Abstract
Background:
Allergic conjunctivitis (AC) is a common ocular inflammatory manifestation of allergen exposure in sensitized individuals. Signs and symptoms of AC can decrease quality of life, interfere with productivity, and lead to considerable economic burden. Consistent suppression of conjunctival inflammation is necessary for managing AC, but currently available medications require frequent administration and exhibit limited duration of action.
Methods:
In this review, we summarized AC pathogenesis, diagnosis, and current treatment options as well as their limitations. Findings from the literature were discussed in the context of the unmet need for a once-daily medication with sustained 24-hour effectiveness.
Results:
Topical pharmacologic treatments are the most common approach for managing extant AC; however, most available medications require multiple daily instillations. Dual-acting antihistamine-mast cell stabilizing agents are currently considered first-line therapeutics for AC because they provide acute relief of signs and symptoms and block persistent inflammation to promote regression of AC. Recent studies of a newly-developed, higher-concentration formulation of a dual-acting antihistamine-mast cell stabilizer have demonstrated that this formulation provides a 24-hour duration of action with once-daily dosing.
Conclusions:
Dual-acting AC medications exhibit a high degree of overall effectiveness and are well tolerated for chronic use. A newly available once-daily medication that manages signs and symptoms of AC for a full 24 hours may be considered a treatment of choice for patients experiencing seasonal or perennial AC. ClinicalTrials.gov NCT01743027 and NCT01479374
Keywords: Allergic conjunctivitis, duration of action, inflammation, mast cell stabilizer, ocular allergy, olopatadine, ocular itching
Allergic conjunctivitis (AC) is a common ocular manifestation of immunoglobulin E (IgE) immune responses to allergen exposure in sensitized individuals and is characterized by itching, conjunctival hyperemia, excessive tearing, and conjunctival and eyelid swelling.1,2 These signs and symptoms can be sufficiently bothersome that patients often experience decreased work productivity, increased work or school absenteeism, limitation of everyday activities, and reduced quality of life.1 As much as 40% of the population is affected by symptoms of AC,3 with the majority of cases (90–95%) attributed to seasonal AC (SAC) or perennial AC (PAC),4 and the prevalence of AC is reportedly increasing.5 Diagnosis and management of AC are complicated by symptomatic resemblance and frequent comorbidity of AC with other conditions, such as dry eye6 or allergic rhinitis.7 As such, AC may be underdiagnosed, underreported, and inadequately treated.
Poorly controlled AC can affect patients in a variety of nonphysical ways, which range from compromised work performance and productivity, increased health care costs, to decreased quality of life.8–10 Among individuals with AC or allergic rhinoconjunctivitis, these effects are mediated directly by the severity or frequency of ocular symptoms and indirectly by secondary factors, e.g., decreased sleep quality.8,11 In a survey of patients with allergy symptoms, respondents reported a work productivity rating decrease of 29% when their symptoms were most severe compared with productivity when the respondents were asymptomatic.12 Similarly, a survey of 404 patients with allergic rhinitis demonstrated significant covariance of ocular symptoms with work and/or school productivity, sleepiness, sleep quality, and mood.13
A survey conducted in the United Kingdom found that employed respondents with SAC lost an average of >3 hours of productive work time per week because of SAC symptoms.9 Respondents with SAC also experienced significantly more pain and discomfort, lower self-perception of their health, and impairment of social function.9 Accrued absences from school and work because of ocular symptoms can be substantial.11 The cost of managing AC, including medication and health care visits as well as decreased productivity, can pose an economic burden to patients: studies in Spain and the United Kingdom estimated costs as high as €349 (U.S. $392) per year and £124 (U.S. $190) per active allergy season, respectively.9,10 In the decade since these findings were published, costs have increased considerably. More recent estimates indicate that the costs attributable to prescription medications alone have increased by ∼25% per year since 2000.14
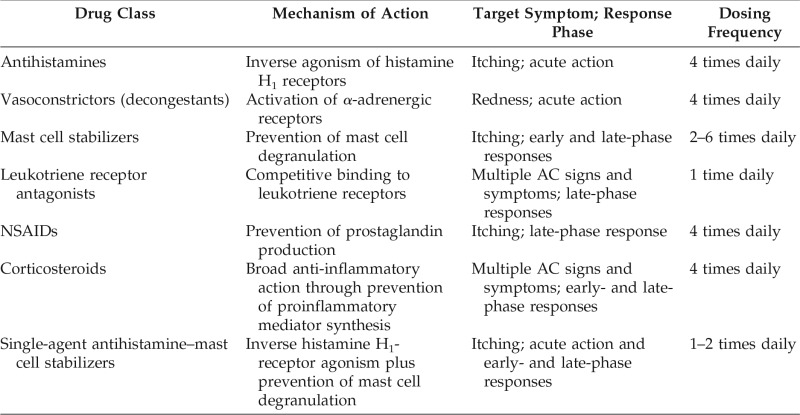
The most common treatment options for AC consist of topical ophthalmic formulations intended to reduce inflammation and provide symptomatic relief.15 As discussed in the section on current treatment options and presented in Table 1, most of these medications require multiple daily doses,16 which can be inconvenient and may reduce treatment compliance.17 The longer-acting medications (e.g., antihistamine–mast cell stabilizing agents) can reduce signs and symptoms of AC for no more than 16 hours. Hence, there is an unmet need for once-daily medications with sustained effectiveness over a full 24-hour period. The purpose of this review was to discuss available treatment options for AC and their limitations, and the need for a 24-hour medication to manage AC.
