Graphical Abstract
Introduction
Mosquitoes are vectors for a variety of devastating arthropod-borne pathogens, such as Plasmodium, dengue virus and West Nile virus. In order to understand the unique mosquito-host interactions and target new ways for disease control, recent studies have found that many blood-derived components are still biologically active when ingested by female mosquitoes. Blood components such as insulin, insulin-like factor, TGF-β can trigger the corresponding signal transduction pathways in mosquitoes and affect mosquitoes’ capacity as disease vectors (Pakpour et al., 2013). Based on recent discoveries, more blood factors that are both functional in mammals and mosquitoes are likely to be reported.
Leukotoxins (EpOMEs) and epoxy eicosatrienoic acids (EETs) are epoxides of C-18 linoleic acid and C-20 arachidonic acid respectively, which are regular components in the mammalian blood. Elevated plasma level of EpOMEs is observed in human patients suffering from extensive burns (Hayakawa et al., 1990). EpOMEs are also associated with acute respiratory distress syndrome (ARDS) (Ozawa et al., 1988). Several studies suggest the diols of EpOMEs, the hydrolysis product of EpOMEs by the soluble epoxide hydrolase (sEH), are the active molecules responsible for the EpOME-associated toxicity (Moghaddam et al., 1997b; Zheng et al., 2001). Epoxyeicosatrienoic acids (EETs) are another important class of epoxide substrates for the mammalian soluble epoxide hydrolase (Yu et al., 2000; Zeldin et al., 1993). EETs, as a group of potent chemicals called eicosanoids, have been extensively studied in terms of human health and drug development (Morisseau and Hammock, 2013). In mammalian systems, a relatively well-studied property of EETs is anti-inflammation, as well as vasodilation, angiogenesis, and analgesic effects (Morisseau and Hammock, 2013). 11,12-EET is reported to reduce tumor necrosis factor-α (TNF-α) induced inflammation by a mechanism that inhibits NF-κB activity (Node et al., 1999). 14,15-EET is also known to be anti-inflammatory (Morin et al., 2008). The role of EETs as anti-inflammatory chemical mediators is also supported by the use of EH inhibitors (Schmelzer et al., 2005; Smith et al., 2005). In mosquitoes, the Toll and Imd pathways are the major immune signaling pathways that are studied in the context of immunity and disease transmission. Both pathways are highly conserved and dependent on the NF-κB transcription factor to play crucial roles in anti-pathogen defense (Dong et al., 2011; Zou et al., 2011). Many immune genes are reported to be regulated by NF-κB, such as diptericin, cecropin, attacin, defensing and nitric oxide synthase (Dong et al., 2006; Hillyer and Estevez-Lao, 2010; Luna et al., 2006; Richman et al., 1997; Vizioli et al., 2000). However, it remains unknown whether EETs and other epoxy fatty acids are endogenous substrates for mosquito EHs, and whether they are chemical mediators that regulate immune responses in a conserved pathway as they do in mammals.
In mosquitoes, we recently reported mammalian sEH homologs, and epoxy fatty acids are endogenous molecules (Xu et al., 2014; Xu et al., 2015). We also found high EH activities on epoxy fatty acids in the midgut of female mosquitoes in comparison to other tissues, and proposed that the EH inhibitor AUDA could be used as a probe to investigate the function of EHs in the mosquito midgut (Xu et al., 2015).
In this study, we tested the hypothesis that epoxy fatty acids are immune response regulators derived from the host that are metabolized by the epoxide hydrolases in the mosquito midgut during blood feeding. We found that the ingestion of the EH inhibitor AUDA triggered early gene expressions of defensin A, cecropin A and cecropin B2 at 6 hours post blood feeding. The ingestion also reduced the bacteria load in the midgut without causing any fitness loss in longevity, fertility and fecundity in our laboratory conditions. Our data supported our hypothesis, suggesting epoxy fatty acids are functional chemical mediators ingested by female mosquitoes, which play roles in regulating immune responses. Because the gut microbiota and the anti-bacterial responses affect the pathogen development in the mosquitoes (Cirimotich et al., 2011; Pan et al., 2012), the effects of EH inhibitor AUDA and related compounds on the biology of pathogens such as Plasmodium and Dengue virus should be tested in Anopheles and Aedes mosquitoes respectively.
Materials and Methods
Mosquito rearing
The larvae of the mosquito Culex quinquefasciatus were reared in plastic water cups, and fed twice daily with a mixture of grounded fish food (TetraMin, Germany) and cat food (Purina, MO). Adults were fed 10% sucrose ad libitum soaked in cotton balls daily in mosquito cages (30 cm × 30 cm ×30 cm) in an insectary incubator at a temperature of 28 ± 1°C and 80 ± 5% relative humidity.
Mosquito blood feeding
Before blood feeding, the 10% sucrose meal was removed from the rearing cages, and the mosquitoes were starved overnight. The next day, 5 ml of sheep blood (Quad Five, MT) was warmed to 37 °C in an incubator with gentle shaking. 5 μl of 10 mM AUDA in DMSO or 5 μl of DMSO was also added into the blood. The final concentration of the ‘AUDA blood’ is 10 μM AUDA, 0.1% DMSO (v/v). The control ‘DMSO blood’ contained only 0.1% DMSO (v/v). The sheep blood is routinely used to maintain the mosquito colony in the lab and contains serum and red blood cells. The concentrations of epoxy fatty acids in the serum were reported previously (Xu et al., 2015), and are comparable to the levels reported in other mammalian blood (Imig, 2012; Jiang et al., 2012; Jiang et al., 2005). Female mosquitoes (4–7 days after eclosion) were allowed to feed for 30 minutes on sheep blood through a glass mosquito feeder, which was connected to a water circulator to keep the blood at a constant 37 °C.
