Abstract
Background
Broccoli sprouts (BS) are the richest source of sulforaphane (SFN), which is a potent inducer of Phase II enzymes, which play a critical role in preventing oxidative stress (OS) and inflammation.
Objectives
To determine if ingestion of whole BS improves airway inflammatory and physiologic outcomes, and OS in adults with asthma and allergic sensitization to an indoor allergen.
Methods
The study is a double-blind, placebo-controlled, randomized trial to compare the effects of BS to placebo (alfalfa sprouts (AS)) on airway inflammation and markers of OS. Forty adults (age 18–50 years) were randomized to eat either: (a) 100g of BS daily or (b) 100g of AS daily for three days. Fractional exhaled nitric oxide (FENO), FEV1, nasal epithelial and PBMC gene expression, inflammatory and OS biomarkers, and symptoms were assessed both before and after ingestion of the sprouts. The primary outcome variable was the change in FENO. Secondary outcome measures included rhinitis and asthma symptoms, lung function, OS and inflammatory biomarkers.
Results
BS ingestion for 3 consecutive days did not reduce FENO despite resulting in a marked increase in serum SFN concentrations (21 vs 22 ppb, p=0.76). Furthermore, BS consumption did not induce cytoprotective antioxidant genes in either PBMCs or nasal epithelial cells, reduce OS and inflammatory markers, or improve lung function.
Conclusions
Ingestion of whole BS for 3 days does not appear to improve eosinophilic pulmonary inflammation, inflammatory and OS biomarkers, or clinical features of asthma among atopic adults with asthma despite resulting in a marked increase in serum SFN levels.
Keywords: Broccoli Sprouts, Asthma, FENO, Antioxidants, Oxidative Stress
Introduction
Asthma is one of the most common chronic diseases, and has increased in prevalence over the past several decades. Current medications target two primary pathophysiologic features of asthma: airways inflammation and bronchoconstriction, yet there are other pathways involved in the pathogenesis of asthma1–3. In addition, there is emerging evidence that certain dietary components or patterns that are thought to be “anti-inflammatory” may modulate asthma severity4, 5.
One of many pathways implicated in the initiation and propagation of inflammation in asthma is the oxidative stress pathway6, and some evidence suggests this pathway can be targeted by certain micronutrients, such as Vitamin C and Vitamin E7. In fact, randomized, placebo-controlled trials of antioxidant micronutrients have shown benefit in asthma, including one trial among children with asthma8. Also, increased dietary intake of fruits and vegetables enriched in antioxidants increased the time to asthma exacerbations compared to low antioxidant diet, suggesting whole food intervention strategies are effective in controlling asthma9. Most recently, there has been growing evidence that the active compound that results from eating certain cruciferous vegetables, SFN enhances antioxidant enzyme gene expression in airways, suggesting that ingestion of these foods could improve the anti-oxidant capacity of airways among asthmatics, thereby reducing airway inflammation, and ultimately improving clinical features of the disease, such as symptoms and lung function.
Studies to date have used a broccoli sprout homogenate in order to both convert the SFN precursors in the broccoli sprouts to SFN and to concentrate the dose of broccoli sprouts, and have found that ingestion of this extract for three consecutive days upregulates nasal lavage antioxidant gene expression10 and attenuates nasal inflammatory responses to diesel exhaust particle challenge11. These studies point to the potential of developing the extract as a drug, but what remains unclear is whether simply increasing intake of cruciferous vegetables high in SFN glucosinolates, which could be implemented as a public health intervention, has the same types of effects as the extract. To answer this question, we conducted a randomized, placebo-controlled trial of 3 consecutive days of broccoli sprouts (BS) ingestion among adults with atopic asthma. AS, which have low SFN content and similar in appearance and texture to BS, served as the placebo. The primary objective of the study was to determine if ingestion of whole broccoli sprouts reduces lower airway inflammation, as measured by FENO, among adults with asthma and a positive skin test to an indoor allergen. Other outcomes examined were markers of oxidative stress, anti-oxidant gene expression, and clinical features including symptoms and lung function in asthmatics.
