Abstract
Background:
There are only a few landmark studies from the Indian subcontinent on fungal rhinosinusitis. The lack of awareness among clinicians regarding the varying clinical presentations of fungal rhinosinusitis prompted us to undertake this study.
Objective:
To determine the prevalence, etiologic basis, clinical features, radiologic features, and microscopic features of fungal rhinosinusitis, and to evaluate the various treatment modalities available.
Methods:
This was a prospective study in which evaluation of 100 patients with chronic rhinosinusitis was done. Specimens collected were subjected to both microbiology and pathologic examination; data collected, including clinical and radiologic features, were analyzed by the Pearson χ2 test and Fisher's exact test.
Results:
The prevalence of fungal rhinosinusitis in our study was 30% (n = 30). Mucor was the most commonly isolated species (n = 15 [50%]) of fungus. Pathologic examination had a higher sensitivity (76.67%) compared with microbiology tests (50%) in the diagnosis of fungal rhinosinusitis. Fungus ball (n = 14 [46.6%]) was the most prevalent entity in the spectrum of fungal rhinosinusitis. Forty percent of cases (n = 12) were of invasive fungal rhinosinusitis. The prevalence of fungal rhinosinusitis was higher among individuals who were immunocompetent (n = 17 [56.6%]). Of patients who were immunocompromised, 84.6% (n = 11) had mucormycosis.
Conclusions:
Unilateral involvement of paranasal sinuses was more in favor of fungal etiology. Complications were more common in fungal rhinosinusitis caused by Mucor species. Mucormycosis, a rare clinical entity, in subjects who were immunocompetent, had a high prevalence in our study.
Keywords: Maxillary sinus, mucor, endoscopy, aspergillus, diabetes mellitus, chronic rhinosinusitis, immunodeficiency, invasive fungal sinusitis, amphotericin-B, endoscopic sinus surgery
Fungal infections can occur in any age group, but symptoms differ based on the immunity status of the individual. A high index of suspicion is needed for the diagnosis of fungal rhinosinusitis when patients present with symptoms similar to chronic sinus infection resistant to conventional antibiotic therapy. Invasive fungal infections occur commonly among individuals who are immunocompromised with systemic illnesses, e.g., diabetes mellitus. Aspergillus is the most common pathogen in fungal rhinosinusitis. This disease entity is increasingly being recognized because of the awareness, improved techniques of specimen collection, processing, fungal culture, and special staining for pathologic examination. A review of literature revealed 17 cases of primary paranasal sinus aspergilloma from Sudan reported by Milosev et al1 in 1969. Stammberger2 reported having treated >140 patients with massive fungal sinusitis during 1976–1985. Hazarika et al.3 reported three cases of rhinocerebral mucormycosis, all of whom were elderly and with diabetes. Chakrabarti et al.4 isolated fungi in 50 of the 119 patients with clinically suspected cases in north India over a 2-year period. According to a literature review, the highest incidence of allergic fungal rhinosinusitis was noted in Mumbai, India, as reported in the study by Ferguson.5
Our institution serves as a referral center for the three states of South India viz. Tamil Nadu, Kerala, and Andhra Pradesh, which have an agricultural economy. The warm moist climate in our geographic region is favorable for fungal sinonasal infections. The presentation of patients with chronic fungal rhinosinusitis can be varied posing a diagnostic challenge to the treating physician. In reviewing the available literature, we realized that all the landmark studies on fungal rhinosinusitis were done in Europe and the American continents. The lack of recognition of this disease entity and the scarcity of reports from this part of the globe prompted us to undertake this study. The aims of our study were to determine the prevalence and the etiologic basis of fungal rhinosinusitis. The objectives included analysis of the clinical, radiologic, and microscopic features, along with evaluation of the various treatment modalities available for fungal rhinosinusitis.