Real-time quantitative PCR
The primers used in this study (Table S1) were designed by the Beacon Designer software (PREMIER Biosoft, CA) except for the bacterial 16S ribosomal RNA primers (Nadkarni et al., 2002). Total RNAs were extracted from 10 blood-fed female mosquitoes from each treatment using Trizol reagent (Invitrogen, MA) at various times post blood feeding. cDNA (from 1 μg total RNA) was synthesized by SuperScript® III reverse transcription (Life Technologies, NY). Real-time quantitative PCR was performed using SYBR® GreenER qPCR SuperMix Universal assay kit (Invitrogen, MA) on a 7500 Fast Real-time PCR System (Applied Biosystems, CA) under manufacturer’s suggested conditions. Gene expression levels were normalized to the S7 ribosomal protein gene, and fold of change between the treatment groups was determined by the ΔΔCt method (Livak and Schmittgen, 2001).
Detection of the inhibitor AUDA or epoxy fatty acids in the midgut by LC-MS/MS
After mosquitoes were allowed to feed on artificial blood meal containing 10 μM AUDA, 0.1% DMSO (v/v) or 0.1% DMSO (v/v) only. Mosquito midguts were dissected at 6 hour intervals and were immediately placed into 1.5 ml eppendorf tubes with 10 μl anti-oxidant solution (0.2 mg/ml of butylated hydroxytoluene and EDTA) and 10 μl of deuterated standards (Yang et al., 2009). 400 μl of methanol was added to each microfuge tube and the tubes were placed in a −80°C freezer for 30 minutes. Subsequently, the midguts were homogenized with a plastic pestle and stored at a −80°C freezer overnight. The next day the homogenates were centrifuged at 10,000×g for 10 minutes and the supernatant was collected. The pellet was washed with 100 μl of ice-cold methanol, containing 0.1% of acetic acid and 0.1% of butylated hydroxytoluene. The samples were centrifuged again, and the supernatants were combined. The following sample preparation by solid phase extraction and analysis by LC-MS/MS was processed as previously described (Yang et al., 2009).
Longevity studies
Adult female mosquitoes were allowed to mate with males after emergence. After 4–7 days, the female mosquitoes were allowed to feed on an artificial blood containing 10 μM EH inhibitor AUDA, 0.1% DMSO or 0.1% DMSO only by a glass mosquito feeder at 37 °C for 30 minutes. Fully ingested females were transferred to a new cage, and were allowed to feed on 10% sucrose meals daily ad libitum. Daily mortality was recorded and dead mosquitoes were removed from the cage until all the mosquitoes died or censored. Analysis of survival curves was conducted by the Kaplan-Meier method (Kaplan E.L., 1958) and significant differences were determined by the nonparametric Wilcoxon test using the Prism 6 software (GraphPad, CA).
Fecundity and fertility studies
Female mosquitoes were allowed to mate and blood-feed as described above. After blood feeding, females that fully ingested were transferred to individual cages and were allowed to feed on 10% sucrose meals daily ad libitum. A cup of water was placed in each cage as an oviposition site. After oviposition, the egg number and the eggs that hatched were counted under a microscope. Egg counts and hatching rate were subjected to statistical analysis by the nonparametric Wilcoxon test using the Prism 6 software (GraphPad, CA).
Bacterial load analysis
All of the dissection tools were sterilized by autoclaving, ethanol or flame before the start of dissecting midguts. Female mosquitoes were cold anesthetized, and placed into a microfuge tube containg 1 mL of 75% ethanol. Subsequently, the mosquito was gently agitated 30 seconds for surface sterilization and then were rinsed three times with sterile phosphate buffered saline (PBS). The mosquitoes were left on ice in the microfuge tube with final PBS rinse until all mosquitoes were sterilized. For the colony forming unit (CFU) assays, mosquitoes were placed on a sterile glass slide under a dissecting microscope, and the midguts were dissected using a needle-tip probe and a fine-tipped forcep. The dissected midguts were transferred to a microfuge tube with 200 μL of sterile PBS. All of the tools were re-sterilized between each mosquito dissection. The midguts were homogenized with sterile plastic pestles, and the homogenates were 10-fold serially diluted four times. All of the serial dilutions (100 μL) were spread onto the LB agar plates, and allowed to dry. The sterile PBS (100 μL) was also plated as a negtive control for contamination. All of the plates were incubated at 37 °C for two days before CFU was counted. The bacterial load was also measured by RT-PCR dependent assays that quantified the relative amount of bacterial 16S rRNA. The midguts were dissected in RNAlater® solutions (Life technology, NY), and the RT-PCR procedure was carried out as described above.
Results
Metabolism of AUDA in the mosquito after blood feeding
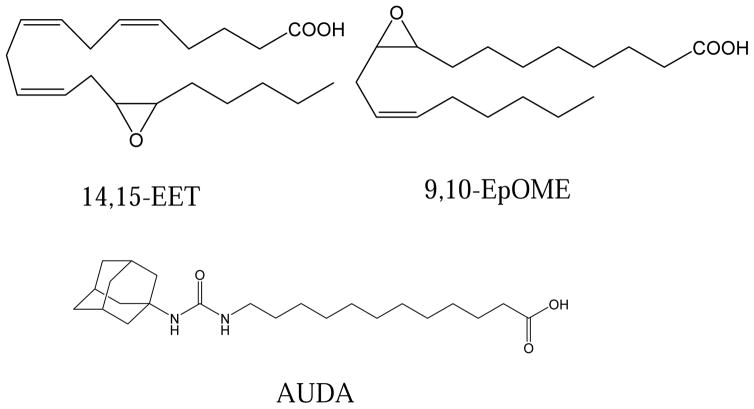
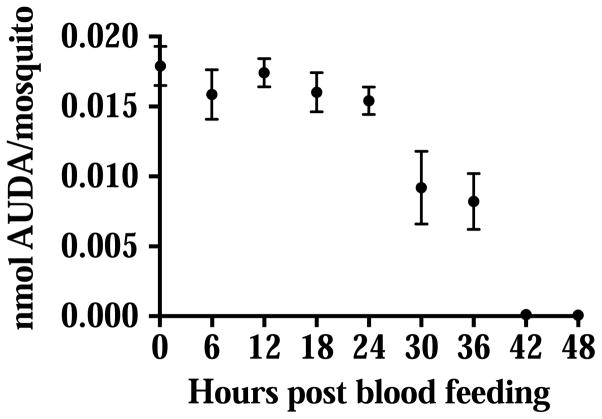
The physiological concentration of epoxy fatty acids in the sheep blood was published previously (Xu et al., 2015). The structures of EETs, EpOMEs and the epoxide hydrolase inhibitor AUDA that was used in the study are shown in Fig. 1. When the ‘AUDA blood’ (10 μM of AUDA, 0.1% v/v DMSO) was ingested by the mosquitoes, AUDA was detected by LC-MS/MS in the mosquito gut immediately after blood feeding (Fig. 2). The concentration of AUDA that was detected in the first 24 hours post blood feeding was relatively stable. At 30 hours post blood feeding, the level of AUDA detected was reduced by half. By the end of the blood digestion (42 and 48 hours post blood feeding), only a trace amount of AUDA (several nmol/mosquito) was detected in the midgut. The blood meal size and body size of female mosquitoes were not statistically different in our experiments (Fig. S2) The metabolism of AUDA in the mosquito midgut suggested the epoxide hydrolase activities were inhibited for a certain period of time, and the inhibition will diminish over time as the inhibitor was metabolized or excreted during the whole blood feeding process.
