Abstract
A 12-day course of high-dose tacrolimus induces tolerance of major histocompatibility complex– mismatched lung allografts in miniature swine but does not induce tolerance of heart allografts unless a kidney is cotransplanted. To determine whether lungs share with kidneys the ability to induce cardiac allograft tolerance, we investigated heart–lung co-transplantation using the same induction protocol. Hearts (n = 3), heart–kidneys (n=3), lungs (n=6), and hearts–lungs (n=3) were transplanted into fully major histocompatibility complex–mismatched recipients treated with high-dose tacrolimus for 12 days. Serial biopsy samples were used to evaluate rejection, and in vitro assays were used to detect donor responsiveness. All heart–kidney recipients and five of six lung recipients demonstrated long-term graft survival for longer than 272 days, while all heart recipients rejected their allografts within 35 days. Tolerant recipients remained free of alloantibody and showed persistent donor-specific unresponsiveness by cell-mediated lympholysis/mixed-lymphocyte reaction. In contrast, heart–lung recipients demonstrated rejection of both allografts (days 47, 55, and 202) and antidonor responsiveness in vitro. In contrast to kidneys, lung cotransplantation leads to rejection of both heart and lung allografts, indicating that lungs do not have the same tolerogenic capacity as kidneys. We conclude that cells or cell products present in kidney, but not heart or lung allografts, have a unique capacity to confer unresponsiveness on cotransplanted organs, most likely by amplifying host regulatory mechanisms.
Introduction
Achieving immunologic tolerance has been a long-standing goal in solid organ transplantation. However, different organs demonstrate varying propensities for tolerance, even when transplanted under the same induction regimen. For example, kidneys and livers seem to be more tolerogenic, with cases of spontaneous kidney or liver acceptance occurring at appreciable rates in murine recipients (1,2) and operational tolerance in human transplant recipients (3,4). In contrast, cardiac allografts rarely exhibit spontaneous tolerance (5).
Using the preclinical, large-animal model of major histocompatibility complex (MHC)-inbred miniature swine (6), we previously showed that isolated, class I–disparate hearts transplanted without immunosuppression all rejected within 8 days (7). The addition of a 12-day course of calcineurin inhibition prolonged class I and full MHC-mismatched heart allograft survival slightly; however, all grafts rejected by postoperative day (POD) 55 (8,9). In contrast, calcineurin inhibition can lead to tolerance of class I and full MHC-mismatched kidney allografts (10,11), as well as full MHC-mismatched lung allografts (12).
Recently, we found that kidney cotransplantation under a 12-day course of tacrolimus is able to induce tolerance of heart allografts across a full MHC mismatch (8,9), reminiscent of the “liver effect” first described in 1969 (13). However, the mechanism underlying kidney-induced cardiac allograft tolerance (KICAT) is unknown. Mechanistic requirements appear to include the thymus (14), a radiosensitive kidney cell population (15), T regulatory cells (16,17), and MHC-matching between heart and kidney parenchyma (18).
To determine whether lung cotransplantation could induce tolerance of fully mismatched heart allografts in an analogous fashion, we conducted a series of heart–lung transplants using the same tolerance induction regimen used in the KICAT experiments.
Materials and Methods
Animals
Transplant donors and recipients were selected from our herd of partially inbred miniature swine (age, 3–6 months; weight, 15–50 kg). The immunogenetic characteristics of this herd have been described previously (6). Donor organs were transplanted into recipients to achieve a two-haplotype full MHC (SLA in swine) class I and class II mismatch [e.g., SLAcc (class Ic/IIc) into SLAdd (class Id/IId)]. Group 1 animals underwent heart transplantation, group 2 animals underwent heart–kidney transplantation, group 3 animals underwent lung transplantation, and group 4 animals underwent heart–lung transplantation. All recipients demonstrated significant in vitro antidonor activity by cell-mediated lympholysis (CML) and/or mixed-lymphocyte reaction (MLR) before organ transplantation. The study was approved by our Institutional Animal Care and Use Committee. All animal care and procedures were in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council and updated and published regularly by the National Academy Press.