Table 1.
Pharmacologic treatments for allergic conjunctivitis
AC = Allergic conjunctivitis; NSAID = nonsteroidal anti-inflammatory drug.
PATHOGENESIS AND DIAGNOSIS
The conjunctiva is a transparent, vascularized mucous membrane that covers and lines the sclera and eyelids, and protects the ocular surface. This membrane contains abundant mast cells, which mediate the inflammatory immune response elicited by AC. Compared with the control subjects, conjunctival mast cell counts are increased by >50% in patients with various forms of AC, including SAC (both in season and out of season).18–21 Further, in healthy control subjects, mast cells are restricted to the conjunctival stroma (i.e., lamina propria), whereas migration to the conjunctival epithelium is evident in patients with SAC.18,20
The immunopathogenesis of AC has been reviewed in detail elsewhere.1,22,23 Briefly, the initial development of AC begins with ocular exposure to an allergen that elicits an immune response through activation of antigen-presenting cells and antibody production. On repeated exposure of sensitized individuals, allergen-activated IgE antibodies bound to primed conjunctival mast cells causes IgE cross-linkage that triggers mast cell degranulation and release of preformed mediators, defined as the early phase response. Subsequent production of histamine, prostaglandins, leukotrienes, and a host of inflammatory cytokines and chemokines contributes to the late-phase response characterized by chronic mast cell activation and mucosal recruitment of proinflammatory cells. This inflammatory response to allergens produces the ocular signs and symptoms associated with AC, exemplified by redness and itching.
Self-referral for AC symptoms is one of the most commonly reported reasons for patient-initiated visits to ophthalmologists or optometrists.12 A diagnosis of AC is dependent on many factors, including clinical signs, patient-reported symptoms, patient history, and a positive skin-prick test.16,24,25 Itching is one of the most frequently reported symptoms of AC; according to some health care providers, ocular itching is such a strong indicator of AC that, in its absence, a diagnosis of AC can often be ruled out.2,26 Other symptoms include dryness, grittiness, burning, photophobia, and foreign body sensation.4,23 Clinical signs of AC include conjunctival redness, tearing and possible clear mucoid discharge, and swelling (Fig. 1). The presence of eosinophils in conjunctival scrapings and elevated histamine, cytokine, or IgE levels in tear samples indicate an immune-mediated ocular condition and indicate a likely diagnosis of AC.27–29 Likewise, skin-prick testing by allergists or immunologists can identify or confirm allergy to one or more substances and may provide insight regarding the degree of patient sensitivity to specific allergens.30 For patients with recurrent, unpredictable AC manifestation or refractory symptoms, referral to a specialist to assess allergen sensitivity is especially warranted.
Figure 1.
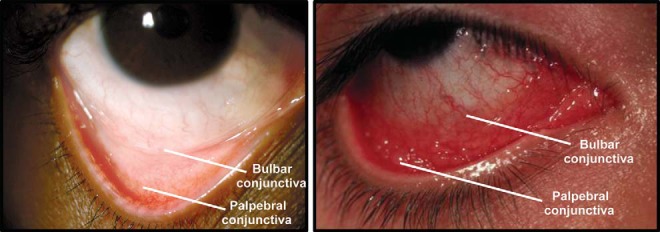
Normal eye (left) and eye with inflammation caused by allergic conjunctivitis (right). The bulbar and palpebral conjunctiva are indicated.