Fig. 1.
Structure of the epoxy fatty acid substrates (14,15-EET and 9,10-EpOME) and the inhibitor AUDA used in the study. The IC50 of AUDA on 14,15-EET is 86 ± 1 nM (Xu et al., 2015).
Fig. 2.
Degradative metabolism of AUDA in the mosquito gut following blood feeding. Circles represent the mean concentration of AUDA detected per mosquito ± standard deviations (n=5), unless the standard deviations are smaller than the datum point. The molecular weight of AUDA is 392.6.
Expression of immune genes following blood feeding on ‘AUDA blood’
To test the hypothesis that epoxy fatty acids are signaling molecules in mosquitoes that regulate NF-κB mediated immune responses, we measured the expression of 5 antimicrobial peptides that are predicted as Toll or Imd pathway marker genes in mosquitoes (Luna et al., 2006; Meister et al., 2005) by quantitative real-time PCR. The expression of these genes in non-blood fed females was also sampled as the expression control (regarded as 1 fold of change).
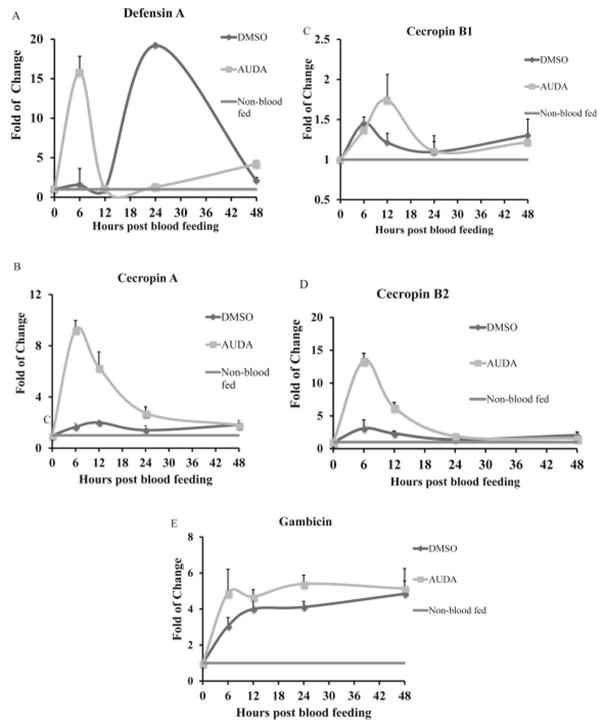
Among the 5 genes evaluated (defensin A, cecropin A, cecropin B1, cecropin B2, gambicin), ingestion of AUDA caused at least a 4 fold of increase in the expression of defensin A, cecropin A and cecropin B2 at certain time points (Fig. 3) compared to DMSO-ingested control mosquitoes. In the control DMSO-fed mosquitoes, there was a peak of defensin A expression at 24 hours post blood feeding (Fig. 3A). The peak of defensin A expression in AUDA-fed mosquitoes was observed at 6 hours post blood feeding. The expression of defensin A in AUDA-fed mosquitoes was up-regulated 10 fold and 2 fold at 6 and 48 hours, and down-regulated 1.6 fold and 14 fold at 12 and 24 hours post blood feeding respectively, in comparison to DMSO-fed mosquitoes. In DMSO-fed mosquitoes, there was a weak but constitutive expression of cecropin A (Fig. 3B) and cecropin B2 (Fig. 3C), but the expression levels did not change by more than 2 fold during the four time points in the 48 hours post blood feeding. Feeding on ‘AUDA blood’ induced a peak of expressions of cecropin A (Fig. 3B), and cecropin B2 (Fig 3D) at 6 hours post blood feeding. The expression of cecropin A and B2 was up-regulated 2 to 6 fold during the first 12 hours post blood feeding and then returned to the same levels as the DMSO-fed controls. The relative expression levels of cecropin B1 (Fig. 3C) and gambicin (Fig. 3E) did not change by more than 2 fold at the four time points that were sampled.
Fig. 3.
Expression of (A) defensin A, (B) cecropin A, (C) cecropin B1, (D) cecropin B2, (E) gambicin following ingestion of AUDA in blood feeding. Mosquitoes were sampled every 6 hours post blood feeding. Non-blood fed females were sampled as controls. Gene expression levels were analyzed by real time quantitative PCR (RT-qPCR). Data are presented as mean fold of change ± standard deviation from three independent experiments compared with the non-blood feeding mosquitoes as controls.