Surgery
The surgical procedures used for heterotopic heart transplantation, combined heart–kidney transplantation, orthotopic lung transplantation, and skin grafting have been described in detail previously (7,19,20). For combined heart–kidney transplantation, the recipients underwent bilateral nephrectomy. The aorta and inferior vena cava were used for end-to-side arterial and venous anastomoses for both the heart and kidney, with the heart placed at least 1 cm caudad to the kidney. The kidney transplantation was completed by performing a vesicoureteral anastomosis. For combined heart–lung transplantation, the recipients first underwent left orthotopic lung transplantation before heterotopic heart transplantation. In brief, after induction of general anesthesia, a left thoracotomy was performed and the recipient hilar structures were isolated. Heparin was administered (200 units/kg) and a pneumonectomy was performed. The donor lung was harvested via median sternotomy. Heparin was administered (400 units/kg). The pulmonary artery was cannulated and flushed in situ with 2 L of Perfadex (XVIVO Perfusion AB, Göteborg, Sweden) containing prostaglandin E1 (500 μg/L). The lung was prepared surgically and immediately transplanted. A thoracostomy tube was placed to evacuate the pleural space and was removed on POD 2.
Two indwelling Silastic central venous catheters were placed surgically into the external or internal jugular veins in all recipients. The catheters facilitated tacrolimus administration and frequent blood sampling for in vitro assays and for monitoring of renal function and whole blood tacrolimus levels.
Skin grafting was performed by placing split-thickness skin grafts on the dorsum of long-term tolerant recipients. Animals received fresh (self) or frozen (donor, third party) skin grafts that were harvested by using a dermatome. Graft beds were prepared with a single pass of the dermatome. An occlusive compression dressing was applied and removed on POD 4 after skin grafting. Day of rejection was defined as the point at which the skin graft became necrotic, as confirmed by biopsy.
Rejection monitoring
Kidney function was monitored by serial serum creatinine levels. Heart function was monitored by daily palpation and electrocardiogram using the AliveCor Veterinary Heart Monitor (AliveCor, Inc, San Francisco, CA). Lung function was monitored by clinical examination of breath sounds and chest radiographs. Routine biopsies were performed on all transplant recipients via flank incisions (for heart–kidneys) or mini-thoracotomies (for lungs) at predetermined time intervals (PODs 20–30, 50–60, 90–100) or whenever there was clinical suspicion for rejection. Cardiac allograft rejection (heart survival time) was defined by either loss of a ventricular impulse on palpation and/or QRS-wave amplitude of less than 0.3 mV and/or the lack of ventricular contraction on echocardiography (21). Renal allograft rejection was defined as sustained rise in serum creatinine level to greater than 10 mg/dL and/or uremia. Allograft rejection was confirmed histologically in all cases.
Immunosuppression
Tacrolimus (Haorui Pharma-Chem Inc, Irvine, CA) was mixed and administered as an intravenous suspension according to the specifications of the manufacturer. Tacrolimus was given as a continuous infusion at a dose of 0.10 to 0.20 mg/kg (adjusted to maintain a whole blood level of 30 to 50 ng/mL) for 12 consecutive days, starting on the day of transplantation (POD 0).
Histopathological examination
Core needle biopsies were performed on cardiac allografts. Wedge biopsies were performed on lung allografts. Acute rejection was scored according to the guidelines promulgated by the International Society for Heart and Lung Transplantation (ISHLT) (22,23) by a transplant pathologist without knowledge of the functional status of the graft. Wedge biopsies were performed on kidney allografts. Acute rejection in the kidney allograft was scored according to the Banff classification (24) by a transplant pathologist without knowledge of the functional status of the graft.
Preparation of peripheral mononuclear cells
Freshly heparinized whole blood was diluted approximately 1:2 with Hanks’ balanced salt solution (HBSS; Gibco BRL, Grand Island, NY), and the mononuclear cells were obtained by means of gradient centrifugation with Histopaque (Sigma, St. Louis, MO). The mononuclear cells were washed once with HBSS, and contaminating red cells were lysed with ammonium chloride potassium lysing buffer (BioWhittaker, Inc, Walkersville, MD). Cells were then washed with HBSS and resuspended in tissue culture medium. All cell suspensions were kept at 4°C until used in cellular assays.
