). There was no difference in wound complication rates for those with a vertical incision and those with an oblique incision. Published rates of seroma, infection, wound breakdown, and lymphedema were collected for comparison.4, 7, 12, 14–16, 19 (Table 3)
Table 3.
Complications after superficial groin dissection
Discussion
The primary purpose of this study was to evaluate whether SGD provided acceptable regional control in patients with metastatic melanoma to the groin. The overall rate of recurrence in the same basin was 4%, which compares favorably to rates reported for DGD +/− IOD.6,12,13,20 (Table 4) Like other published studies, we found that number of positive nodes and extracapsular extension are better predictors of poor outcome than palpable tumor burden in the groin.20–22 Thus, even in patients with palpable disease, SGD is appropriate for clearance of lymph nodes from the groin. The obvious caveat to this is that patients must be evaluated preoperatively and have no evidence of iliac metastases prior to SGD.
Table 4.
Literature review of groin dissection recurrence rates
| Reference | Year | No. of patients | Regional recurrence rates (%) | Comments |
|---|---|---|---|---|
| Shada and Slingluff | 2010 | 52 | 4 | SGD subset only |
| Hughes et al.14 | 2000 | 29 | 7 | SGD subset only |
| Guggenheim et al.6 | 2008 | 43 | 12 | SGD subset only, nonpalpable disease only |
| van der Ploeg et al.17 | 2008 | 18 | 11 | SGD subset only |
| Sabel et al.16 | 2007 | 212 | 9 | SGD +/− IOD, Includes concurrent systemic recurrence |
| Essner et al.13 | 2006 | 89 | 4 | DGD +/− IOD |
| Singletary et al.24 | 1992 | 264 | 15 | DGD +/− IOD |
| Kretschmer et al.20 | 2001 | 104 | 34 | SGD/DGD +/− IOD, palpable disease only |
| Lawton et al.15 | 2002 | 56 | 2 | SGD + IOD, includes negative ELND |
| Slingluff et al.25 | 1994 | 911 | 5 | ELND, axillary and cervical included |
| van Akkooi et al.7 | 2006 | 129 | 11 | DGD + IOD |
| Allan et al.12 | 2008 | 22 | 8 | DGD + IOD, palpable disease only |
SGD: superficial groin dissection. IOD: iliac-obturator dissection. DGD: deep groin dissection ELND: elective lymph node dissection.
Hughes et al evaluated both SGD and SGD+IOD in melanoma patients and found that 34% of patients without evidence of iliac disease on CT scan had positive pelvic lymph nodes.14 The current study has a 4% disease recurrence rate in patients with a negative CT scan, and the discrepancy may be due to an improvement in CT scan resolution over time. Allan et al used CT to evaluate patients with palpable adenopathy and had a sensitivity of 60% and NPV of 86% and concluded that CT is not reliable to exclude pelvic nodal involvement.12 However, they found no significant difference in disease free survival between patients with and without pelvic node involvement. Van der Ploeg et al. proposed a different mechanism to direct operative strategy: use of lymphoscintigraphy to determine whether pelvic nodes received substantial “second echelon” drainage. They concluded that a strategy to use the location of second-tier nodes to determine extent of groin dissection after positive SLN was warranted.17 Their recurrence rate following SGD directed by lymphoscintigraphy was 11.1%.17
Few studies compare palpable and microscopic disease burden. Kretschmer et al. evaluated patients with palpable inguinal disease, and found no difference in overall survival between those receiving inguinal dissection only and those receiving inguinal dissection with iliac and obturator lymphadenectomy. However, overall recurrence rate was 33.6%, and the fact that the study evaluated palpable disease may factor into that rate.20 The current study included patients with palpable disease and did not see as high a rate of local recurrence after SGD. Perhaps our use of preoperative evaluation to exclude patients with iliac disease produced a patient population with lower overall regional disease burden. Guggenheim et al. reported an 11.9% local recurrence rate after SGD performed in patients with nonpalpable disease, and concluded that a limited procedure in a defined patient subgroup was appropriate.6 Sabel et al reported lower overall survival and higher distant recurrence in patients undergoing groin dissection for palpable disease when compared with nonpalpable disease.16 Our study also found increased distant recurrences as the initial site in patients with palpable groin nodes, but this did not translate to a different overall distant recurrence rate or reduced overall survival rate. We suspect that absence of differences in overall survival in the present study is due to exclusion of patients with clinical evidence of iliac metastases, which removes some of the patients with palpable inguinal disease who have the worst prognosis. Since such preoperative staging is recommended in the patient care setting, the data from the present study are relevant to patient subsets selected for SGD.