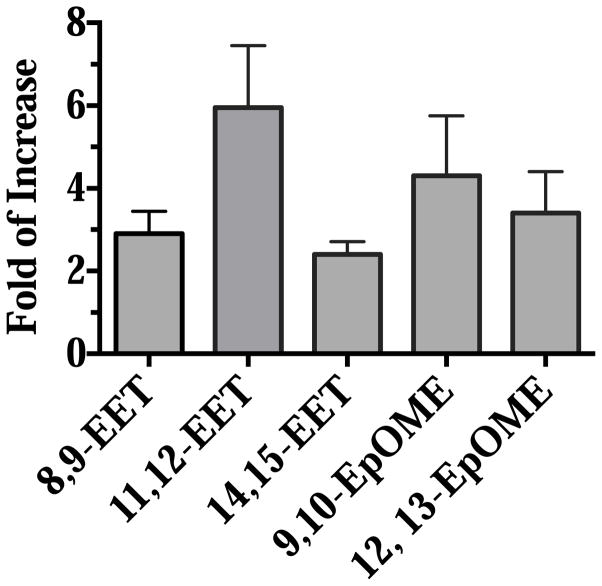
AUDA ingestion increased the concentrations of epoxy fatty acids in the midgut at 6 hours post blood feeding
At 6 hours post blood feeding, 50 mosquito midguts from each treatment were dissected, and homogenized in methanol. Lipids were extracted as previously described, and the amount of epoxy fatty acids (EpOMEs and EETs) in the midgut were analyzed by LC-MS/MS. The concentration was represented as pmol detected/midgut, and the concentrations of epoxy fatty acids in the midgut of AUDA-fed mosquitoes were compared to the ones of DMSO-fed mosquitoes. The data are presented as fold of increase in Fig. 4 with primary analytical data in supplemental material (Table S2). The midgut concentrations of EpOMEs and EETs were increased by 2 to 6 fold in mosquitoes that fed on ‘AUDA blood’ in comparison to mosquitoes that fed on ‘DMSO’ blood. The corresponding diol concentrations did not change significantly following feeding on ‘AUDA blood’ (Fig. S2). The data suggest the epoxide hydrolase inhibitor AUDA protects the epoxy fatty acids from the metabolism by the epoxide hydrolases in the midgut. Conversely, the epoxide hydrolase activities appear to be involved in the metabolism of epoxy fatty acids in blood that is ingested from the host blood.
Fig. 4.
Ingestion of AUDA increases the concentration of epoxy fatty acids in the midgut of female mosquitoes at 6 hours post blood feeding. 50 midguts from each treatment were dissected and homogenized in methanol. The concentration of epoxy fatty acids (nM/midgut) from the midguts of the AUDA-fed mosquitoes is compared to those of the DMSO-fed mosquitoes. Data are presented as mean fold of increase ± standard deviation of three independent experiments.
Mosquito fecundity, fertility and longevity were not affected by the ingestion of ‘AUDA blood’
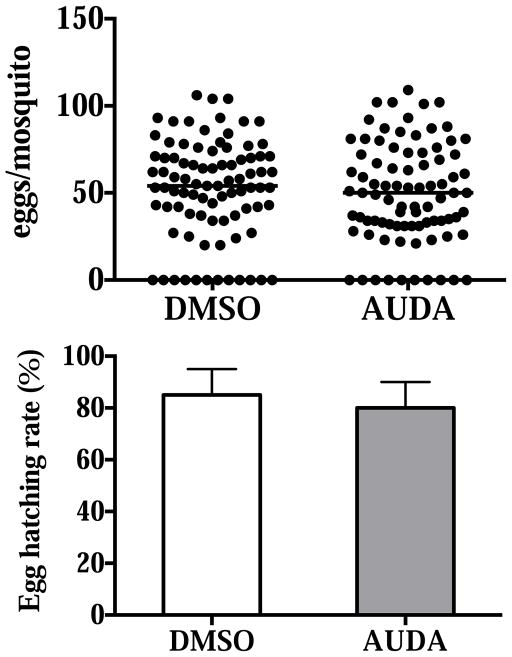
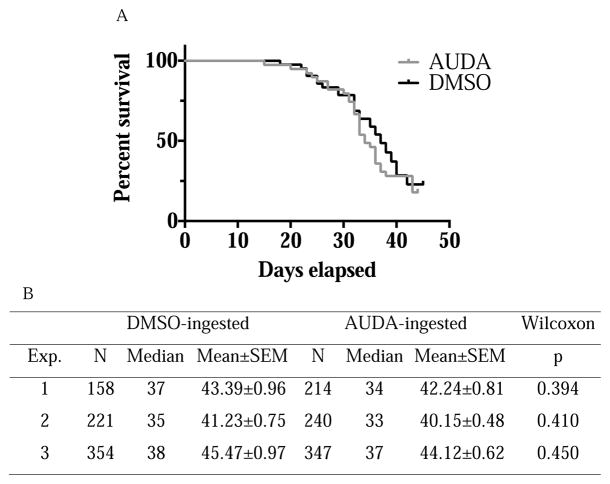
If epoxy fatty acids are signaling molecules that remain functional when ingested by mosquitoes from host blood, and epoxide hydrolases are the major enzymes in the mosquito gut that degrade epoxy fatty acids, the inhibition of epoxide hydrolases may result in fitness costs, such as reductions in fecundity, fertility or longevity. These parameters have direct effects on mosquitoes as disease vectors. We examined whether there were any differences in the number of eggs laid, egg hatching rate, and longevity between DMSO- and AUDA-fed mosquitoes. In the pooled data including four independent experiments, there were no significant differences in eggs laid and egg hatching rates between DMSO- and AUDA-fed mosquitoes (Fig. 5). In the three independent experiments on mosquito longevity, there were no significant differences between the two treatments under our laboratory conditions (Fig. 6).
Fig. 5.
Fecundity and fertility of female mosquitoes that fed on 0.1% (v/v) DMSO or 10 μM AUDA in blood feeding. Eggs laid and hatched from 4 independent experiments are pooled and shown. Each circle represents the eggs laid by an individual. The horizontal black bar represents the median egg counts in each treatment. In the egg hatching rate, data are presented as mean ± standard deviation. The values were not statistically different (p=0.33 and p=0.62 respectively) between the two treatments determined by the Mann-Whitney test.
Fig. 6.
Survival analysis of female mosquitoes that fed on 0.1% (v/v) DMSO or 10 μM AUDA in blood feeding. A. Representative survival analysis comparing DMSO- or AUDA-fed mosquitoes under identical conditions after blood feeding. Female mosquitoes were only blood-fed once and fed on 10% sucrose daily after blood feeding. The survival analysis showed the treatments were not statistically different (p=0.3935) by the Wilcoxon test. B. Summary of the number of individuals, median, mean ±SEM and statistical significance of three independent survival analysis.