Cell-mediated lympholysis
Cell-mediated lympholysis (CML) assays with porcine cells have been described previously (25). Briefly, lymphocyte cultures containing 4×106/mL responder and 4×106/mL stimulator peripheral blood mononuclear cells (PBMCs) (irradiated with 2500 cGy) were incubated for 6 days at 37°C in 5% carbon dioxide and 100% humidity in CML media. Bulk cultures were harvested, and effectors were tested for cytotoxic activity on 51Cr-labeled (Amersham, Arlington Heights, IL) lymphoblast targets generated from phytohemagglutinin (M-form;Life Technologies, Gaithersburg, MD) stimulation. Effector cells were incubated for 5.5 hours with target cells at effector:target ratios of 100:1, 50:1, 25:1, and 12.5:1. Two target cells were tested in each assay: (a) PBMCs matched by MHC to the donor and (b) third-party PBMCs. Supernatants were then harvested using the Skatron collection system (Skatron, Sterling, VA), and 51Cr release was determined on a gamma counter (Micromedics, Huntsville, AL). The results were expressed as a percentage of specific lysis of counts per minute (cpm) and calculated as follows:
Mixed-lymphocyte reaction
Mixed-lymphocyte reaction (MLR) assays with porcine cells have been described previously (25). Briefly, cultures containing 4×106 responder and 4×106 irradiated (2500 cGy) stimulator PBMCs were incubated in 200 μL of media in 96-well flat-bottomed plates (Costar Corning, Lowell, MA) for 5 days at 37°C in 5% CO2 and 100% humidity. After the 5-day incubation, 1 μCi of [3H]thymidine was added to each well, followed by an additional 5-hour incubation under the same conditions. [3H]Thymidine incorporation was determined in triplicate samples by beta-scintillation counting. Absolute counts were compensated for background and then expressed as stimulation indices (SI), calculated as SI = average cpm for a responder– stimulator pair per cpm of the same responder stimulated by an autologous stimulator.
Assessment of alloantibody
The presence of antidonor immunoglobulin (IgM and IgG) in the serum of experimental swine was examined by indirect flow cytometry using a Becton Dickinson FACScalibur (Sunnyvale, CA) to determine the MHC-binding specificity of the antibody. Fluorescein isothiocyanate (FITC)-labeled goat anti-swine IgM or IgG polyclonal antibodies were used as secondary reagents (Kirkegaard & Perry Laboratories Inc, Gaithersburg, MD). For staining, 1×106 cells per tube of donor-type PBMCs were resuspended in 100 μL HBSS containing 0.1% bovine serum albumin and 0.05% sodium azide and incubated for 30 minutes at 4°C with 10 μL of undiluted, decomplemented test sera. After two washes, a saturating concentration of FITC-labeled goat anti-swine IgM or IgG was added and incubated for 30 minutes at 4°C. After a final wash, cells were analyzed by means of flow cytometry with propidium iodide gating to exclude dead cells. Both normal pig serum and pretransplant sera from each experimental animal were used as controls for specific binding.
Statistical analysis
Graft survival times were compared using a two-tailed Mann– Whitney U test. Differences in graft survival time were deemed significant when p < 0.05.
Results
Lung cotransplantation, in contrast to kidney cotransplantation, was unable to induce long-term acceptance of heart allografts
To determine whether lung cotransplantation imparts the same tolerogenic effect as kidney cotransplantation, three animals underwent combined heart–lung transplantation across a full MHC mismatch (group 4, Table 1). Two animals (nos. 22008 and 22334) lost their heart impulse by POD 55 and showed evidence of severe heart rejection (Figures 1 and 2). One animal (no. 22063) maintained its heart impulse and had only mild to moderate acute cellular rejection on serial biopsy samples (Figures 1 and 2). However, on POD 202, this animal showed evidence of chronic rejection with cardiac allograft vasculopathy and endothelialitis (Figures 1 and 2). In comparison, previously published results of combined heart–kidney transplantation across a full MHC mismatch demonstrated long-term rejection-free survival of heart allografts (group 2, Table 1), while transplantation of isolated hearts across a full MHC mismatch in the same study resulted in heart rejection within 35 days (group 1, Table 1) (9).
Table 1.
Histology and survival of allografts in recipients of MHC-mismatched transplants treated with 12 days of tacrolimus
| Full MHC mismatches (class I/class II)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Organ(s) transplanted | Animal no. | Recipient | Donor | Heart graft survival | Final heart pathology* | Final kidney pathology† | Lung graft survival | Final lung pathology‡ |
| 1§ | Heart | 21109 | CC | DD | 35 | 3R | — | — | — |
| 21189 | CC | DD | 32 | 3R | — | — | — | ||
| 21257 | CC | DD | 18 | 3R | — | — | — | ||
| 2§ | Heart–kidney | 21019 | CC | DD | >295 | 0 | 0 | — | — |
| 21018 | CC | DD | >284 | 0 | 0 | — | — | ||
| 20977 | CC | DD | >272 | 0 | 0 | — | — | ||
| 3¶ | Lung | 15515 | DD | AA | — | — | — | >515 | A2 |
| 15616 | DD | CC | — | — | — | 103 | A4 | ||
| 15841 | DD | AA | — | — | — | >429 | A2 | ||
| 15843 | DD | CC | — | — | — | >481 | 0 | ||
| 15895 | DD | AA | — | — | — | >389 | 0 | ||
| 16052 | DD | CC | — | — | — | >438 | 0 | ||
| 4 | Heart–lung | 22008 | CC | DD | 47 | 3R | — | 47 | A4 |
| 22334 | DD | CC | 55 | 3R | — | 55 | A4 | ||
| 22063 | CD | DC | >202 | CAV | — | 202 | A2 | ||
CAV, cardiac allograft vasculopathy; ACR, acute cellular rejection.