METHODS
This was a prospective study of 100 cases of patients with chronic rhinosinusitis who underwent treatment on an outpatient or inpatient basis in the department of Otorhinolaryngology at our institution, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India, from July 2011 to July 2013 (Fig. 1). Informed consent was obtained from all the patients included in the study. Approval was obtained from the institutional review board, PSG Institute of Medical Sciences and Research, Coimbatore (approval identification 11/259; approval date, June 28, 2012).
Figure 1.
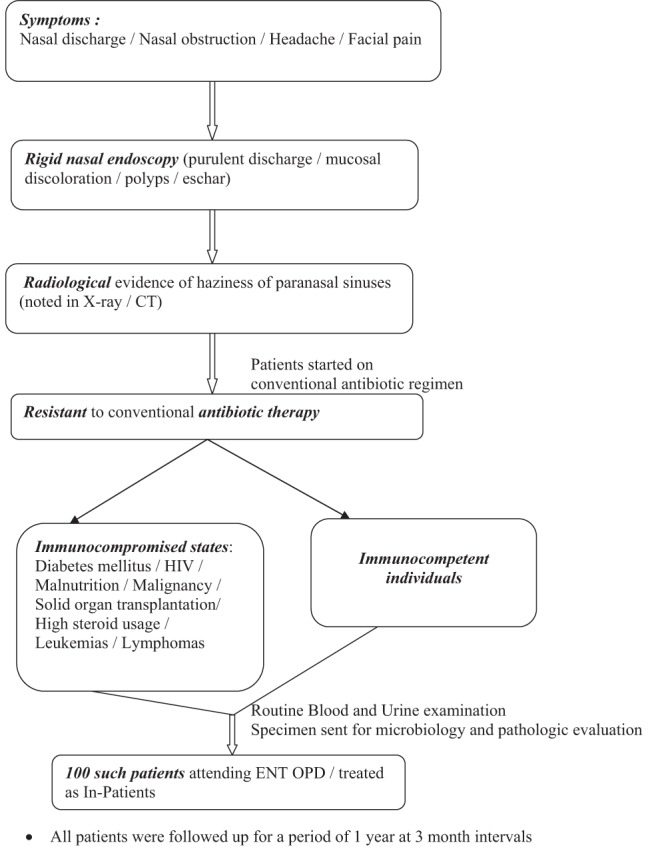
Flowchart, which depicts patient flow into the study.
A detailed history was obtained from all the patients, with emphasis on a history of immunocompromised states. An immunocompromised host is an individual who does not have the ability to respond normally to an infection due to an impaired immune system. Immunocompromised states include diabetes mellitus, human immunodeficiency virus infection, malnutrition, cancers, and solid organ transplantation. The inclusion criteria of the study were individuals >14 years of age, both sexes, and those who satisfied the established diagnostic criteria for chronic rhinosinusitis. In the case of children between 14 and 18 years old, informed permission was taken from the parents for diagnosis and treatment of children, with the assent of the child.
There were 168 patients who were screened during the study period. Seven patients who did not give their consent for the study; 31 who could not afford the cost of treatment, including a computed tomography and cost of surgery; 19 patients who were lost to follow up; 4 patients who had paranasal sinus tumors proven on histology; 5 patients who had high-risk systemic illnesses, e.g., acute coronary events (in whom anticoagulation medications could not be stopped for a biopsy); and 2 patients who were hemodynamically unstable were excluded from the study. After excluding these 68 patients from the study, the remaining 100 patients were worked up based on a questionnaire (Supplemental Appendix 1). To avoid observer bias, each patient was examined by at least three of the six authors (G.Z., S.P. and any of the remaining four authors). The data collected from the patients included age, sex, associated comorbidities, and clinical symptoms and signs, including details of any complications the patients had at the time of presentation. The variables also included the findings of imaging studies, the reports of microbiology and pathology examination (Supplemental Appendix 2). The patients were categorized according to their age groups, sex, and profession to identify the category with the highest prevalence of fungal rhinosinusitis. The patients included in the study presented with nasal discharge, nasal obstruction, headache, or facial pain; with radiologic evidence of sinus involvement; and without any response to conventional antibiotic therapy. Statistical significance was assessed to establish if the presence of certain symptoms could be an alarming sign for the likelihood of fungal rhinosinusitis. The above-mentioned symptoms were part of the inclusion criteria, although other symptoms of chronic rhinosinusitis were also taken into account.