Ingestion of AUDA during blood feeding affected the growth of bacteria in the midgut of female mosquitoes
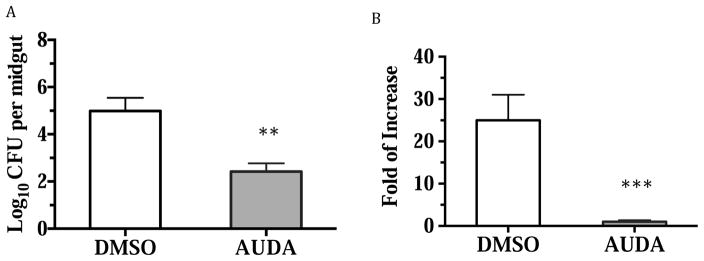
AUDA-fed mosquitoes showed an increase in the expression of several antimicrobial peptides at 6 hours post blood feeding. In order to examine if this up-regulation of expression will affect the microbiota in the midgut, the bacteria in the midgut were cultured on common LB agar plates. Concurrently, the relative amount of bacteria was determined using the RT-PCR dependent assay targeting bacterial 16S ribosomal RNA to confirm the results of the bacterial load experiments.
Mosquito midgut homogenates were spread on LB agar plates, and the colony forming units (CFU) were counted. The midguts of AUDA-fed mosquitoes had less bacteria that could be cultured on LB agar plates (Fig. 7). Because we noticed that probably not all the bacteria can be cultured on the LB agar plates, we used another RT-PCR-based assay to quantify the relative amount of bacterial 16S RNA in the midgut of each treatment. The primers used are shown in Table S1. We found in the RT-PCR-based assay that there were significantly less bacteria in the midgut of AUDA-ingested mosquitoes, in accordance with the CFU assays.
Fig. 7.
Ingestion of AUDA during blood feeding affects the bacteria load in the midgut of adult female mosquitoes. A. Number of bacteria from the midgut of female mosquitoes that can be cultured on LB plate. 6 midguts of each treatment were dissected and homogenized individually. The lysate was plated on LB agar plate after several serial dilutions in a sterile environment. The LB agar plates were cultured at 37 °C for two days before the colony formed unit (CFU) were counted. B. Bacteria load measured by quantifying the relative amount of bacterial 16s rRNA from the midgut of female mosquitoes by RT-PCR. 10 midguts from each treatment were dissected individually in RNAlater solutions. The relative amount of bacterial 16s rRNA from the midguts of AUDA-ingested mosquitoes is regarded as 1 fold of change when compared to those of the DMSO-ingested mosquitoes. Values are mean ± standard deviation. The statistical analysis was done by the Student’s t test (**p<0.01, ***p<0.001 vs. DMSO).
Spiked EpOMEs and DiHOMEs reduced bacterial load in the mosquito midgut, while EETs reversed the effects of the inhibitor AUDA
As shown in Fig. 4, ingestion of AUDA resulted in increased levels of EpOMEs and EETs. To determine which of the lipid mediators contributed to the biology we have observed, we spiked epoxides or diols into the blood with or without AUDA and measured their effects on midgut bacteria.
Interestingly, we found that spiking EpOMEs and DiHOMEs reduced midgut bacterial load, similar to the effect of the inhibitor AUDA (Fig. 8). EpOMEs and DiHOMEs, however, did not synergize or reverse the effect of AUDA (Fig. 8). On the other hand, EETs alone had no effects on the midgut bacteria, but can reverse the effect of AUDA to increase the level of bacteria to the level observed in DMSO ingested mosquitoes (Fig. 8). DHETs, the corresponding diols of EETs, had no effect on the bacterial load (Fig. 8).
Fig. 8.
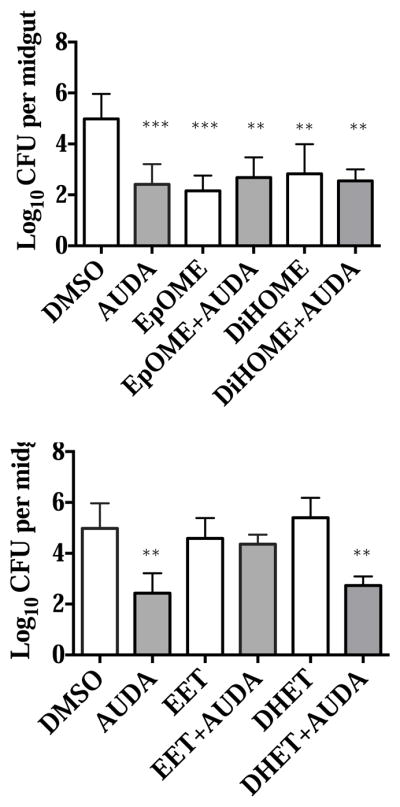
Effects of AUDA, spiked EpOME, DiHOME, EET and DHET on the gut bacteria at 6 hours post blood feeding. The EpOMEs are a mixture of 500 nM 9,10-EpOME and 12,13-EpOME, respectively, and the DiHOMEs are a mixture of corresponding diols of the two at 500 nM each; The EETs are a mixture of 200 nM 8,9-EET, 11,12-EET and 14,15-EET respectively and the DHETs are a mixture of the corresponding diols of the three at 200 nM each. Every group contains at least 6 mosquitoes for statistical analysis (**p<0.01, ***p<0.001 vs. DMSO).
Discussion
Epoxy fatty acids, such as EpOMEs and EETs, are epoxides of long chain fatty acids. EpOMEs and their metabolites have been reported to be potent chemical mediators of acute respiratory distress syndrome (ARDS) (Moghaddam et al., 1997b). EETs are oxygenated product of arachidonic acids, and belong to a group of eicosanoids including prostaglandins and leukotrienes. Eicosanoids have been studied extensively in terms of human health and drug development. In mammals, the products of cyclooxygenase and lipoxygenase pathways are largely but not exclusively pro-inflammatory, while the p450 branch of the cascade producing oxylipins which are largely but not completely anti-inflammatory. In insects, eicosanoids are known to play roles in ion transport, reproduction, and immunity (Stanley and Kim, 2014; Stanley and Miller, 2006), although most studies have focused on prostaglandins and leukotrienes. We recently reported that EETs are endogenous molecules in mosquitoes, and detected epoxide hydrolase activities similar to the mammalian soluble epoxide hydrolase (Xu et al., 2014; Xu et al., 2015). The soluble epoxide hydrolase is the main enzyme that hydrolyzes EETs to their corresponding diols in mammals, inhibition of which has been found to be beneficial in many mammalian disease models (Morisseau and Hammock, 2013). In this study we applied an epoxide hydrolase inhibitor (AUDA) discovered earlier (Xu et al., 2015) to be active on mosquito epoxide hydrolase to study the roles of epoxide hydrolases during the process of blood feeding. However, this study is by no means a thorough study on epoxide hydrolases and epoxy fatty acids in mosquitoes. Epoxy fatty acids, such as EETs, can also by synthesized from arachidonic acid by cytochrome p450s in vitro (Xu et al., 2015). EpOMEs and EETs are also detected in vivo in larvae and adults (Xu et al., 2015). As a result, epoxide hydrolases may affect mosquito physiology by regulating epoxy fatty acids from host blood or synthesized in vivo, at different developmental stages and in different biological contexts.