Grading of rejection from 0 (no rejection) to 3R (severe rejection) based on ISHLT scoring system (22).
Grading of rejection from 0 (no rejection) to ACR3 (severe rejection) based on Banff scoring system (24).
Grading of rejection from 0 (no rejection) to A4 (severe rejection) based on ISHLT scoring system (23).
Group 1 and 2 animals previously published (9).
Group 3 animals previously published (12).
Figure 1. Pathological grading of heart and lung biopsies.
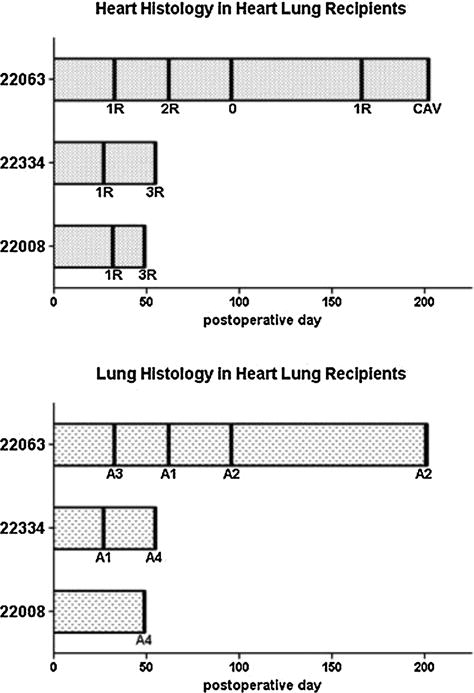
Serial biopsies of heart (top) and lung (bottom) allografts were graded according to the ISHLT grading system.
Figure 2. Histology of representative heart and lung biopsies.
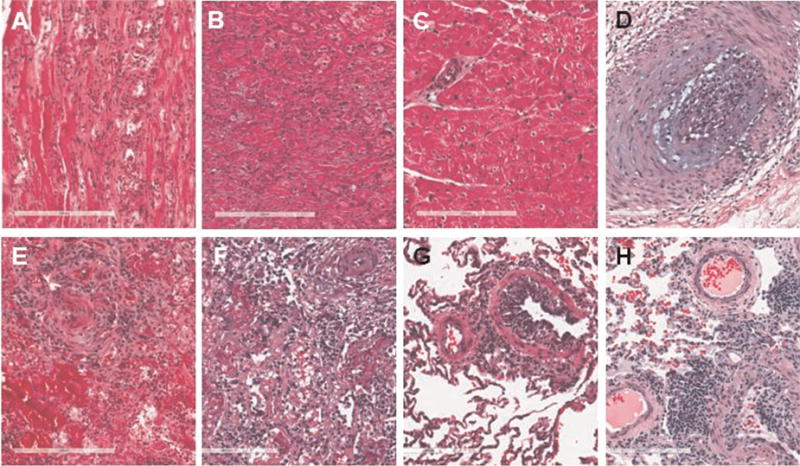
(A and E) On POD 49, animal no. 22008 showed severe rejection of the heart (3R) and severe rejection of the lung (A4B2C×D1). (B and F) On POD 55, animal no. 22334 showed severe rejection of the heart (3R) with diffuse myocyte necrosis and severe rejection of the lung (A4B×C×D×) with superimposed pneumonia. (C and G) On POD 97, animal no. 22063 showed no rejection of the heart (0) and mild rejection of the lung (A2B1C0D0). (D and H) On POD 202, animal no. 22063 showed cardiac allograft vasculopathy with endothelialitis of the heart and mild rejection of the lung (A2B1C×D×). POD, postoperative day.