All the patients underwent diagnostic nasal endoscopy to rule out any anatomic variations of the osteomeatal complex, the presence of polyps, and nasal discharge. Specimens for fungal culture and pathologic examination were obtained by diagnostic nasal endoscopy and during surgery. Nasal swabs from the middle meatus were subjected to potassium hydroxide mount, and, if fungal elements were identified, then fungal culture was done. After surgery, tissue removed from the sinuses was sent to a single pathologist (not an author on this article). The specimens were fixed in 10% buffered formalin. All the specimens were routinely processed and paraffin embedded. Periodic acid–Schiff stain, Gomori methenamine silver, and lactophenol cotton blue stains were used for fungal organisms. All specimens were subjected to potassium hydroxide wet mount and fungal culture (Sabouraud dextrose agar). For fungal culture, the specimen was incubated at 25°C and 37°C for 4 weeks. Aspergillus species (Fig. 2) was identified by the presence of typical conidiophores, Mucor by the presence of right-angled aseptate hyphae (Figs. 3 and 4), and Candida appeared as budding yeasts, occasionally with pseudohyphae. Negative cultures were documented after 4 weeks of incubation.
Figure 2.
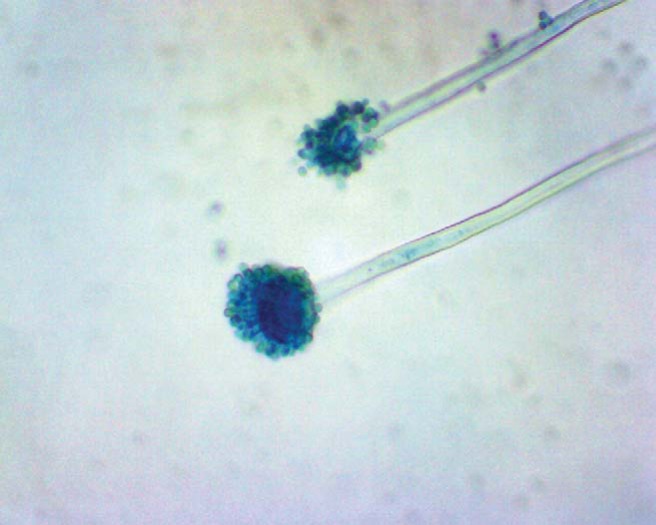
Aspergillus on Lactophenol Cotton Blue staining, showing septate hyphae and swollen vesicle giving rise to phialides from which chains of conidia arise (original magnification ×400).
Figure 3.
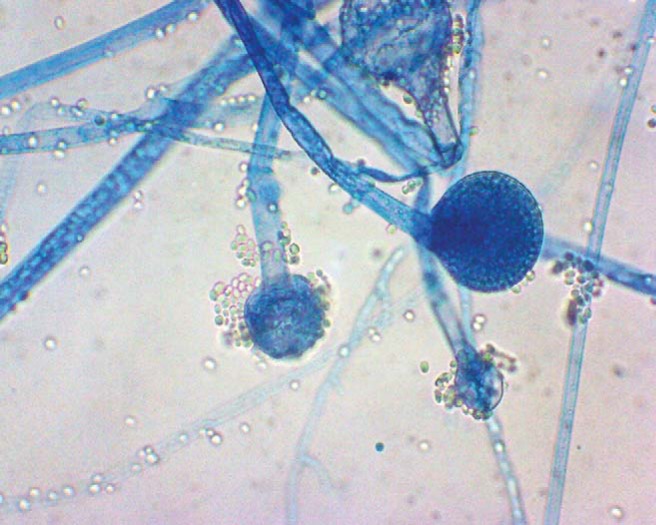
Mucor on Lactophenol Cotton Blue staining, showing broad aseptate hyphae, with extension of columella into sporangium and aggregation of sporangiospores (original magnification ×400).