Methods
Study Population
The study is a double-blind, placebo-controlled, randomized trial to examine the biological and clinical effect of BS in asthmatic adults with a positive skin test to an indoor allergen. Participants were recruited by advertising and had to meet the following eligibility criteria at the screening visit to be randomized: (1) doctor diagnosis of asthma, (2) sensitization to at least one major indoor allergen (cat, dog, dust mite, cockroach, mouse), (3) non-smoker, defined as no cigarettes smoked in the past month and absence of high urine cotinine by a rapid urine cotinine test, (4) normal thyroid stimulating hormone (TSH) and no history of hypothyroidism, (5) no other major pulmonary disease, and (6) age 18–50 years. Participants who took oral corticosteroids in the previous two weeks or had had an intensive care unit admission for asthma in the past 12 months were excluded as well as those with significant medical issues, such as heart disease and type 1 diabetes.
Intervention
Participants were randomized 1:1 to either BS or AS. The active intervention consisted of consumption of 100 grams of BS daily and the placebo consisted of 100g of AS daily for three days. AS have low SFN precursor content and a similar texture and appearance to BS and have been used successfully in other BS trials10, 12. BS or AS were added to sandwiches prepared by the Johns Hopkins ICTR Nutrition Core on the day of the study visit. The sprouts were not heated or mechanically disrupted and other ingredients needed for the food were cooled to room temperature before being incorporated into the sandwiches so that active ingredient would not be inactivated. The sandwich options were: (1) buffalo chicken wrap, (2) barbeque chicken wrap, (3) tuna salad wrap, (4) seafood salad wrap, (5) vegetarian breakfast burrito, (6) ham and cheese croissant, (7) open face lox bagel, (8) beef tacos.
The BS and AS were obtained from a local grower, Sunsprouts (parent company-Hanover Foods) that grows sprouts for commercial sale. The BS were supplied directly from the commercial grower to ensure that the BS would be consumed within 11 days of sprouting (for the highest SFN precursor content). The participants were observed eating the sandwiches and were instructed to thoroughly chew the sandwich, as glucoraphanin is converted to SFN through the mastication process. Sandwiches were weighed before they were eaten, and any remaining sandwich that the participant was unable to eat was weighed to determine the percentage of the sandwich ingested by the participant. All the study participants ate >99% sandwiches. On the first and third days of either BS or AS, before ingesting the first sandwich and after ingesting the final sandwich, outcome measures, including FENO, pulmonary function testing, blood, urine, and nasal epithelial cells were collected. Blood was also drawn for measurement of thyroid stimulating hormone (TSH) at baseline and after the sandwich ingestion on the 3rd day of food ingestion as high intake of cruciferous vegetables may induce hypothyroidism13.
Three days was selected for the duration of the intervention as three consecutive days of broccoli sprout extract ingestion was sufficient to upregulate phase II antioxidant genes in nasal lavage in a previous study10. A 100g serving of BS was selected because it approximated the dose in the previous study that demonstrated induction of anti-oxidant genes in nasal lavage cells, and was the amount that could reasonably be eaten during a day.
Study Procedures
Skin prick testing was performed using standard procedures to a panel of 14 common aeroallergens at the screening visit. The participant was instructed to be off antihistamines for 3–5 days prior to skin testing. A positive control in the form of histamine was placed and a negative control in the form of saline-glycerin was placed. If the participant otherwise qualified for the study but could not undergo skin testing or did not have a valid skin test panel (for example, due to absence of a response to the histamine positive control), specific IgE testing to house dust mite, cockroach, mouse, cat and dog allergens was performed instead to determine sensitization to indoor allergens (ImmunoCAP, Phadia, Uppsala, Sweden). Spirometry was performed at the baseline screening visit and pre and post-intervention. Spirometry was performed according to ATS guidelines to obtain FEV1 and FVC14, 15
Fractional exhaled nitric oxide (FENO) is a known marker of pulmonary inflammation and provided a means of assessing pulmonary inflammation16–18. FENO was measured before and after the intervention. Measurement of exhaled nitric oxide was obtained according to the American Thoracic Society Guidelines and prior to lung function19. FENO concentrations were measured using a chemiluminescent analyzer (NIOX Mino, Aerocrine, Sweden).
Nasal epithelial cells were obtained both before and after the intervention. The sampling was performed with a Rhinoprobe, a commercially available, disposable curette designed for such sampling, and the cell sample was used to measure Phase II enzyme gene expression. Samples were obtained from the inferior turbinates. Blood was collected both before and after the intervention by venipuncture to obtain serum and peripheral blood mononuclear cells (PBMCs). PBMCs were isolated by BD vacutainer cell preparation tube protocol.