Induction of tolerance to lung allografts is disrupted in the setting of combined heart–lung transplantation
We have previously shown (12) that a short course of tacrolimus induced long-term tolerance of lung allografts across a full MHC mismatch in five of six recipients (group 3, Table 1). However, when hearts and lungs were transplanted into the same recipient across a full MHC mismatch, lung allografts demonstrated severe rejection (group 4, Table 1, Figures 1 and 2). Animal no. 22063 was graded as having mild rejection of the lung on POD 202; however, gross appearance of the lung at this time was notable for friable, nonaerated tissue (Figures 1 and 2). Of note, as previously reported (9), when hearts and kidneys were transplanted into the same recipient across a full MHC mismatch, both hearts and kidneys remained free of rejection (group 2, Table 1).
Heart–lung transplant recipients showed antidonor responsiveness in vitro
To assess the ability of lung cotransplantation to induce donor-specific unresponsiveness, MLR and CML assays were performed before and after heart–lung transplantation. Two of three heart–lung recipients showed persistent antidonor responsiveness by MLR (nos. 22334 and 22063, Figure 3). The remaining heart–lung recipient (no. 22008) showed loss of donor-specific responsiveness by MLR, but antidonor response was regained by day of allograft rejection (Figure 3). Two of three heart–lung recipients showed donor-specific hyporesponsiveness by CML at the time of graft rejection (nos. 22008 and 22334); the remaining heart–lung recipient (no. 22063) showed donor-specific responsiveness by CML postoperatively (Figure 4). In contrast, previous studies showed persistent loss of donor-specific responsiveness in MLR and CML assays in all heart–kidney recipients (group 2, Table 1) (9). In another earlier study, five of six lung transplant recipients demonstrated donor-specific hyporesponsiveness postoperatively; animal no. 15616 demonstrated antidonor responsiveness on day of lung rejection (POD 103, group 3, Table 1) (12). Of note, one heart recipient (no. 21109) maintained donor responsiveness by MLR and CML assays, while the other two heart recipients developed donor-specific hyporesponsiveness (group 1, Table 1) (9).
Figure 3. MLR assays from heart–lung recipients.
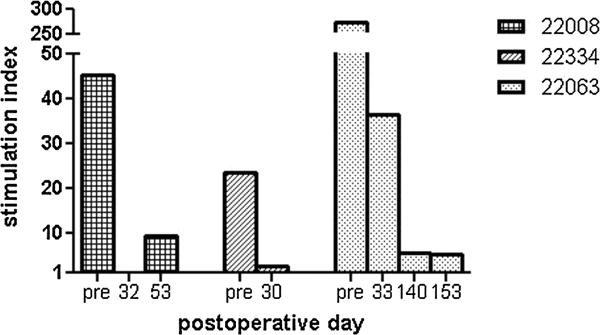
SIs to donor-type PBMCs are plotted as a function of postoperative day for each animal. MLR, mixed-lymphocyte reaction; PBMCs, peripheral blood mononuclear cells.
Figure 4. CML assays from heart–lung recipients.
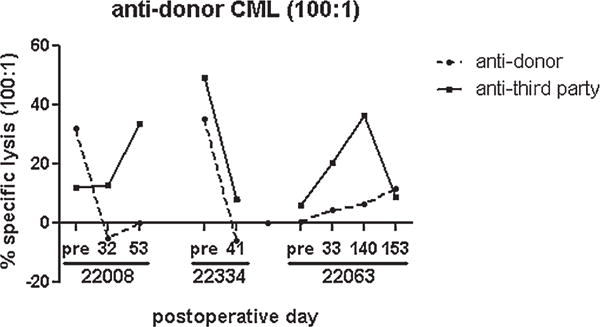
Percentage specific lysis at 100:1 effector: target ratio is plotted as a function of POD. Responses against donor-type targets (dotted line) and third-party Yorkshire targets (solid line) are shown for each animal. POD, postoperative day.
Circulating alloantibody is detectable in select heart and heart–lung recipients after heart rejection
To determine whether rejection of heart–lung allografts led to alloantibody formation, flow cytometry analysis of antidonor antibodies was performed. IgG alloantibody was elevated in one of three heart–lung recipients and one of three heart recipients (nos. 22008 and 21109, Figure 5, Table 1) (9). In contrast, in previous studies, heart– kidney recipients never showed detectable levels of circulating IgM and IgG alloantibody (9). Alloantibody data for lung recipients are not available (12).
Figure 5. Alloantibody response.