Figure 4.
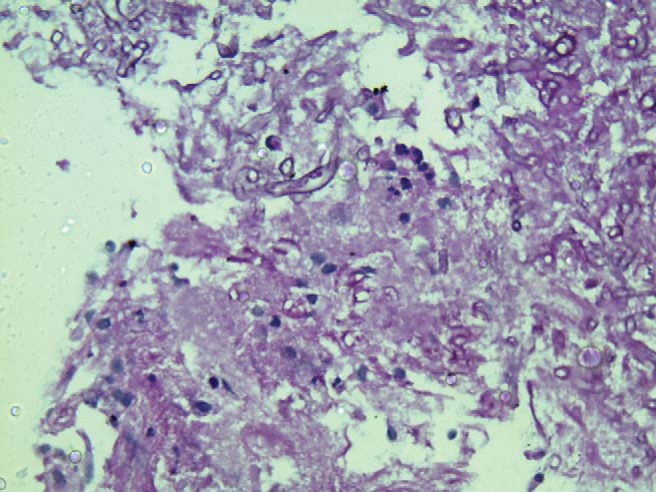
Tissue section, showing Mucor on Periodic acid-Schiff stain with broad aseptate hyphae (original magnification ×400).
Radiographic and computed tomography imaging of nose and paranasal sinuses were done in all the cases of fungal rhinosinusitis to assess the patency of the osteomeatal complex, involvement of sinuses, and erosion of bony margins or expansion of the sinus cavity. Patients were categorized based on the comorbid systemic diseases they had since immunocompromised states that arise from some of these conditions that make them more susceptible to fungal infections. The prevalence of fungal rhinosinusitis among immunocompromised and immunocompetent groups of patients was determined. Complications of fungal rhinosinusitis, including orbital cellulitis, cranial nerve palsies, orbital apex syndrome, and cavernous sinus thrombosis were also accounted for, and species that caused them were identified.
In cases of fungus ball, the mainstay of treatment was endoscopic-assisted surgical clearance. For cases of invasive fungal rhinosinusitis, treatment included a combination of endoscopic surgical debridement, followed by antifungal therapy. Antifungal therapy included use of intravenous amphotericin-B and itraconazole. Parenteral amphotericin-B was the drug of choice for invasive fungal rhinosinusitis; the dose was titrated based on periodic monitoring of renal function parameters and electrolytes. The patients were discharged on oral itraconazole. During the follow-up, hepatic function parameters were monitored and itraconazole was continued for up to a year in all cases of invasive fungal rhinosinusitis. All the patients were advised to perform routine saline solution douching during the postoperative period. The patients were asked to follow up 1 week after surgery for suction clearance of the sinonasal cavity after surgery. Allergic fungal rhinosinusitis was treated by exteriorization of the sinuses through endoscopic sinus surgery, along with long-term systemic steroids. Serum immunoglobulin E was monitored as a prognostic indicator for recurrence of allergic fungal rhinosinusitis.
All the patients were followed-up in our department for up to 1 year. The patients were evaluated clinically for improvement in symptoms, clinical examination, and periodic diagnostic nasal endoscopy at 3-month intervals to assess for any relapse of fungal infection. The outcome measures that were evaluated prospectively were the presence of discharge or fungal material in the sinonasal cavity after surgery, the status of nasal mucosa, presence of crusts or eschar, recurrence of polyps, and patency of osteomeatal complex.