Questionnaires were administered to capture demographic information, cruciferous vegetable intake, and clinical information, including the Asthma Control Test (ACT) and the Rhinitis Quality of Life Score. Urine was collected at the screening visit for rapid urine cotinine measurement on all participants and pregnancy testing on women of childbearing potential. Rapid urine cotinine testing was performed with NicAlert test strips and a NicAlert of 4 or greater excluded a participant from proceeding. A positive pregnancy test also excluded a participant from proceeding to randomization.
Laboratory Methods
RNA isolation and RT-PCR
Total RNA was extracted from nasal epithelial cells and PBMCs using the RNAqueous Micro total RNA isolation kit (Ambion) and quantified by ultraviolet absorption spectrophotometry. The reverse transcription reaction was performed using a high capacity cDNA synthesis kit (Sensiscript RT kit, Qiagen). Gene expression for Nrf2 target genes was evaluated using quantitative reverse transcription real-time polymerase chain reaction (qRT-PCR) and assay-on-demand primers and probe sets from Applied Biosystems. The probe sets used for the target genes are NADPH-quinone oxidoreductase 1 NQO1 (Hs02512143_s1), Heme oxygenase I HMOX1 (Hs01110250_m1), glutamate cysteine ligase catalytic subunit GCLC (Hs00155249_m1), glutamate cysteine ligase modifier subunit GCLM (Hs00157694_m1), β-Actin ACTB (Hs99999903_m1). SFN is known to induce these antioxidant genes through activation of NRF2. β-Actin was used for normalization.
Measurement of Total Antioxidant Capacity
The total antioxidant capacity in serum was measured using Cayman’s antioxidant assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). The capacity of antioxidants in the sample was compared with that of Trolox, a water-soluble tocopherol analog and the results are expressed as millimolar Trolox equivalents.
Thiobarbituric acid reactive substances (TBARS) assay
TBARS were measured in serum using commercially available kit (Cell Biolabs). Results were expressed as nanomole of MDA/ml serum.
Protein Carbonyls
Estimation of total protein carbonyl content in serum was carried out by 2,4-dinitrophenylhdrazine method and expressed as nanomole/mg protein20.
Isoprostane levels
Estimation of Isoprostane in urine was measured using commercially available kit (Cell Biolabs). Results were normalized to creatinine (Cayman chemicals) and expressed as pg/mg creatinine.
Cytokine ELISA
Levels of IL-4, IL-13 and IL-6 in serum were measured by enzyme immunoassays using high sensitivity human ELISA kits (eBioscience) and quantified as per the manufacturer instructions.
SFN measurement
Blood levels of SFN were measured as SFN conjugates by TNO Triskelion (Netherlands) according to their published literature21, 22. Briefly, serum was incubated with n-butanethiol in formic acid-triethylamine-methanol. Free iberin and SFN and their conjugates react to form a stable butanethiol conjugate which was determined using UPLC-MS/MS. Phenyl isothiocyanate was used as an internal standard and quantification was performed using external calibration with MRM at m/z 254->164 (iberin), 268->178 (SFN) and 226->136 (PITC). Iberin and SFN were obtained from LKT laboratories/Biomol. A Waters Acquity I-class UPLC and AB Sciex API 4000 MS were used.
Statistical Analyses
Power Estimate
With at least 18 subjects in each arm, at alpha=0.05, the study was powered to detect a reduction in log10 (FENO) of 0.25. This magnitude of effect corresponds to an approximately 10ppb greater decrease in FENO in the BS than AS group if the baseline geometric mean FENO is 20 ppb. A clinically important decrease of FENO is defined as a change of 10 ppb for values lower than 50 ppb23.
Statistical Analyses
Distributions of variables were examined between study groups and at two time points: immediately before ingesting the sandwich on day 1 and then on day 3, after ingesting the sandwich. Changes in the outcome variables from before to after ingestion of the sandwiches were calculated. Differences in the change in each outcome between the two groups (BS vs. AS) were tested using non-parametric tests. A p value <0.05 was considered statistically significant. All analyses were performed with R (version 3.2.1, Vienna, Austria).