CURRENT TREATMENT OPTIONS
Optimal management of AC necessitates a broad approach that involves allergen avoidance, symptomatic relief, and pharmacologic suppression of inflammatory responses.2,31 For SAC, closing windows and avoiding the outdoors during allergenic seasons can reduce exposure; likewise, patients with PAC may benefit from frequent cleaning and use of air filters to reduce environmental allergens such as pet dander and household dust. Allergen avoidance can be difficult to implement for aeroallergens but may improve symptoms by ≥30%.25 Nonpharmacologic measures, such as artificial tears or saline solution to dilute the antigen load and inflammatory mediators23 or cold compresses, can provide a degree of temporary relief of ocular symptoms and may decrease redness.31,32
To suppress the inflammation that underlies AC signs and symptoms, interventions that target one or more points in the inflammatory response cascade are necessary (Table 1). The most common treatment approach for management of extant AC is use of a topical pharmacologic medication (e.g., a dual-acting antihistamine–mast cell stabilizer agent) to reduce inflammation combined with nonpharmacologic remedies (e.g., cold compresses or artificial tears) to provide temporary symptomatic relief.23,32 Although this approach is effective for most patients with mild symptoms of SAC or PAC, none of these medications last a full 24 hours. In addition, patients who experience moderate to severe symptoms that significantly interfere with daily activities and quality of life may require more effective and longer-lasting treatment. There have been few recent developments in strategies for treatment of AC. Existing drug classes and immunotherapies have been modified to improve safety and efficacy profiles, but AC remains inconvenient and costly to manage. A key limitation of many topical AC treatments is the need for multiple daily instillations to maintain symptomatic relief.2,16,33
Allergen-Specific Immunotherapy
Targeted immunotherapy, in which the immune system is desensitized to triggering allergens through chronic exposure to low doses of specific allergens delivered subcutaneously or sublingually, is intended to desensitize individuals to triggering allergens and prevent the activation of inflammatory signaling pathways.34,35 Allergen immunotherapy is indicated for patients with inadequate response to pharmacologic agents or who experience unacceptable adverse effects from medications.36 Over time, immunotherapy can effectively reduce immune responses to seasonal or environmental allergens in sensitized individuals to prevent the activation of inflammatory cascades and development of AC. Immunotherapy improves symptoms of SAC (e.g., itchiness, watery eyes, and red eyes) and may reduce AC medication use.34,35 Despite the effectiveness of immunotherapy, this treatment approach is not used by most patients.
In the Allergies, Immunotherapy, and Rhinoconjunctivitis study, only 22% of patients with allergic rhinoconjunctivitis reported receiving allergen immunotherapy.37 Further, many patients do not pursue desensitizing immunotherapy options recommended by their health care providers, and only a fraction of these patients complete therapy.38 Results of the Allergies, Immunotherapy, and Rhinoconjunctivitis study indicate that this may be because of treatment inconvenience, cost, or ineffectiveness.37 The availability and efficacy of topical treatments for acute AC symptoms may also be a factor; many patients reported using prescription or nonprescription medications to manage their symptoms.37
Topical Antihistamines
Topical antihistamines are widely available without a prescription. Antihistamines competitively block histamine receptors (e.g., H1 or H4) on nerve endings and blood vessels of the mucosal surface, thereby reducing itchiness and conjunctival hyperemia.39 First-generation antihistamines were associated with a range of systemic adverse effects (e.g., sedation, dizziness, cognitive impairment, blurred vision) caused by anticholinergic actions and nonspecific binding to histamine H2 receptors in addition to drying of the ocular surface. Newer oral, intranasal, and topical ocular antihistamines demonstrate improved H1 receptor selectivity, with fewer adverse effects; however, ocular adverse effects, such as burning and dryness, remain a concern. Topical antihistamines (e.g., levocabastine, emedastine difumarate) are useful for providing rapid relief of AC symptoms, but their duration of action is limited; most topical antihistamines require dosing four times daily.2,15,16
Antihistamine–Vasoconstrictor Combinations
Topical vasoconstrictors are highly effective at reducing ocular and conjunctival hyperemia through stimulation of vascular α-adrenergic receptors. Vasoconstrictors are commonly available in nonprescription combination formulations that contain an antihistamine (e.g., naphazoline-antazoline, naphazoline-pheniramine). These formulations exhibit a rapid onset of action and relieve redness and itchiness associated with AC. However, they are not recommended for long-term use because of reduced effectiveness over time and a potential rebound effect that can produce persistent red eye on discontinuation.23 As with topical antihistamines, combination antihistamine–vasoconstrictor formulations have a relatively short duration of action and are administered four times daily.2,15,16
Leukotriene Receptor Antagonists
Leukotriene receptor antagonists (e.g., montelukast), which are currently available for oral dosing, prevent leukotrienes from binding to their conjunctival receptors to decrease inflammatory signaling and improve multiple ocular symptoms of AC. Leukotriene receptor antagonists have a slower onset of action, are less effective than topical antihistamines, and are not used as first-line therapy or monotherapy for AC.39
Nonsteroidal Anti-inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) prevent formation of proinflammatory mediators and disrupt the inflammatory cascade that contributes to itching in AC. All topical NSAIDs (e.g., ketorolac, nepafenac, bromfenac) can be used chronically to reduce itching.40 NSAIDs require dosing four times daily, and their efficacy in managing AC is limited because they inhibit the production of only one type of inflammatory mediator (i.e., prostaglandins). A systematic review revealed that topical NSAIDs significantly reduced conjunctival itching associated with AC but had no effect on other symptoms, such as chemosis or swelling.41 Topical NSAIDS are rarely used today because of their lack of efficacy.