RESULTS
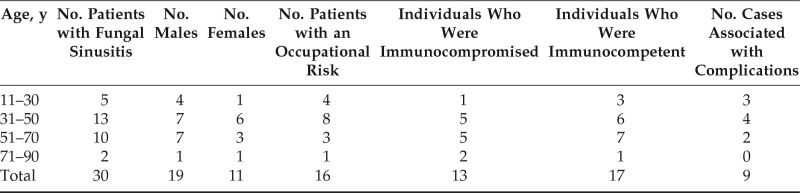
In our study of 100 cases of chronic rhinosinusitis, 30% of the patients had a fungal etiology, with a highest prevalence in the third and fourth decades of life 43.3% (n = 13), with a male preponderance (n = 19 [63.3%]) (Table 1). The population of patients with fungal rhinosinusitis were mainly composed of manual laborers (n = 8 [26.7%]) and farmers (n = 8 [26.7%]) (Table 1). The patients presented with symptoms of nasal obstruction (n = 14 [46.6%]), nasal discharge (n = 12 [40%]), headache (n = 12 [40%]), facial pain (n = 4 [13.3%]), and facial swelling (n = 2 [6.6%]). Nasal obstruction as a symptom of fungal rhinosinusitis was not statistically significant (p = 0.846) contrary to the other symptoms which were significant. Diagnostic nasal endoscopy revealed nasal polyps in 43.3% (n = 13), purulent nasal discharge in 30% (n = 9), and the presence of eschar in 13.3% of the patients (n = 4) with fungal rhinosinusitis. Radiologic evaluation showed unilateral involvement of sinuses in 83.3% of patients (n = 25). Maxillary sinus was most commonly involved in 46.6% of cases (n = 14), followed by ethmoids in 40% (n = 12) and sphenoid sinus in 36.6% of fungal sinusitis (n = 11). Among the 30 cases, 23 patients (76.6%) had typical radiologic features of fungal rhinosinusitis. Radiodense shadows in central soft tissue (Fig. 5) was noted in 50% of fungal rhinosinusitis cases (n = 15).
Table 1.
Age and sex distribution along with immunity status, occupational risk, and complications among patients with fungal rhinosinusitis
Figure 5.
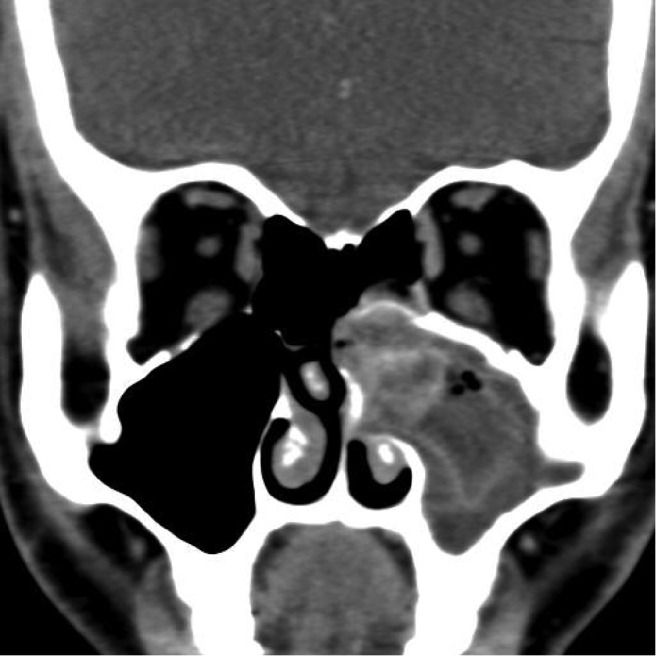
Computed tomography image showing soft tissue density lesion, with heterogenous hyperdensities in the left maxillary sinus and posterior ethmoids in a patient with fungal rhinosinusitis.