Results
Study Population
The consort diagram (Figure 1) depicts the flow of study participants from assessment of eligibility to completion of the study. Overall, the two groups were similar in terms of demographic and clinical characteristics, controller medication use and dose, as well as baseline cruciferous vegetable intake (Table I).
Figure 1.
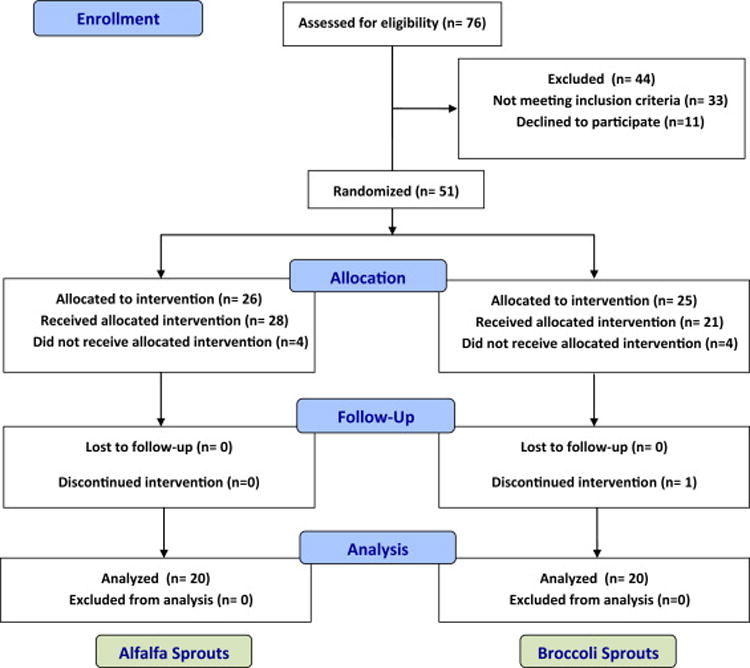
Consort diagram.
Table 1.
Study Population Characteristics
| Broccoli Sprouts (n=20) |
Alfalfa Sprouts (n=20) |
|
|---|---|---|
|
| ||
| SOCIODEMOGRAPHIC CHARACTERISTICS | ||
|
| ||
| Female | 11 (55%) | 13 (65%) |
|
| ||
| Age, mean (SD) | 33.1 (9.62) | 34.2 (9.17) |
|
| ||
| Race/ethnicity | ||
| Black/African American | 11 (55%) | 16 (80%) |
| White/Other | 9 (45%) | 4 (20%) |
|
| ||
| Educational attainment | ||
| Some college or more | 14 (70%) | 10 (50%) |
|
| ||
| Smoking status | ||
| Current | 0 (0%) | 0 (0%) |
| Former | 3 (15%) | 2 (10%) |
| Never | 17 (85%) | 18 (90%) |
|
| ||
| Cruciferous vegetable intake (Total number of servings over one week), mean (SD) |
5.2 (3.08) | 5.4 (4.55) |
|
| ||
| CLINICAL CHARACTERISTICS | ||
|
| ||
| Age of asthma diagnosis (years), mean(SD) | 11.4 (7.7) | 11.9 (10.0) |
|
| ||
| Controller Medication | ||
| None | 11 (55%) | 11 (55%) |
| Low-dose or Medium dose ICS | 3 (15%) | 4 (20%) |
| High dose ICS or High-dose ICS+LABA | 6 (30%) | 5 (25%) |
|
| ||
| Lung Function, mean (SD) | ||
| FVC (liters) | 3.94 (0.89) | 3.80 (1.01) |
| FVC % predicted | 97% (17%) | 100% (13%) |
| FEV1 (liters) | 3.06 (0.78) | 2.89 (0.87) |
| FEV1 % predicted | 89% (17%) | 91% (17%) |
| FEV1/FVC % | 77.5% (10%) | 75.9% (9%) |
|
| ||
| BMI kg/m2, median (25 – 75th%iles) | 30.9 (25.1 – 37.0) | 32.2 (25.5 – 35.8) |
|
| ||
| FENO (ppb), median (25 75 th%iles) | 21 (15–42) | 25.5 (15–42) |
|
| ||