Corticosteroids
Corticosteroids prevent production of multiple classes of late-phase response mediators, including prostaglandins, leukotrienes, histamine, and some cytokines. The numerous points of intervention in the inflammatory cascade make glucocorticoids an effective pharmacologic therapy for AC,23 but long-term topical use can lead to serious adverse effects, including increased intraocular pressure and corneal abnormalities. Long-term systemic use increases the risk of posterior subcapsular cataract formation.42 For this reason, patients at risk (e.g., those with glaucoma or diabetic retinopathy) or patients who receive higher doses or longer treatment courses of corticosteroids should be monitored by an ophthalmologist or optometrist.
Corticosteroids (e.g., loteprednol etabonate, given four times daily)2,16 are generally not used as primary therapy for AC unless there is persistent or moderate-to-severe inflammation that the eye care professional does not feel will respond sufficiently to antihistamine–mast cell stabilizer medications alone. When corticosteroids are prescribed, they typically are used for short durations in the early stages of AC or during flare-ups until AC can be controlled with safer medications such as antihistamines, mast cell stabilizers, or dual-acting, single-molecule antihistamine–mast cell stabilizer agents.31 Topical corticosteroids are important in severe cases of allergic eye disease to break the cycle of inflammation and can be discontinued once the condition is under control. Most cases of SAC or PAC do not often require corticosteroid intervention. For patients who require long-term use of corticosteroids, close observation by an eye care professional is warranted.
Mast Cell Stabilizers
Topical mast cell stabilizers act to prevent mast cell degranulation and subsequent release of proinflammatory molecules triggered by IgE binding to sensitized conjunctival mast cells after allergen exposure.23 Topical mast cell stabilizers (e.g., cromolyn sodium, lodoxamide tromethamine, nedocromil sodium, pemirolast potassium) effectively prevent activation of the early phase response by preventing release of histamine, cytokines, and other inflammatory and chemotactic mediators. Preventing the early phase response blocks downstream inflammation events, including production of prostaglandins and leukotrienes, eosinophil infiltration, chemokine and adhesion molecule expression, and chronic mast cell activation that perpetuate the late-phase response in AC. Most mast cell stabilizers require administration four to six times daily; nedocromil sodium can be given twice daily.2,15,16 Because of the required loading time for maximal efficacy of mast cell stabilizers, these medications are most effective when treatment is initiated before symptoms manifest23; their effectiveness is limited when AC cascades have been activated and mast cell degranulation and histamine release have already occurred.