Candida was the most common organism isolated (n = 8 [26.6%]) on microbiologic examination (Fig. 6). The sensitivity of microbiology examination in diagnosing fungal rhinosinusitis was 50%, but specificity was 100% (Table 2). Mucor (n = 13 [43.3%]) was the most commonly identified fungus on histology (Fig. 7). The sensitivity and specificity of pathologic examination was 76.67% and 100%, respectively (Table 3). There was a statistically significant correlation for microbiology and pathologic examination in the diagnosis of fungal rhinosinusitis. Orbital cellulitis was the most common complication of fungal rhinosinusitis (n = 7 [23.3%]) (Table 1). Fungal rhinosinusitis had a lesser prevalence among individuals who were immunocompromised (Table 1). Among the patients who were immunocompromised (n = 13), 84.6% of cases (n = 11) were mucormycosis. Among the individuals who were immunocompetent (n = 17), four had mucormycosis. Fungal rhinosinusitis was common among individuals with diabetes mellitus and patients who were on long-term steroid therapy for conditions such as glomerulonephritis and rheumatoid arthritis (Fig. 8). Some individuals in the study had more than one condition that affected their immunity status. The immunity levels of patients had a statistically significant correlation (p = 0.039), with prevalence of fungal rhinosinusitis.
Figure 6.
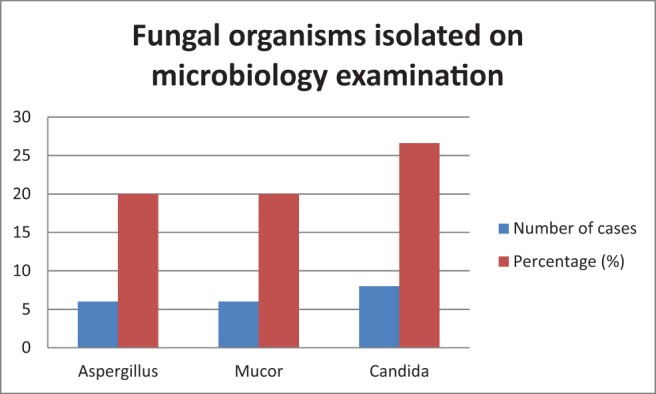
Fungal organisms isolated on microbiology examination.
Table 2.
Microbiology examination of the study population
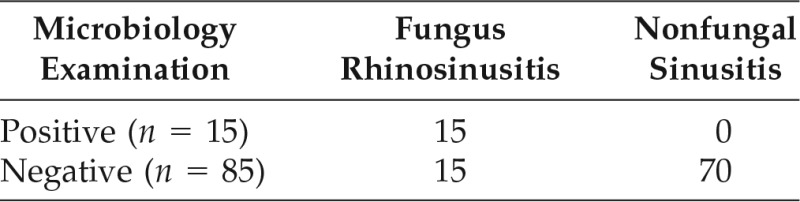
True positive = 15; false positive = 0; false negative = 15; true negative = 70.
Sensitivity, 50%; specificity, 100%; positive predictive value, 100%; negative predictive value, 82.35%; accuracy, 0.85.
Figure 7.
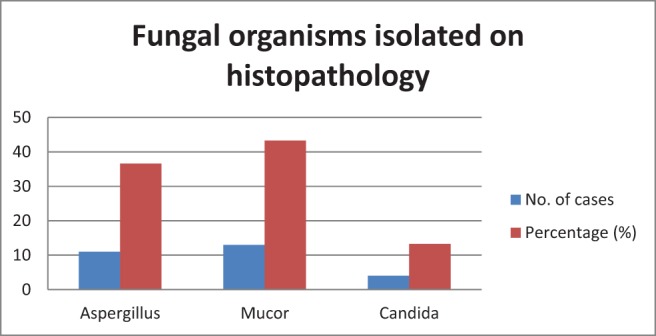
Fungal organisms isolated on histopathology.
Table 3.
Pathology examination of the study population

True positive = 23; false positive = 0; false negative = 7; true negative = 70.
Sensitivity, 76.6%; specificity, 100%; positive predictive value, 100%; negative predictive value, 90.9%; accuracy, 0.93.
Figure 8.