| Number of allergic sensitivities,* median (25th 75th%ile) | 2 (1–3) | 3 (2–4) |
|
| ||
| Specific Sensitizations n (%) | ||
| Cat | 11 (55%) | 15 (75%) |
| Dog | 7 (35%) | 12 (60%) |
| Dust mite | 12 (60%) | 11 (55%) |
| Cockroach | 8 (40%) | 14 (70%) |
| Mouse | 7 (35%) | 8 (40%) |
defined as either specific IgE ≥0.35 or SPT net wheal≥ 3mm to indoor allergen panel
Effect of BS ingestion on FENO, Lung Function, and Clinical Outcomes
All of the participants had very low to undetectable SFN in blood at baseline. Every participant in the BS group had a marked increase in blood SFN levels, while there was no change in SFN levels in the participants who consumed AS sandwiches (Figure 2). There were no differences between the BS and the AS groups in FENO, which was the primary outcome (Figure 3). FENO concentrations at baseline were similar between the two groups and on day 3, after having consumed three days of sandwiches, FENO concentrations were also similar (19.5 vs 22 ppb) in the AS and BS groups, respectively, p =0.48). Similarly, there were no differences between groups in lung function, Asthma Control Test score, or the Rhinitis Quality of Life Score (Table II). Overall, BS were well tolerated. One participant in the BS group had an elevated TSH on day 3 (5.23 mIU/L) that returned to normal when rechecked (3.56 mIU/L), one participant had a TSH that was below normal on day 3 that returned to normal when rechecked, and one participant in the BS group dropped out because of gastrointestinal symptoms.
Figure 2.
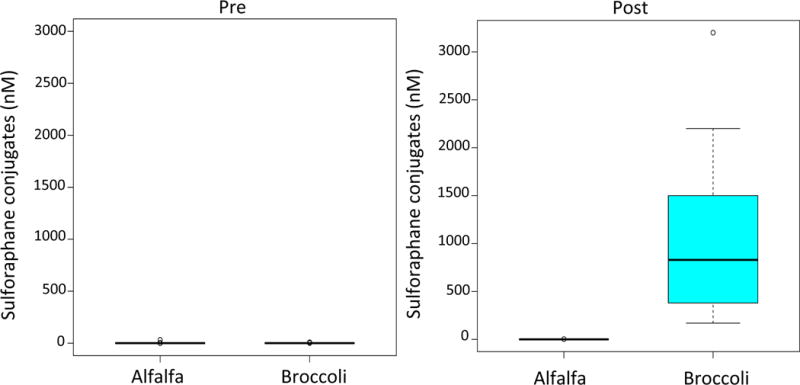
Boxplots of serum SFN conjugates (nM) pre and post intervention by treatment group alfalfa sprouts, broccoli sprouts.
Figure 3.
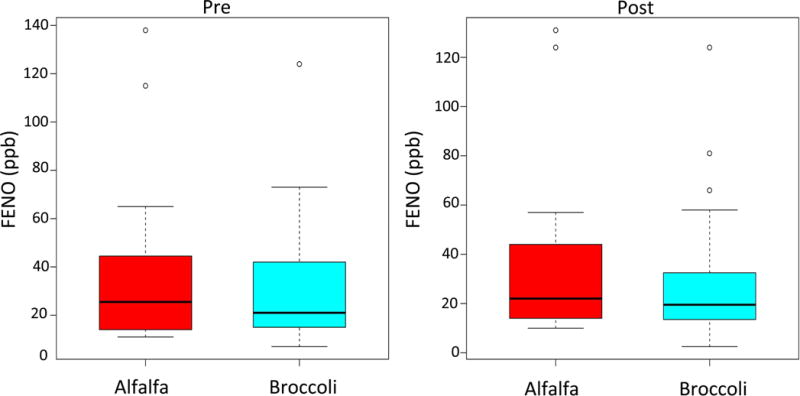
Effect of BS ingestion on fractional exhaled nitric oxide. Boxplots of FENO pre and post intervention by alfalfa sprouts and broccoli sprouts.
Table II.