In our laboratory artificial blood feeding system, mosquitoes usually takes 1–2 μl of blood when not interrupted. Assuming 2 μl of blood was ingested, the recovery of AUDA by LC-MS/MS was 90%. We chose a dose of AUDA that is more than 100 times higher than its IC50 (Xu et al., 2015), which is expected to knock out more than 99% epoxide hydrolase activities on epoxy fatty acids during the process of blood feeding based on the in vitro data. At this dose (10 μM in blood or in water), we did not observe any toxic effects of AUDA on mosquito larvae and adults. We also did not observe any effects that could result from the inhibition of the juvenile hormone epoxide hydrolase, because the development, survival rate and fecundity were not changed in our experiments.
Epoxy fatty acids are autocrine and paracrine lipid signaling molecules. They are present in tissues in small amount, and are efficiently hydrolyzed by epoxide hydrolases. In many experimental models, the lack of epoxide hydrolase inhibition will result in failure to observe physiological functions of epoxy fatty acids. Taking all the arguments into account, we used 10 μM of AUDA as the dose to probe the function of epoxide hydrolases and epoxy fatty acids in mosquitoes during the blood feeding process. In this study our hypothesis is that AUDA is acting as an epoxide hydrolase inhibitor. However, AUDA was originally synthesized to mimic 14,15-EET. Further, Olearczyk (Olearczyk et al., 2006) showed AUDA can mimic EETs in mammals in addition to stabilizing them by inhibiting the mammalian soluble epoxide hydrolase. AUDA also has detergent and possibly other unknown physical and biological properties.
In order to test the hypothesis that ingestion of AUDA will stabilize the epoxy fatty acids in the midgut, which are signaling molecules that affect immune gene expression, we allowed the mosquitoes to feed on ‘AUDA blood’ and measured the expression of several NF-κB dependent genes. Based on the gene expression data, the control blood feeding process seemed to trigger a delayed (defensin A at 24 hours) or weak (cecropin A, B1, B2, gambicin) responses on the expression of these anti-bacterial genes, which were similar to the reported immune responses when mosquitoes ingested pathogen-containing blood meals (Bartholomay et al., 2010b; Richman et al., 1997; Vaughan et al., 1992). We observed the up-regulation of defensin A, cecropin A and cecropin B2 during the early period post blood feeding by ingestion of AUDA. The ingestion of AUDA also increased the concentrations of several epoxy fatty acids (EpOME and EETs) in the midgut, suggesting that epoxy fatty acids in mosquitoes are lipid signaling molecules that regulate immune responses. The epoxy fatty acids were reported to be either inflammatory (EpOMEs) or anti-inflammatory (EETs), and the diols of these epoxy fatty acids are also reported to be more or less potent than their parent molecules in different models (Ng et al., 2007; Norwood et al., 2010; Viswanathan et al., 2003). As a result, we interpreted our data as the overall effects of all the epoxy fatty acids and their corresponding diols that can affect immune gene expression when epoxide hydrolases are inhibited.
To further determine which lipid mediators are responsible for the observed biology, we spiked epoxides or diols into the blood and fed it to mosquitoes with or without AUDA. We did not observe any significant differences when spiking epoxides or diols that were 10 times more than the physiological level in the blood. When we spiked 100 times more than the physiological level, we found EpOMEs and DiHOMEs were as effective as AUDA in reducing midgut bacteria. It also seemed EpOMEs and DiHOMEs were equally potent. We did not observe any effects of DHETs, but found that EETs were able to reverse the effect of AUDA. This finding is the first indication that the linoleic acid-derived EpOMEs, DiHOMEs and the arachidonic acid-derived EETs are functional lipid chemical mediators in mosquitoes. The mechanism by which these fatty acid derivatives act is debated in mammals, and to that regard is also not known in mosquitoes. In mammals, it is reported that EpOMEs, and in some systems the DiHOMEs, are toxic or are associated with disease patterns (acute respiratory distress syndrome) (Hayakawa et al., 1990; Moghaddam et al., 1997a; Zheng et al., 2001). EpOMEs and DiHOMEs are also shown to induce chemotactic activity of the neutrophils (Totani et al., 2000), which are blood cells that regulate innate immune responses. The insect immune system shares a variety of things in common functionally and structurally with the mammalian systems. For instance, a number of hemocyte proteins are found homologous to those in the human neutrophils (Bergin et al., 2005). The insect hemocytes are known to phagocytose and kill microbes in a way similar to human neutrophils, and the superoxide production and microbial killing of both cells can be inhibited by a NADPH oxidase inhibitor (Bergin et al., 2005). Interestingly, a study also indicates DiHOMEs can inhibit the respiratory burst of neutrophils, which is a microbial elimination process mediated by the NADPH oxidase (Thompson and Hammock, 2007). EETs are mainly regarded as anti-inflammatory molecules in mammalian systems acting by reducing the activation of NF-κB activities (Inceoglu et al., 2011; Liu et al., 2013; Morin et al., 2010; Node et al., 1999). The IMD and Toll pathways are two pathways insect utilize to regulate immune response, and both pathways are dependent on the NF-κB activities. Modulation of the NF-κB activities in mosquitoes has been shown to make mosquitoes more refractory to human pathogens they can vector (Dong et al., 2011; Garver et al., 2009). Taken all the information into account, it’s tempting to test hypothesis that EpOMEs, EETs, and their metabolites mediate their biology in similar ways to the mammalian systems.