Dual-Acting Antihistamine–Mast Cell Stabilizing Agents
Agents with dual antihistamine and mast cell stabilizing actions are more suitable for extant AC than single-action medications because they block binding of free histamine to receptors and inhibit further release of proinflammatory mediators from mast cells. This dual action rapidly alleviates multiple signs and symptoms of AC in the short term and blocks the feed-forward cycle of persistent inflammation caused by continuous mast cell activation in the long term to promote regression of AC. Antihistamine–mast cell stabilizing agents (e.g., olopatadine, alcaftadine, epinastine, bepotastine besilate) are currently considered first-line therapeutics for AC because they offer acute symptomatic relief and control inflammation, and can be used chronically without long-term safety concerns. Most dual-acting agents require twice-daily dosing.2,15,16 Olopatadine 0.2% and alcaftadine are indicated for once-daily dosing and maintain effectiveness through 16 hours after administration in conjunctival allergen challenge (CAC) studies.43–45
BENEFITS OF A TRUE 24-HOUR TOPICAL MEDICATION
There is a recognized need for treatments that act on multiple phases of inflammatory responses to ocular allergen exposure and that demonstrate rapid onset and prolonged duration of action.2,31 Availability of a true 24-hour medication would maintain efficacy through a full dosing period. Therefore, patients would not be administering a daily rescue dose but rather a maintenance dose during symptomatic periods. Sustained anti-inflammatory action and less ocular exposure to preservatives because of fewer daily instillations may also benefit the large proportion of patients with AC who also have or are at risk for dry eye.6,46
Results of studies of treatment adherence in other ocular conditions, e.g., glaucoma, have indicated that nonadherence is increased in treatment regimens that require more daily instillations.47–49 Although symptoms of AC are bothersome and interfere with patients' quality of life, most cases of SAC and PAC are relatively benign, unlike other AC manifestations, such as vernal or atopic keratoconjunctivitis, and are not typically severe or vision threatening.2,31 Therefore, limitations of currently available medications, such as the need for multiple daily doses and ocular adverse effects, can lead to poor treatment adherence or treatment discontinuation, particularly if the negative impact on quality of life is viewed as a burden beyond that imposed by the AC itself. Antihistamine–mast cell inhibitor formulations with once-daily instillation regimens are currently available and provide an effective and convenient treatment option for patients with AC; however, the maximum duration of efficacy of these medications is actually closer to 16 hours. The lack of a once-daily medication that maintains full efficacy for 24 hours remains an unmet need for management of AC.6,43–46,50,51
Olopatadine 0.77%: A New Treatment Option
Olopatadine 0.2% is approved for once-daily dosing. A higher-concentration formulation, once-daily olopatadine 0.77%, was developed with the goal of extending the duration of effectiveness beyond that of the 16-hour duration olopatadine 0.2%. The safety and efficacy of olopatadine HCl 0.77% (equivalent to 0.7% olopatadine free base) 24 hours after administration were recently evaluated in two phase III multicenter, randomized, double-masked, active-controlled clinical trials.52,53
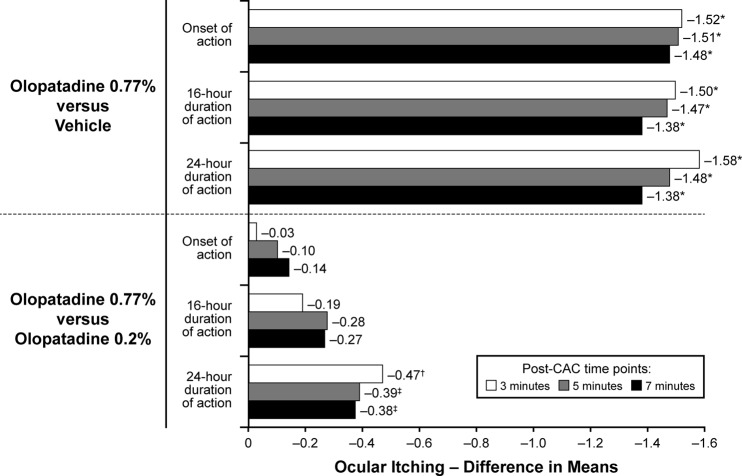
In a study of 202 patients with AC, olopatadine 0.77% maintained efficacy and superiority compared with olopatadine 0.2% or vehicle, measured by using the CAC model.52 Post-CAC scores for multiple symptoms of inflammation, including ocular itching, conjunctival redness, and total redness, at 24 hours after instillation were significantly lower in patients who received olopatadine 0.77% compared with those who received olopatadine 0.2% or vehicle (p < 0.05).52 Olopatadine 0.77% was also superior to vehicle at onset and at 16 hours after instillation (p < 0.001 and p ≤ 0.01, respectively). Compared with vehicle, treatment differences in self-reported ocular itching (potential score range: 0 [no itch] to 4 [incapacitating itch]) ranged from −1.48 to −1.52 at onset and from −1.38 to −1.58 at 24 hours, favoring olopatadine 0.77% (Fig. 2). Post-CAC ocular itch scores with olopatadine 0.77% were similar to olopatadine 0.2% at onset (treatment difference, −0.03 to −0.14) but were significantly lower with olopatadine 0.77% at 24 hours (treatment difference, −0.38 to −0.47) (Fig. 2). Scores were also lower with olopatadine 0.77% at 16 hours after instillation, which is the established duration of action for olopatadine 0.2%.
Figure 2.
Mean treatment differences in ocular itching after conjunctival allergen challenge. Ocular itching was assessed at 3, 5, and 7 minutes. *p < 0.001, †p < 0.01, ‡p < 0.05. Reproduced with permission (from Ref. 52).