The current study found a difference in pattern of recurrence for patients with palpable disease as compared to those with microscopic disease, indicating a potential difference n tumor biology between the two groups. This is the first series to compare recurrence patterns in these two patient groups. Patients with microscopic lymph node metastases were more likely to harbor in-transit metastases as a first site of metastasis, but many of these patients went on to develop distant metastases. The higher rate of initial distant metastasis for those with palpable disease may speak to the increased total systemic disease burden at the time of resection. Wagner et al. found increased distant metastasis as initial site of recurrence in patients with positive SLN when compared to those with negative SLN.23 Although these populations are not the ones evaluated in the current study, it supports the hypothesis that overall increase in disease burden increases risk of distant metastases.
A secondary aim of this study was to evaluate morbidity following SGD. Morbidity rates were similar for palpable and micrometastatic disease in this study. We hypothesized that morbidity would be equivalent to or better than that published for DGD +/− IOD. Review of the literature reveals wide ranges for reported morbidity rates, and rates of seroma, wound breakdown, and lymphedema after SGD in our study are within those range reported after deep groin dissection.6,12,14,16,17,19 (Table 3) The rate of wound infections we found was slightly greater than the upper end of the reported range. This may be due to the variance in defining wound infection across multiple studies. Our definition was strict and potentially included some false positives. Importantly, we had no major vascular complications even though the Sartorius muscle was not rotated to cover the femoral vessels. We believe that preservation of the fascia lata covering the vessels over most of their length in the wound sufficiently protects the femoral vessels. Though the Sartorius flap is useful in some cases, such as wound dehiscence with exposed vessels, our approach captures the majority of nodal tissue in the groin, has good outcomes, and preserves fascia. A retrospective assessment of lymphadenectomy with preservation of fascia has been shown to decrease morbidity.15
In contrast to our findings, some studies have found increased morbidity in patients presenting with clinically palpable disease.12,16 Sabel et al reported that patients undergoing groin dissection for palpable disease had increased peri-operative morbidity when compared to those undergoing groin dissections after positive sentinel node.16 The fact that this study evaluated SGD and SGD + IOD may alter the morbidity rate. Though the current study does not find a clear morbidity benefit for SGD over a more extensive dissection, we must acknowledge the limitations of the current study.
Pitfalls of the current study are its retrospective nature limiting the assessment of morbidity and the effort to compare to other published retrospective studies. Also confounding a more in-depth comparison is the inability to separate SGD and DGD, with or without IOD, in prior studies. Some studies do not differentiate between SGD and DGD, while others refer to ilioinguinal dissection as DGD. More universally applied terminology to define extent of dissection in the inguinal, iliac, and obturator regions would be helpful. An additional limitation involves the length of time over which this cohort was collected (15 years). During this time period, sentinel node biopsy became standard, and the entire nonpalpable group of patients was evaluated after advent of SLN biopsy at our institution. Thus, patients with palpable disease tended to be spread out over a longer period, and may have had increased disease burden earlier in the study when imaging was less precise. Despite these limitations, until randomized prospective trials comparing morbidity and clinical outcome after SGD, DGD alone and in combination with IOD are performed, such retrospective data may be useful for informing patients about perioperative risks and in guiding future study design.
Conclusion
Our results lend support to our standard procedure of using SGD for patients with inguinal metastases without clinical evidence of iliac metastases. DGD is appropriate for patients with extensive disease involving the fascia lata, and IOD is appropriate for patients with clinical evidence of metastases in the iliac or obturator basins, or after resection of a positive Cloquet’s node. In properly selected patients with microscopic or clinically evident inguinal metastases, superficial groin dissection, superficial to the fascia lata, provides good regional control and comparable morbidity rates.
Synopsis.
Groin dissection in melanoma has significant morbidity, and there is not universal agreement upon the extent of dissection required. We evaluated our institutional experience with superficial groin dissection in melanoma, with a focus on postoperative groin recurrence and morbidity.