Effect of Whole Broccoli Sprouts on Clinical Outcomes
| Alfalfa Sprouts | Broccoli Sprouts | p-value* | |||||
|---|---|---|---|---|---|---|---|
| Pre Ingestion |
Post Ingestion |
Difference (post-pre) |
Pre Ingestion |
Post Ingestion |
Difference (post-pre) |
||
| FVC (l), mean (SD) | 3.80 (0.99) |
3.78 (0.97) |
− 0.02 (0.21) |
3.95 (0.89) |
3.90 (0.86) |
− 0.05 (0.10) |
0.07 |
| FEV1 (l), mean (SD) |
2.88 (0.86) |
2.85 (0.86) |
− 0.03 (0.18) |
3.00 (0.77) |
3.00 (0.77) |
0 (0.09) |
0.99 |
| FEV1/FVC %, mean (SD) | 76% (10%) |
75% (11%) |
−1 % (3%) |
76% (11%) |
77% (10%) |
1% (2%) |
0.12 |
| ACT score, median(25th–75 %ileth | 20 (18–23) |
22 (20–23) |
0 (0–1.5) |
21 (20–22) |
21 (19–22) |
0 (−1.0–1.3) |
0.43 |
| Mini Rhinitis Quality of Life Questionnaire Score | 1.5 (0.8–2.3) |
1.4 (0.8–1.7) |
−0.3 (−0.6–0.04) |
1.6 (1.1–2.2) |
1.5 (0.7–2.3) |
−0.3 (−0.5–0.04) |
0.76 |
ranksum test comparing difference between pre and post measurements between BS and AS groups
Effect of BS ingestion on SFN levels and phase II enzyme gene expression
We measured transcript levels of NRF2 target antioxidant genes HMOX-1, NQO1, GCLC and GCLM in nasal epithelial cells as a measure of SFN activity in a target tissue relevant to asthma. There were no differences in the expression of any of these antioxidant genes between the treatment groups from pre-to post-treatment measurement (Figure 4A). We also examined antioxidant gene expression in PBMCs to assess whether SFN appeared to have activity in circulating cells. Similar to the findings with respect to nasal epithelial antioxidant gene expression, there was no significant difference between the groups (Figure 4B).
Figure 4.
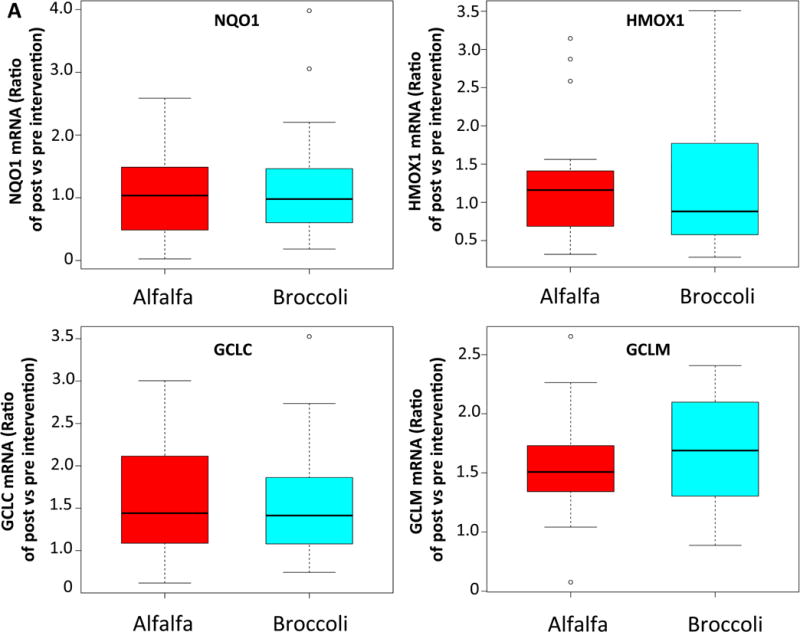
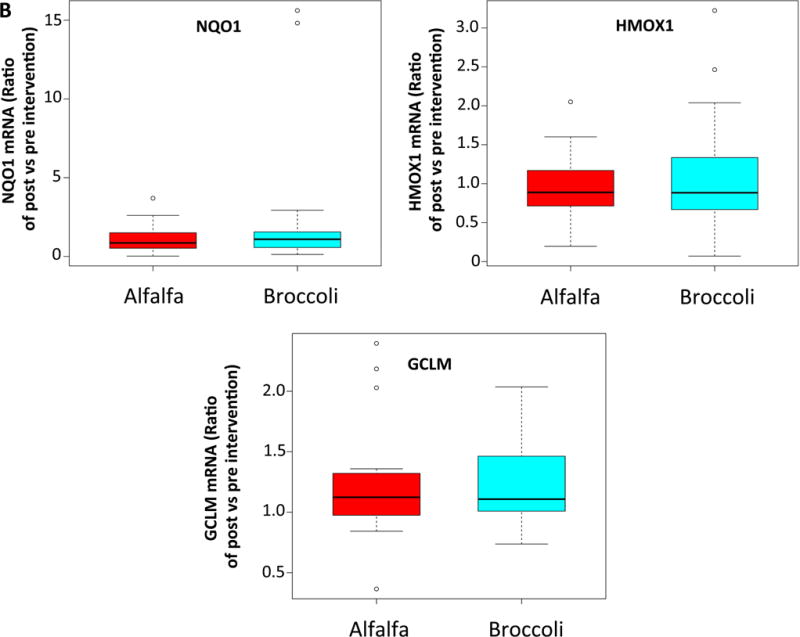
BS consumption failed to upregulate anti-oxidant gene expression in nasal epithelial cells and peripheral blood mononuclear cells. Boxplots showing relative change in the phase 2 enzyme gene expression from baseline to post-intervention in nasal epithelial cells (A) and peripheral blood mononuclear cells (B).