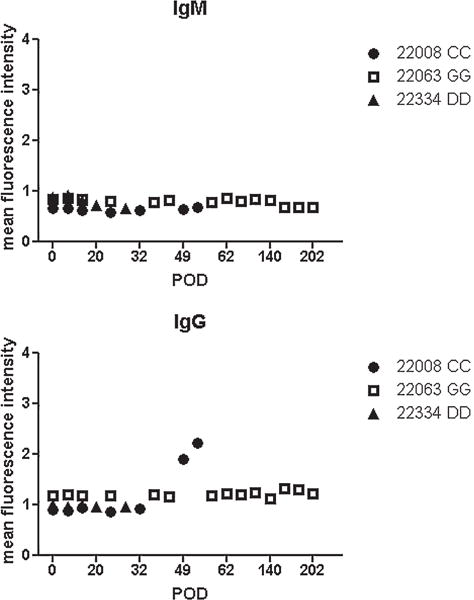
Levels of circulating antidonor IgM (left) and IgG (right) alloantibody were measured by flow cytometry in heart–lung recipients. Data were normalized to the mean fluorescence intensity of negative control values to plot normalized mean fluorescence intensity as a function of POD. POD, postoperative day.
Donor skin graft survival is not prolonged in a heart–lung recipient
To test the immune competence of heart–lung recipients, animal no. 22063 underwent skin grafting on POD 140. The donor skin graft (class Id, class IIc) rejected in 20 days and the challenge skin graft (class Id, class IIa) rejected in 10 days (data not shown). In contrast, a donor skin graft remained intact for 95 days on long-term tolerant heart– kidney recipient no. 21019 (9). Animal no. 22063 demonstrated rejection of heart and lung allografts 42 days after donor skin graft rejection (Figure 1). In contrast, heart and kidney allografts from animal no. 21019 remained free of rejection despite donor skin rejection (9).
Discussion
We have previously shown that tolerance of both kidney (10,11) and lung (12,26) allografts can be induced in MHC-inbred miniature swine using a short course of high-dose calcineurin inhibition. However, we have been unable to induce tolerance of heart transplants despite using similar tolerance induction strategies (7,8). Taking advantage of the tolerogenic properties of kidney allografts, we previously demonstrated that kidney cotransplantation could induce tolerance of heart allografts across a full MHC mismatch (9). To date, the mechanism underlying this “kidney effect” is unknown.
To investigate whether the mechanism underlying KICAT is unique to the kidney or is also a property of the lung, we evaluated whether lung cotransplantation could provide a similar tolerogenic influence on the heart. The data presented here show that, in contrast to kidney cotransplantation, which results in indefinite heart allograft survival, lung cotransplantation failed to protect cotransplanted heart allografts. Although the sample size is too small to determine whether the difference in graft survival of hearts in heart recipients versus heart–lung recipients is statistically significant, it is clear that the lung cannot provide the same tolerogenic effect as the kidney. Indeed, both heart and lung allografts in heart–lung recipients demonstrate decreased survival of both grafts compared with heart–kidney recipients or lung recipients.
It is intriguing to consider what unique cell or cell product present in the kidney but not (or to a lesser extent) heart or lungs allografts is able to confer tolerance on heart allografts. The tolerogenic capacity of the kidney has been attributed to its ability to induce immune regulation (27) [reviewed in (37)]. It is hypothesized that regulatory T cells generated by the kidney migrate to the thymus and/or to the heart allograft, providing immune regulation. Indeed, renal tubular epithelial cells are known to induce FOXP3+ regulatory T cells via transforming growth factor-β signaling (28), and renal endothelial cells express indoleamine 2,3-dioxygenase, an enzyme that leads to T-cell suppression, via interferon-γ signaling (29). Histological analysis of tolerant kidney grafts also demonstrate the presence of “T reg-rich organized lymphoid structures,” defined as nodular infiltrates rich in FOXP3+ -expressing cells and plasmacytoid dendritic cells associated with the vasculature of the kidney (17). Similar regulatory T cell–rich organized lymphoid structures have also been identified in tolerant mouse lung allografts (30). However, in contrast to the T-cell “acceptance reaction” that occurs in kidneys (31), the immune network of the lung, being a barrier organ, may favor alloreactivity. After lung transplantation, alveolar macrophages upregulate signaling molecules such as CD40 and CD80 to enable more-efficient antigen presentation and activate T cells (32). Nonhematopoietic cells in the lung such as vascular endothelium and airway epithelium express MHC class II constitutively (33) and can also serve as potent antigen presenting cells (34,35).