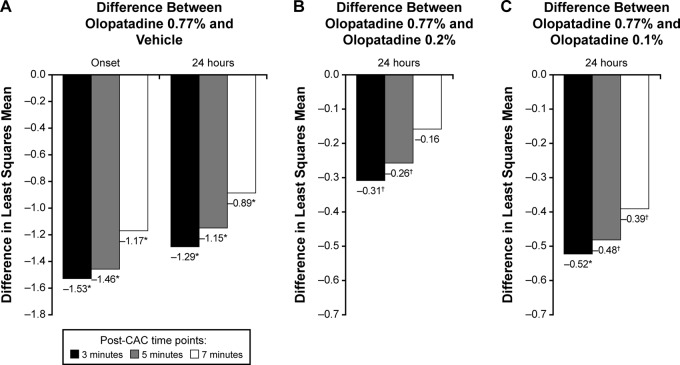
In a recently published study of 345 adults with a history of AC, patients received a single dose of olopatadine 0.77%, olopatadine 0.2%, olopatadine 0.1%, or vehicle, and were subjected to CAC at treatment onset of action and at 24 hours after instillation.53 At 3, 5, and 7 minutes after CAC, ocular itch reduction with olopatadine 0.77% was superior to vehicle at onset and 24 hours after instillation (Fig. 3 A) and to active control formulations 24 hours after instillation (Fig. 3, B and C).53 At 24 hours, the percentage of ocular itch responders (i.e., patients with a self-reported ocular itch score of 0 or a score reduction of ≥2 units relative to a baseline confirmatory CAC score) was significantly higher with olopatadine 0.77% (41% of patients) compared with vehicle (4% of patients, p < 0.0001) and olopatadine 0.2% (26% of patients, p < 0.05). At onset of action, conjunctival redness, total redness, and chemosis were significantly reduced with olopatadine 0.77% compared with vehicle, olopatadine 0.1%, and olopatadine 0.2%. The most frequently reported adverse drug reaction with olopatadine 0.77% was dysgeusia (2%). In both studies, adverse events and other safety parameters were similar across treatment groups, and the safety profile of olopatadine 0.77% was similar to that of olopatadine 0.2%.52,53 Together, these trials indicate that olopatadine 0.77% is more effective and maintains a longer duration of action compared with currently available olopatadine formulations and that once-daily olopatadine 0.77% provides an effective 24-hour control of AC signs and symptoms.
Figure 3.
Ocular itching examined after conjunctival allergen challenge. (A) Olopatadine 0.77% versus vehicle at onset and 24 hours after instillation. (B) Olopatadine 0.77% versus olopatadine 0.2% 24 hours after instillation. (C) Olopatadine 0.77% versus olopatadine 0.1% at 24 hours after instillation. *p < 0.0001 versus olopatadine 0.77%, †p < 0.05 versus olopatadine 0.77%. Ocular itching scores were assessed on a 0–4 scale with 0.5-unit increments (0, none; 4, incapacitating itch). Reproduced with permission (from Ref. 53).
CONCLUSION
Allergic conjunctivitis affects a large percentage of the population and is believed to be increasing in prevalence.2 The symptoms of AC contribute to decreased productivity and quality of life, and the effects of AC can be markedly exacerbated by comorbid conditions, such as allergic rhinitis or dry eye. Topical ocular antihistamine–mast cell stabilizer formulations are first-choice AC medications because these formulations rapidly alleviate acute ocular signs and symptoms, and reduce or prevent late-phase responses perpetuated by mast cell degranulation. Furthermore, these dual-acting agents exhibit a high degree of overall effectiveness and are well tolerated for chronic use.
Currently available once-daily antihistamine–mast cell stabilizer agents (e.g., olopatadine 0.2%, alcaftadine) demonstrated efficacy of only 16 hours, which can result in recurrence of inflammation and ocular symptoms in the 8 hours remaining before the next dose. Newly available once-daily olopatadine 0.77% has demonstrated superior effectiveness with regard to ocular itching, conjunctival redness, and chemosis, at onset and through 24 hours after instillation when directly compared with once-daily olopatadine 0.2%. The tolerability profile was comparable between olopatadine 0.2% and 0.77%. As the first true once-daily medication for managing signs and symptoms of AC for a full 24 hours, olopatadine 0.77% may be considered the treatment of choice for patients who experience SAC or PAC.