There are more than 200 immunity-related genes in the genome of Culex quinquefasciatus (Bartholomay et al., 2010a), and the immune response is a continuous and dynamic process. In many cases the expression of several anti-microbial peptides is not essential for the immune responses or their functions are redundant, leading to a lack of phenotypic changes. Thus we were unable to conclude the effects of the inhibitor AUDA on mosquito physiology based on gene expression data alone.
If an early expression of defensin A, cecropin A and B2 was triggered by ingestion of AUDA at 6 hours post blood feeding, the bacteria colonizing in the midgut may be affected. We measured the bacterial load in the midgut by culturing midgut bacteria on common LB agar plates and measuring bacterial 16S ribosomal RNAs by RT-PCR. We detected a decrease in the number of bacteria in the midgut of AUDA-fed mosquitoes, but we did not observe any fitness loss in fecundity, fertility and longevity under our laboratory conditions. Our data suggest that the AUDA ingestion caused a transient immune response, which diminished following the metabolism of AUDA in the midgut. It has been reported in Drosophila melanogaster (Libert et al., 2006) that a chronic and sustained immune response will affect fitness, but not acute and transient immune responses. In Anopheles gambiae, a transient immune response was also reported to render mosquitoes refractory to the human malaria parasite without affecting mosquito longevity and fecundity (Dong et al., 2006; Garver et al., 2009).
A late or weak immune response is one of the critical reasons pathogens can survive and successfully infect the midgut, which will allow the transmission cycle to continue. When the immune response is turned on earlier and at a higher level by transgenic techniques or by infection of the bacterium Wolbachia, mosquitoes become refractory to pathogens, such like Plasmodium falciparum and West Nile virus (Corby-Harris et al., 2010; Dong et al., 2011; Pan et al., 2012). In this study, we showed that the epoxide hydrolase inhibitor AUDA was an immune gene regulator during the process of blood feeding of Culex quinquefasciatus. Ingestion of AUDA also affected the growth of bacteria in the midgut. Because the anti-bacterial responses and microbes in the midgut are reported to affect mosquitoes’ capacity as disease vectors, further research of the impact of the EH inhibitor AUDA on Anopheles and Aedes mosquitoes may not only lead to a deeper understanding of host-mosquito interactions, but also new targets to manipulate immune responses for disease transmission control.
Supplementary Material
Fig. S1. Ingestion of AUDA does not change the concentration of diols of corresponding epoxy fatty acids in the midgut at 6 hours post blood feeding. 50 midguts from each treatment were dissected and homogenized in methanol. The concentration of epoxy fatty acids (nM/midgut) from the midguts of the AUDA-fed mosquitoes is compared to those of the DMSO-fed mosquitoes. Data are presented as mean fold of increase ± standard deviation of three independent experiments
Fig. S2. Blood meal size and wing length of female mosquitoes. 15 females from each group were selected immediately after blood feeding. The midguts were dissected and the blood protein concentration was measured by BCA assay with BSA as the standard. The wing length was also measured to evaluate body size. Values are presented as mean ± standard deviations. The differences in blood protein ingested and wing length are not statistically significant by Student’s t test (p=0.20 and p=0.49 respectively).
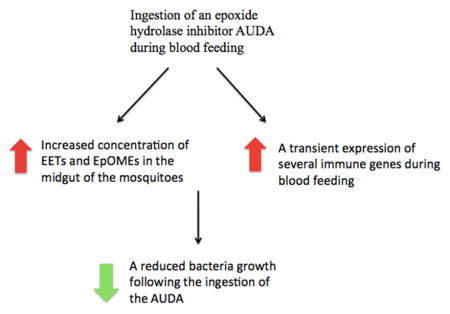
Ingestion of an EH inhibitor (AUDA) increased the concentration of epoxy fatty acids in the midgut of the mosquito.
The ingestion also triggered a transient expression of several immune genes.
The growth of midgut bacteria was affected by the ingestion of AUDA.
Acknowledgments
This study was funded in part by NIEHS (R01 ES002710 and P42 ES004699), the West Coast Metabolomics Center at UC Davis (NIH/NIDDK U24 DK097154), the UC Davis Jastro-Shields Graduate Research Award, and the China Scholarship Council. We thank Professor George Dimopoulos for detailed discussions and suggestions on how to measure bacterial load in mosquito midguts.