Interestingly, tolerance of lungs was achieved in the setting of lung transplantation (12) but not in the setting of combined heart–lung transplantation. This suggests that the proinflammatory milieu of thoracoabdominal surgery (which involves a longer operation in two body cavities) and the presence of a rejecting heart graft overcame the tolerogenic potential of lung allografts. The ability of one rejecting organ to promote rejection of another was previously demonstrated when heart rejection abrogated tolerance of kidneys in recipients of full MHC-mismatched heart and kidney allografts that were also fully MHC mismatched to each other (18). The lung may be more susceptible than the kidney to proinflammatory changes accompanying heart transplantation, as rejection of both heart and lungs occurred when both donor organs were MHC matched. Alternatively, the cardiac allografts may establish an immunologic milieu particularly conducive for rejection, and the lung allografts may be more vulnerable to this than the kidney allografts. Importantly, the mechanism of KICAT cannot be solely dependent on increased antigen load, as neither double heart transplantation (36) nor combined heart–lung transplantation leads to heart tolerance.
The results of our CML, MLR, and alloantibody assays demonstrated that the relationship between in vitro antidonor responsiveness and in vivo graft outcome was imperfect. For instance, despite in vitro evidence of preserved antidonor responsiveness (by both CML and MLR), one of the lung–heart recipients (no. 22063) failed to develop severe acute cellular rejection. While this recipient ultimately developed cardiac allograft vasculopathy, it did not develop more severe acute cellular rejection, which is interesting because it was the only recipient in this group to maintain antidonor responsiveness by CML assay throughout the entire experiment. This disparity could be explained by the contributions of regulatory mechanisms in vivo that are not recapitulated in vitro. Of note, however, all tolerant heart–kidney recipients and lung recipients consistently show loss of donor-specific responsiveness in vitro.
We recognize that the use of historical data could affect our results because of the potential for subtle changes in the operator, the procedure, or the genetics of the herd over time. However, the same surgeons were involved in all the transplants using the same procedures, and the breeding of our swine is carefully controlled to mitigate genetic drift. Finally, the same swine strains (SLAcc and SLAdd) were used as donor and recipients in most experiments. We have not found that the direction of the transplant, SLAcc into SLAdd or the reverse, affects the outcome of heart, lung, or kidney transplantation.
In conclusion, we find that lung cotransplantation, in contrast to kidney cotransplantation, is unable to induce tolerance of heart allografts, suggesting that kidneys possess a unique and organ-specific ability to confer tolerance. Further work will address how kidney allografts are able to foster an immunological environment that allows tolerance induction of resistant organs such as the heart.
Acknowledgments
Dr. Madariaga is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital and a recipient of a fellowship from the International Society for Heart and Lung Transplantation and a National Research Service Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health (F32HL117540). Dr. Spencer is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital, and recipient of a Training Fellowship Grant in Transplantation Biology (T32AI07529). Dr. Michel is a recipient of the 2013 ASTS-Novartis Scientist Scholarship Grant. We acknowledge C06RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine. We are indebted to Mr. J. Scott Arn for herd management and quality control typing. We thank Nicole Brousaides for preparing biopsy samples for histological analysis.
Abbreviations
- CML
cell-mediated lympholysis
- ISHLT
International Society for Heart and Lung Transplantation
- KICAT
kidney-induced cardiac allograft tolerance
- MHC
major histocompatibility complex
- MLR
mixed-lymphocyte reaction
- PBMC
peripheral blood mono-nuclear cell
- POD
postoperative day
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Russell PS, Chase CM, Colvin RB, Plate JM. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J Exp Med. 1978;147:1449–1468. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouard S, Pallier A, Renaudin K, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu XQ, Hu ZQ, Pei YF, Tao R. Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects. Hepatobiliary Pancreat Dis Int. 2013;12:12–33. doi: 10.1016/s1499-3872(13)60002-8. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekharan D, Issa F, Wood KJ. Achieving operational tolerance in transplantation: How can lessons from the clinic inform research directions? Transpl Int. 2013;26:576–589. doi: 10.1111/tri.12081. [DOI] [PubMed] [Google Scholar]