Footnotes
Medical writing support was funded by Alcon. Medical writing support was provided by Heather D. Starkey, Ph.D., of Complete Healthcare Communications, LLC (Chadds Ford, Pennsylvania)
J. Schaeffer has received honoraria or compensation or has served as an adviser to Alcon, Allergan, and Bausch & Lomb. E. Donnenfeld has served as a consultant for Allergan, Alcon, and Bausch & Lomb. W. Carr has no conflicts of interest pertaining to this article
REFERENCES
- 1. Chigbu DI. The pathophysiology of ocular allergy: A review. Cont Lens Anterior Eye 32:3–15; quiz 43–44, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Bielory L, Meltzer EO, Nichols KK, et al. An algorithm for the management of allergic conjunctivitis. Allergy Asthma Proc 34:408–420, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J Allergy Clin Immunol 126:778–783.e776, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Butrus S, Portela R. Ocular allergy: Diagnosis and treatment. Ophthalmol Clin North Am 18:485–492, v, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Gomes PJ. Trends in prevalence and treatment of ocular allergy. Curr Opin Allergy Clin Immunol 14:451–456, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Hom MM, Nguyen AL, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann Allergy Asthma Immunol 108:163–166, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Gelardi M, Leo ME, Quaranta VN, et al. Clinical characteristics associated with conjunctival inflammation in allergic rhinoconjunctivitis. J Allergy Clin Immunol Pract 3:387–391.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Maspero J, Lee BW, Katelaris CH, et al. Quality of life and control of allergic rhinitis in patients from regions beyond western Europe and the United States. Clin Exp Allergy 42:1684–1696, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Pitt AD, Smith AF, Lindsell L, et al. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol 11:17–33, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Smith AF, Pitt AD, Rodruiguez AE, et al. The economic and quality of life impact of seasonal allergic conjunctivitis in a Spanish setting. Ophthalmic Epidemiol 12:233–242, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Virchow JC, Kay S, Demoly P, et al. Impact of ocular symptoms on quality of life (QoL), work productivity and resource utilisation in allergic rhinitis patients—An observational, cross sectional study in four countries in Europe. J Med Econ 14:305–314, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Bielory L, Skoner DP, Blaiss MS, et al. Ocular and nasal allergy symptom burden in America: The Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Allergy Asthma Proc 35:211–218, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Stull DE, Schaefer M, Crespi S, Sandor DW. Relative strength of relationships of nasal congestion and ocular symptoms with sleep, mood and productivity. Curr Med Res Opin 25:1785–1792, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Bielory L, Syed BA. Pharmacoeconomics of anterior ocular inflammatory disease. Curr Opin Allergy Clin Immunol 13:537–542, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Azari AA, Barney NP. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA 310:1721–1729, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern® Guidelines. Conjunctivitis. American Academy of Ophthalmology. Available online at www.aao.org/ppp; accessed May 16, 2016.
- 17. Vandenbroeck S, De Geest S, Dobbels F, et al. Prevalence and correlates of self-reported nonadherence with eye drop treatment: The Belgian Compliance Study in Ophthalmology (BCSO). J Glaucoma 20:414–421, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Anderson DF, MacLeod JD, Baddeley SM, et al. Seasonal allergic conjunctivitis is accompanied by increased mast cell numbers in the absence of leucocyte infiltration. Clin Exp Allergy 27:1060–1066, 1997. [DOI] [PubMed] [Google Scholar]
- 19. Macleod JD, Anderson DF, Baddeley SM, et al. Immunolocalization of cytokines to mast cells in normal and allergic conjunctiva. Clin Exp Allergy 27:1328–1334, 1997. [PubMed] [Google Scholar]
- 20. Morgan SJ, Williams JH, Walls AF, et al. Mast cell numbers and staining characteristics in the normal and allergic human conjunctiva. J Allergy Clin Immunol 87:111–116, 1991. [DOI] [PubMed] [Google Scholar]
- 21. Leonardi A. The central role of conjunctival mast cells in the pathogenesis of ocular allergy. Curr Allergy Asthma Rep 2:325–331, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Irkec MT, Bozkurt B. Molecular immunology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol 12:534–539, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Bielory BP, O'Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: Guide to therapy. Acta Ophthalmol 90:399–407, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Pawankar R, Canonica G, Holgate S, et al. WAO White Book on Allergy. Milwaukee, WI: World Allergy Organization, 2011. [Google Scholar]
- 25. Van Cauwenberge P, De Belder T, Vermeiren J, et al. Global Resources in Allergy (GLORIA): Allergic rhinitis and allergic conjunctivitis. Clin Exp Allergy Rev 3:46–50, 2003. [Google Scholar]