Abbreviations
- EH
epoxide hydrolase
- JH
juvenile hormone
- sEH
soluble epoxide hydrolase
- JHEH
juvenile hormone epoxide hydrolase
- EET
epoxyeicosatrienoic acid
- EpOME
epoxy octadecenoic acid
- DiHOME
dihydroxyoctadecanoic acid
- DHET
dihydroxyeicosatrienoic acid
- AUDA
12-(3-adamantan-1-yl-ureido) dodecanoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SH, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MA. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010a;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SHPP, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MAT. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010b;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun. 2005;73:4161–4170. doi: 10.1128/IAI.73.7.4161-4170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. Natural microbe-mediated refractoriness to Plasmodium Infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby-Harris V, Drexler A, de Jong LW, Antonova Y, Pakpour N, Ziegler R, Ramberg F, Lewis EE, Brown JM, Luckhart S, Riehle MA. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. Plos Pathogens. 2010;6 doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Das S, Cirimotich C, Souza-Neto JA, McLean KJ, Dimopoulos G. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7:e1002458. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M, Kosaka K, Sugiyama S, Yokoo K, Aoyama H, Izawa Y, Ozawa T. Proposal of leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin. Biochemistry international. 1990;21:573–579. [PubMed] [Google Scholar]
- Hillyer JF, Estevez-Lao TY. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev Comp Immunol. 2010;34:141–149. doi: 10.1016/j.dci.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiological Reviews. 2012;92:101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Anderson GD, McGiff JC. The red blood cell participates in regulation of the circulation by producing and releasing epoxyeicosatrienoic acids. Prostaglandins & Other Lipid Mediators. 2012;98:91–93. doi: 10.1016/j.prostaglandins.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HL, Quilley J, Reddy LM, Falck JR, Wong PYK, McGiff JC. Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids. Prostaglandins & Other Lipid Mediators. 2005;75:65–78. doi: 10.1016/j.prostaglandins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, MP Nonparametric estimation from incomplete observations. Journal of American Statistical Association. 1958;53:457–481. [Google Scholar]
- Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFκB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Liu JY, Lin YP, Qiu H, Morisseau C, Rose TE, Hwang SH, Chiamvimonvat N, Hammock BD. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. European Journal of Pharmaceutical Sciences. 2013;48:619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luna C, Hoa NT, Lin H, Zhang L, Nguyen HL, Kanzok SM, Zheng L. Expression of immune responsive genes in cell lines from two different Anopheline species. Insect Mol Biol. 2006;15:721–729. doi: 10.1111/j.1365-2583.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- Meister S, Kanzok SM, Zheng X-l, Luna C, Li TR, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nature Medicine. 1997a;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997b;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echave V, Albadine R, Rousseau E. 17,18-epoxyeicosatetraenoic acid targets PPAR-gamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. American journal of respiratory cell and molecular biology. 2010;43:564–575. doi: 10.1165/rcmb.2009-0155OC. [DOI] [PubMed] [Google Scholar]
- Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. EET displays anti-inflammatory effects in TNF-alpha stimulated human bronchi: putative role of CPI-17. American journal of respiratory cell and molecular biology. 2008;38:192–201. doi: 10.1165/rcmb.2007-0232OC. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annual review of pharmacology and toxicology. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology (Reading, England) 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor α. Drug Metabolism and Disposition. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood S, Liao J, Hammock BD, Yang GY. Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis. American journal of translational research. 2010;2:447. [PMC free article] [PubMed] [Google Scholar]
- Olearczyk JJ, Field MB, Kim IH, Morisseau C, Hammock BD, Imig JD. Substituted adamantyl-urea inhibitors of the soluble epoxide hydrolase dilate mesenteric resistance vessels. The Journal of pharmacology and experimental therapeutics. 2006;318:1307–1314. doi: 10.1124/jpet.106.103556. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Sugiyama S, Hayakawa M, Satake T, Taki F, Iwata M, Taki K. Existence of leukotoxin 9,10-epoxy-12-octadecenoate in lung lavages from rats breathing pure oxygen and from patients with the adult respiratory distress syndrome. The American review of respiratory disease. 1988;137:535–540. doi: 10.1164/ajrccm/137.3.535. [DOI] [PubMed] [Google Scholar]
- Pakpour N, Akman-Anderson L, Vodovotz Y, Luckhart S. The effects of ingested mammalian blood factors on vector arthropod immunity and physiology. Microbes and Infection. 2013;15:243–254. doi: 10.1016/j.micinf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman AM, Dimopoulos G, Seeley D, Kafatos FC. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. The EMBO journal. 1997;16:6114–6119. doi: 10.1093/emboj/16.20.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Kim Y. Eicosanoid signaling in insects: from discovery to plant protection. Critical Reviews in Plant Sciences. 2014;33:20–63. [Google Scholar]
- Stanley DW, Miller JS. Eicosanoid actions in insect cellular immune functions. Entomologia Experimentalis Et Applicata. 2006;119:1–13. [Google Scholar]
- Thompson DA, Hammock BD. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. Journal of biosciences. 2007;32:279–291. doi: 10.1007/s12038-007-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totani Y, Saito Y, Ishizaki T, Sasaki F, Ameshima S, Miyamori I. Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP. The European respiratory journal. 2000;15:75–79. doi: 10.1183/09031936.00.15107500. [DOI] [PubMed] [Google Scholar]
- Vaughan JA, Noden BH, Beier JC. Population dynamics of Plasmodium falciparum sporogony in laboratory-infected Anopheles gambiae. The Journal of parasitology. 1992;78:716–724. [PubMed] [Google Scholar]
- Viswanathan S, Hammock BD, Newman JW, Meerarani P, Toborek M, Hennig B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. Journal of the American College of Nutrition. 2003;22:502–510. doi: 10.1080/07315724.2003.10719328. [DOI] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Charlet M, Lowenberger C, Blass C, Muller HM, Dimopoulos G, Hoffmann J, Kafatos FC, Richman A. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol Biol. 2000;9:75–84. doi: 10.1046/j.1365-2583.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Morisseau C, Hammock BD. Expression and characterization of an epoxide hydrolase from Anopheles gambiae with high activity on epoxy fatty acids. Insect Biochemistry and Molecular Biology. 2014;54:42–52. doi: 10.1016/j.ibmb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Morisseau C, Yang J, Mamatha DM, Hammock BD. Epoxide hydrolase activities and epoxy fatty acids in the mosquito Culex quinquefasciatus. Insect Biochem Mol Biol. 2015 doi: 10.1016/j.ibmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical Chemistry. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZG, Xu FY, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circulation Research. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, Snapper JR, Capdevila JH. Regiofacial and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. Journal of Biological Chemistry. 1993;268:6402–6407. [PubMed] [Google Scholar]
- Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. American journal of respiratory cell and molecular biology. 2001;25:434–438. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]
- Zou Z, Souza-Neto J, Xi Z, Kokoza V, Shin SW, Dimopoulos G, Raikhel A. Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS pathogens. 2011;7:e1002394. doi: 10.1371/journal.ppat.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Ingestion of AUDA does not change the concentration of diols of corresponding epoxy fatty acids in the midgut at 6 hours post blood feeding. 50 midguts from each treatment were dissected and homogenized in methanol. The concentration of epoxy fatty acids (nM/midgut) from the midguts of the AUDA-fed mosquitoes is compared to those of the DMSO-fed mosquitoes. Data are presented as mean fold of increase ± standard deviation of three independent experiments
Fig. S2. Blood meal size and wing length of female mosquitoes. 15 females from each group were selected immediately after blood feeding. The midguts were dissected and the blood protein concentration was measured by BCA assay with BSA as the standard. The wing length was also measured to evaluate body size. Values are presented as mean ± standard deviations. The differences in blood protein ingested and wing length are not statistically significant by Student’s t test (p=0.20 and p=0.49 respectively).