- 6.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Tx. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine. I. Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 8.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Tx. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Madariaga ML, Michel SG, Tasaki M, et al. Induction of cardiac allograft tolerance across a full MHC barrier in miniature swine by donor kidney cotransplantation. Am J Transplant. 2013;13:2558–2566. doi: 10.1111/ajt.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Tx. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Tx. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 12.Shoji T, Sahara H, Muniappan A, et al. An MHC class II disparity raises the threshold for tolerance induction in pulmonary allografts in miniature swine. Transplant Proc. 2006;38:3268–3270. doi: 10.1016/j.transproceed.2006.10.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calne RY, Sells RA, Pena JR. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Choo JK, Allan JS, et al. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Tx. 1999;68:485–491. doi: 10.1097/00007890-199908270-00007. [DOI] [PubMed] [Google Scholar]
- 15.Mezrich JD, Yamada K, Lee RS, et al. Induction of tolerance to heart transplants by simultaneous cotransplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Tx. 2003;76:625–631. doi: 10.1097/01.TP.0000079926.80833.42. [DOI] [PubMed] [Google Scholar]
- 16.Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature Swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant. 2003;3:1107–1115. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 17.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madariaga ML, Michel SG, La Muraglia GM, et al. Kidney-induced cardiac allograft tolerance in miniature swine is dependent on MHC-matching of donor cardiac and renal parenchyma. Am J Transplant. 2015 Mar 30; doi: 10.1111/ajt.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkman RL, Colvin MW, Flye GS, et al. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Tx. 1979;28:18–3. [PubMed] [Google Scholar]
- 20.Allan JS, Wain JC, Schwarze ML, et al. Modeling chronic lung allograft rejection in miniature swine. Tx. 2002;73:447–453. doi: 10.1097/00007890-200202150-00020. [DOI] [PubMed] [Google Scholar]
- 21.Avitall B, Payne DD, Connolly RJ, et al. Heterotopic heart transplantation: Electrophysiologic changes during acute rejection. J Heart Transplant. 1988;7:176–182. [PubMed] [Google Scholar]
- 22.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 25.Kirkman RL, Colvin RB, Flye MW, Williams GM, Sachs DH. Transplantation in miniature swine. VII. Evidence for cellular immune mechanisms in hyperacute rejection of renal allografts. Tx. 1979;28:24–30. [PubMed] [Google Scholar]
- 26.Shoji T, Muniappan A, Guenther DA, et al. Long-term acceptance of porcine pulmonary allografts without chronic rejection. Transplant Proc. 2004 doi: 10.1016/j.transproceed.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 27.Gianello PR, Yamada K, Fishbein JM, et al. Long-term acceptance of primarily vascularized renal allografts in miniature swine. Systemic tolerance versus graft adaptation. Tx. 1996;61:503–506. doi: 10.1097/00007890-199602150-00032. [DOI] [PubMed] [Google Scholar]
- 28.Frasca L, Marelli-Berg F, Imami N, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53:679–689. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 29.Thebault P, Condamine T, Heslan M, et al. Role of IFNgamma in allograft tolerance mediated by CD4+CD25+regulatory T cells by induction of IDO in endothelial cells. Am J Transplant. 2007;7:2472–2482. doi: 10.1111/j.1600-6143.2007.01960.x. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5:544–554. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu A, Yamada K, Meehan SM, Sachs DH, Colvin RB. Acceptance reaction: Intragraft events associated with tolerance to renal allografts in miniature swine. J Am Soc Nephrol. 2000;11:2371–2378. doi: 10.1681/ASN.V11122371. [DOI] [PubMed] [Google Scholar]
- 32.Nicod LP, Joudrier S, Isler P, Spiliopoulos A, Pache JC. Upregulation of CD40, CD80, CD83 or CD86 on alveolar macrophages after lung transplantation. J Heart Lung Transplant. 2005;24:1067–1075. doi: 10.1016/j.healun.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Kreisel D, Richardson SB, Li W, et al. Cutting edge: MHC class II expression by pulmonary nonhematopoietic cells plays a critical role in controlling local inflammatory responses. J Immunol. 2010;185:3809–3813. doi: 10.4049/jimmunol.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krupnick AS, Gelman AE, Barchet W, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 35.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med. 2009;179:344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- 36.Yamada K, Mawulawde K, Menard MT, et al. Mechanisms of tolerance induction and prevention of cardiac allograft vasculopathy in miniature swine: The effect of augmentation of donor antigen load. J Thorac Cardiovasc Surg. 2000;119(Pt 1):709–719. doi: 10.1016/S0022-5223(00)70005-5. [DOI] [PubMed] [Google Scholar]
- 37.Mezrich J, Yamada K, Sachs DH, Madsen JC. Combined heart and kidney transplantation: Why two organs may be better than one? Surgery. 2004;135:473–478. [Google Scholar]