Footnotes
Disclosures: The authors have no commercial interest in the subject of study
References
- 1.Gutzmer R, et al. Sentinel lymph node status is the most important prognostic factor for thick (> or = 4 mm) melanomas. J Dtsch Dermatol Ges. 2008;6:198–203. doi: 10.1111/j.1610-0387.2007.06569.x. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242:302–11. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 311–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spratt JS, Shieber W, Dillard MB. Anatomy and surgical technique of groin dissection. Saint Louis, MO: The C.V. Mosby Company; 1965. [Google Scholar]
- 4.Swan MC, Furniss D, Cassell OC. Surgical management of metastatic inguinal lymphadenopathy. BMJ. 2004;329:1272–1276. doi: 10.1136/bmj.329.7477.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarnaik AA, Puleo CA, Zager JS, Sondak VK. Limiting the morbidity of inguinal lymphadenectomy for metastatic melanoma. Cancer Control. 2009;16:240–247. doi: 10.1177/107327480901600306. [DOI] [PubMed] [Google Scholar]
- 6.Guggenheim MM, et al. Morbidity and recurrence after completion lymph node dissection following sentinel lymph node biopsy in cutaneous malignant melanoma. Ann Surg. 2008;247:687–693. doi: 10.1097/SLA.0b013e318161312a. [DOI] [PubMed] [Google Scholar]
- 7.van Akkooi AC, et al. Morbidity and prognosis after therapeutic lymph node dissections for malignant melanoma. Eur J Surg Oncol. 2007;33:102–108. doi: 10.1016/j.ejso.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Shen P, et al. Is the node of Cloquet the sentinel node for the iliac/obturator node group? Cancer J. 2000;6:93–97. [PubMed] [Google Scholar]
- 9.Balch CM, et al. Sentinel node biopsy and standard of care for melanoma. J Am Acad Dermatol. 2009;60:872–875. doi: 10.1016/j.jaad.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 10.Judson PL, et al. A prospective, randomized study analyzing sartorius transposition following inguinal-femoral lymphadenectomy. Gynecol Oncol. 2004;95:226–230. doi: 10.1016/j.ygyno.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Abbas S, Seitz M. Systematic review and meta-analysis of the used surgical techniques to reduce leg lymphedema following radical inguinal nodes dissection. Surg Oncol. doi: 10.1016/j.suronc.2009.11.003. in press. [DOI] [PubMed] [Google Scholar]
- 12.Allan CP, Hayes AJ, Thomas JM. Ilioinguinal lymph node dissection for palpable metastatic melanoma to the groin. ANZ J Surg. 2008;78:982–986. doi: 10.1111/j.1445-2197.2008.04716.x. [DOI] [PubMed] [Google Scholar]
- 13.Essner R, et al. Surgical management of the groin lymph nodes in melanoma in the era of sentinel lymph node dissection. Arch Surg. 2006;141:877–82. doi: 10.1001/archsurg.141.9.877. discussion 882–4. [DOI] [PubMed] [Google Scholar]
- 14.Hughes TM, A’Hern RP, Thomas JM. Prognosis and surgical management of patients with palpable inguinal lymph node metastases from melanoma. Br J Surg. 2000;87:892–901. doi: 10.1046/j.1365-2168.2000.01439.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawton G, Rasque H, Ariyan S. Preservation of muscle fascia to decrease lymphedema after complete axillary and ilioinguinofemoral lymphadenectomy for melanoma. J Am Coll Surg. 2002;195:339–351. doi: 10.1016/s1072-7515(02)01230-9. [DOI] [PubMed] [Google Scholar]
- 16.Sabel MS, et al. Inguinal node dissection for melanoma in the era of sentinel lymph node biopsy. Surgery. 2007;141:728–735. doi: 10.1016/j.surg.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 17.van der Ploeg IM, Valdes Olmos RA, Kroon BB, Nieweg OE. Tumor-positive sentinel node biopsy of the groin in clinically node-negative melanoma patients: superficial or superficial and deep lymph node dissection? Ann Surg Oncol. 2008;15:1485–1491. doi: 10.1245/s10434-008-9840-2. [DOI] [PubMed] [Google Scholar]
- 18.McElearney ST, Dengel LT, Vaughters AB, Patterson JW, McGahren ED, Slingluff CL., Jr Neonatal congenital malignant melanoma with lymph node metastasis. J Clin Oncol. 2009;27:2726–8. doi: 10.1200/JCO.2008.20.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonouchi H, et al. Operative morbidity associated with groin dissections. Surg Today. 2004;34:413–418. doi: 10.1007/s00595-003-2738-5. [DOI] [PubMed] [Google Scholar]
- 20.Kretschmer L, Neumann C, Preusser KP, Marsch WC. Superficial inguinal and radical ilioinguinal lymph node dissection in patients with palpable melanoma metastases to the groin–an analysis of survival and local recurrence. Acta Oncol. 2001;40:72–78. doi: 10.1080/028418601750071091. [DOI] [PubMed] [Google Scholar]
- 21.Coit DG, Brennan MF. Extent of lymph node dissection in melanoma of the trunk or lower extremity. Arch Surg. 1989;124:162–166. doi: 10.1001/archsurg.1989.01410020032004. [DOI] [PubMed] [Google Scholar]
- 22.Mann GB, Coit DG. Does the extent of operation influence the prognosis in patients with melanoma metastatic to inguinal nodes? Ann Surg Oncol. 1999;6:263–271. doi: 10.1007/s10434-999-0263-5. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JD, et al. Patterns of initial recurrence and prognosis after sentinel lymph node biopsy and selective lymphadenectomy for melanoma. Plast Reconstr Surg. 2003;112:486–497. doi: 10.1097/01.PRS.0000070989.23469.1F. [DOI] [PubMed] [Google Scholar]
- 24.Singletary SE, Shallenberger R, Guinee VF. Surgical management of groin nodal metastases from primary melanoma of the lower extremity. Surg Gynecol Obstet. 1992;174:195–200. [PubMed] [Google Scholar]
- 25.Slingluff CLJ, Stidham KR, Ricci WM, Stanley WE, Seigler HF. Surgical management of regional lymph nodes in patients with melanoma. Experience with 4682 patients. Ann Surg. 1994;219:120–130. doi: 10.1097/00000658-199402000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]