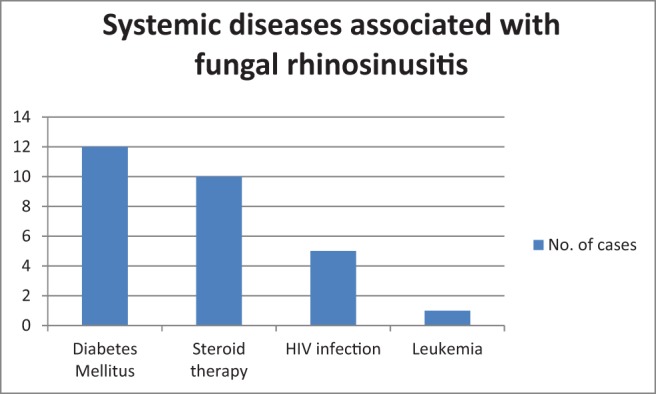
Systemic diseases associated with fungal rhinosinusitis.
Mucor was the most commonly identified fungus in as many as 15 patients (50%), followed by Aspergillus in 13 patients (43.3%), and Candida species in 9 patients (30%) in our study. In the entire spectrum of fungal rhinosinusitis, fungus ball had the highest prevalence (n = 14 [46.6%]) in our study, followed by acute fulminant fungal rhinosinusitis (n = 12 [40%]), allergic fungal rhinosinusitis (n = 2 [6.6%]), and indolent fungal rhinosinusitis (n = 2 [6.6%]). Four cases of fungal rhinosinusitis showed features of angioinvasion and tissue invasion on histology, the causative organisms being Mucor or Aspergillus species. All the invasive fungal rhinosinusitis cases (n = 12 [40%]) and chronic indolent fungal rhinosinusitis (n = 2 [6.6%]) were successfully managed by a combination of surgical debridement, followed by antifungal therapy, whereas the majority of the cases were fungus ball (n = 14 [46.6%]) were treated by endoscopic-assisted sinus surgery. Allergic fungal rhinosinusitis cases (n = 2 [6.6%]) were treated with endoscopic sinus surgery and long-term systemic steroids.
The average (standard deviation) time interval for follow-up in our study were 11.06 ± 1.23 months. After validation of outcome measures, 90% of the patients (n = 27) were found to have complete resolution of symptoms and nasal mucosa reverted to normal by the end of 1 year. The three patients who could not be followed up had invasive mucormycosis and developed renal failure and later succumbed to cardiopulmonary arrest during treatment with parenteral amphotericin-B.
DISCUSSION
Milosev et al.1 found that Aspergillus rhinosinusitis occurred more frequently in male patients in their teens and twenties. In contrast, the majority of the patients (n = 13 [43.3%]) in our study were in the age group of 31–50 years. Occupation had a significant role because individuals from low socioeconomic status who were experiencing malnutrition were susceptible to fungal rhinosinusitis. Facial swelling was noted in two patients (6.6%), both of whom had sinonasal mucormycosis, which was similar to the results of the study done by Hazarika et al.,3 in which cheek swelling was present in all three cases of mucormycosis reported.
Stammberger et al.6 first described a radiologic finding of a double-density appearance of maxillary antrum due to deposition of calcium salts such as calcium phosphate and calcium sulfate, which was pathognomonic of fungal rhinosinusitis. According to a study by Killeen et al.,7 fungus balls had higher-density and more heterogeneous components on radiology, and allergic fungal mucin was generally more radiodense. Similar findings were noted in half of our cases. Chakrabarti et al.,4 in north India, found that the most common species that caused fungal rhinosinusitis was Aspergillus; however, Mucor was the most common species isolated in our study. According to Cho et al.,8 cranial neuropathy, visual loss, and orbital pain were the most common complications encountered in cases of fungal rhinosinusitis. Similarly, in our study, orbital cellulitis and cranial nerve palsies were the most common complications (Table 1).