- 26. Ono SJ, Abelson MB. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol 115:118–122, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Leonardi A, Bogacka E, Fauquert JL, et al. Ocular allergy: Recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy 67:1327–1337, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy 36:777–784, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Mimura T, Usui T, Yamagami S, et al. Relation between total tear IgE and severity of acute seasonal allergic conjunctivitis. Curr Eye Res 37:864–870, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Bousquet J, Heinzerling L, Bachert C, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 67:18–24, 2012. [DOI] [PubMed] [Google Scholar]
- 31. O'Brien TP. Allergic conjunctivitis: An update on diagnosis and management. Curr Opin Allergy Clin Immunol 13:543–549, 2013. [DOI] [PubMed] [Google Scholar]
- 32. Bilkhu PS, Wolffsohn JS, Naroo SA, et al. Effectiveness of nonpharmacologic treatments for acute seasonal allergic conjunctivitis. Ophthalmology 121:72–78, 2014. [DOI] [PubMed] [Google Scholar]
- 33. Sanchez M, Parra BF, Matheu V, et al. Allergic conjunctivitis. J Investig Allergol Clin Immunol 21:1–19, 2011. [PubMed] [Google Scholar]
- 34. Calderon MA, Penagos M, Sheikh A, et al. Sublingual immunotherapy for allergic conjunctivitis: Cochrane systematic review and meta-analysis. Clin Exp Allergy 41:1263–1272, 2011. [DOI] [PubMed] [Google Scholar]
- 35. Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: A systematic review. JAMA 309:1278–1288, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; and Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: A practice parameter second update. J Allergy Clin Immunol 120(suppl. 3):S25–S85, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Blaiss MS, Dykewicz MS, Skoner DP, et al. Diagnosis and treatment of nasal and ocular allergies: The Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Ann Allergy Asthma Immunol 2014;112:322–328.e321, 2014. [DOI] [PubMed] [Google Scholar]
- 38. Anolik R, Schwartz AM, Sajjan S, Allen-Ramey F. Patient initiation and persistence with allergen immunotherapy. Ann Allergy Asthma Immunol 113:101–107, 2014. [DOI] [PubMed] [Google Scholar]
- 39. Gane J, Buckley R. Leukotriene receptor antagonists in allergic eye disease: A systematic review and meta-analysis. J Allergy Clin Immunol Pract 1:65–74, 2013. [DOI] [PubMed] [Google Scholar]
- 40. Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol 55:108–133, 2010. [DOI] [PubMed] [Google Scholar]
- 41. Swamy BN, Chilov M, McClellan K, Petsoglou C. Topical non-steroidal anti-inflammatory drugs in allergic conjunctivitis: Meta-analysis of randomized trial data. Ophthalmic Epidemiol 14:311–319, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol 11:478–483, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Greiner JV, Edwards-Swanson K, Ingerman A. Evaluation of alcaftadine 0.25% ophthalmic solution in acute allergic conjunctivitis at 15 minutes and 16 hours after instillation versus placebo and olopatadine 0.1%. Clin Ophthalmol 5:87–93, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ackerman S, D'Ambrosio F, Jr, Greiner JV, et al. A multicenter evaluation of the efficacy and duration of action of alcaftadine 0.25% and olopatadine 0.2% in the conjunctival allergen challenge model. J Asthma Allergy 6:43–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abelson MB, Gomes PJ. Olopatadine 0.2% ophthalmic solution: The first ophthalmic antiallergy agent with once-daily dosing. Expert Opin Drug Metab Toxicol 4:453–461, 2008. [DOI] [PubMed] [Google Scholar]
- 46. Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther 18:205–215, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy? Ophthalmology 112:863–868, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: Objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol 144:533–540, 2007. [DOI] [PubMed] [Google Scholar]
- 49. Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology 113:431–436, 2006. [DOI] [PubMed] [Google Scholar]
- 50. McLaurin E, Narvekar A, Gomes PJ. Phase 3 study of efficacy and safety of once-daily olopatadine hydrochloride, 0.77% ophthalmic formulation in patients with allergic conjunctivitis using the ora conjunctival allergen challenge model (Ora-CAC™) (NCT01743027). Invest Ophthalmol Vis Sci 55:2488, 2014. [Google Scholar]
- 51. Pataday (olopatadine hydrochloride ophthalmic solution 0.2%). Full Prescribing Information. Alcon Laboratories, Inc., Fort Worth, TX, 2010. [Google Scholar]
- 52. Torkildsen G, Narvekar A, Bergmann M. Efficacy and safety of olopatadine hydrochloride 0.77% in patients with allergic conjunctivitis using a conjunctival allergen-challenge model. Clin Ophthalmol 9:1703–1713, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McLaurin E, Narvekar A, Gomes P, et al. Phase 3 randomized double-masked study of efficacy and safety of once-daily 0.77% olopatadine hydrochloride ophthalmic solution in subjects with allergic conjunctivitis using the conjunctival allergen challenge model. Cornea 34:1245–1251, 2015. [DOI] [PubMed] [Google Scholar]