Similar to the work of Ferguson,5 in our study, endoscopic sinus surgery was the mainstay of treatment of fungus ball. However, treatment of invasive mucormycosis included reversal of immunocompromised states, systemic amphotericin-B, and surgical debridement, which was similar to the study done by Gillespie and O'Malley.9 Overall antifungal chemotherapy combined with surgery offered a better treatment outcome, which was similar to the study done by Gillespie et al.10 A recent study by Patro et al.11 concluded that preoperative itraconazole administration decreased the disease load significantly and also reduced the extent of surgery. Approximately 11.3% (n = 19) of the initially screened population (n = 168) was lost to follow-up, which was probably because many of our patients who live in distant towns had to travel several hours to reach our institute and, for some, treatment was not affordable.
CONCLUSION
The high prevalence of fungal rhinosinusitis in our study (30%) could have been due to the high prevalence of type 2 diabetes mellitus in our country. Unilateral involvement of paranasal sinuses is more in favor of fungal etiology. Maxillary sinus was most commonly involved in fungal rhinosinusitis, which is probably because the sinus ostium is located lowest in the middle meatus compared with other sinus ostia, which provides easy access to microorganisms. The presence of facial swelling, eschar, features of angioinvasion, and tissue invasion on histology were suggestive invasive mucormycosis.
Mucor was the most common species that caused fungal rhinosinusitis, and complications were more associated with this species, which could have been due to mucormycosis being an opportunistic infection and the presence of a higher number of immunocompromised individuals in our study. Pathologic examination had a higher sensitivity compared to microbiology examination as an investigation in the diagnosis of fungal rhinosinusitis. The prevalence of fungal rhinosinusitis was higher among individuals who were immunocompetent. Among them, four cases were of patients who were immunocompetent and with mucormycosis, which is a rare clinical entity, with very few such reports in the world literature. Among the patients who were immunocompromised and with fungal rhinosinusitis, the majority had sinonasal mucormycosis.
ACKNOWLEDGMENTS
I thank the faculty members of the pathology and microbiology departments for their valuable academic inputs. I thank the Information Technology department staff, Otorhinolaryngology department staff and ward nursing staff for helping me collect patient details.
Footnotes
No external funding sources reported
The authors have no conflicts of interest to declare pertaining to this article
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1. Milosev B, el-Mahgoub S, Aal OA, el-Hassan AM. Primary aspergilloma of the paranasal sinuses in Sudan. A review of seventeen cases. Br J Surg 56:132–137, 1969. [DOI] [PubMed] [Google Scholar]
- 2. Stammberger H. Endoscopic surgery for mycotic and chronic recurring sinusitis. Ann Otol Rhinol Laryngol Suppl 119:1–11, 1985. [DOI] [PubMed] [Google Scholar]
- 3. Hazarika P, Ravikumar V, Nayak RG, et al. Rhinocerebral mycosis. Ear Nose Throat J 63:464–468, 1984. [PubMed] [Google Scholar]
- 4. Chakrabarti A, Sharma SC, Chandler J. Epidemiology of pathogenesis of paranasal sinus mycoses. Otolaryngol Head Neck Surg 107:745–750, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson BJ. Fungus balls of the paranasal sinuses. Otolaryngol Clin North Am 33:389–398, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Stammberger H, Kennedy DW. Paranasal sinuses: Anatomic terminology and nomenclature. Ann Otol Rhinol Laryngol Suppl 167:7–16, 1995. [PubMed] [Google Scholar]
- 7. Killeen DE, Sedaghat AR, Cunnane ME, Gray ST. Objective radiographic density measurements of sinus opacities are not strong indicators of noninvasive fungal diseases. Am J Rhinol Allergy 28:483–486, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Cho HJ, Jang MS, Hong SD, et al. Prognostic factors for survival in patients with acute invasive fungal rhinosinusitis. Am J Rhinol Allergy 29:48–53, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Gillespie MB, O'Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am 33:323–334, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Gillespie MB, O'Malley BW, Jr, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg 124(5):520–526, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Patro SK, Verma RK, Panda NK, et al. Efficacy of preoperative itraconazole in allergic fungal rhinosinusitis. Am J Rhinol Allergy 29:299–304, 2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.