Effect of BS ingestion on biomarkers of oxidative stress and inflammation
We measured urinary isoprostane and serum TBARS levels as in vivo biomarkers of lipid peroxidation levels. Protein carbonyl levels, a marker of protein oxidation, and total antioxidant capacity in the serum samples from BS and AS group were also determined. Similar to the gene expression data, we observed no significant reduction in lipid peroxidation or protein oxidation or increase in total antioxidant capacity from baseline in the BS and AS group (Table III). Lastly, to evaluate the BS ingestion induced changes in the expression of serum inflammatory cytokines, we measured the levels of IL-4, IL-13 and IL-6. Consistent with the gene expression and oxidative stress biomarkers data, serum cytokine levels were not changed with ingestion of BS (Table III).
Table III.
Effect of Whole Broccoli Sprouts on oxidative and inflammatory biomarker outcomes.
| Alfalfa Sprouts | Broccoli Sprouts | p-value* | |||||
|---|---|---|---|---|---|---|---|
| Pre Ingestion |
Post Ingestion |
Difference (post-pre) |
Pre Ingestion |
Post Ingestion |
Difference (post-pre) |
||
| Urine isoprostane (pg/mg creatinine) | 598 (393– 787) |
698 (413– 1117) |
0.8 (−400 –358) |
719 (470– 833) |
754 (502– 1146) |
28 (−246– 271) |
0.78 |
| Serum cytokines (pg/ml) | |||||||
| IL - 4 | 1.8 (0.7–2.2) |
2.0 (1.3–2.6) |
0.5 (0.3–1.0) |
1.4 (0.8–2.3) |
2.2 (1.1–3.1) |
0.6 (0.1–1.0) |
0.97 |
| IL - 6 | 1.6 (0.5–2.6) |
1.3 (0.4–2.8) |
0.0 (−1.5–0.5) |
0.6 (0.1–1.6) |
0.7 (0.0–1.4) |
−0.1 (−0.6–0.0) |
0.51 |
| IL-13 | 0.6 (0.0–10.9) |
1.1 (0.0 – 10.0) |
0.0 (−0.4–0.7) |
0.9 (0.2–8.8) |
1.8 (0.2–9.7) |
0.3 (0.0 – 1.5) |
0.28 |
| TBARS (nanomole MDA/ml) | 6.1 (4.6–8.1) |
6.0 (4.9–7.2) |
0.5 (−0.1–1.3) |
7.1 (6.3–8.0) |
7.5 (6.5–9.2) |
0.1 (−0.4–1.5) |
0.79 |
| Protein carbonyls (nanomole/mg protein) | 1.2 (1.0–1.6) |
1.2 (1.1–1.3) |
0.0 (−0.4–0.2) |
1.5 (1.2–1.6) |
1.4 (1.2–1.5) |
0.0 (−0.2–0.1) |
0.86 |
| Total antioxidant capacity (mM rolox equivalents) | 1.2 (1.0–1.2) |
1.2 (1.1–1.3) |
0.1 (−0.1–0.2) |
1.3 (1.1–1.5) |
1.4 (1.3–1.6) |
0.2 (−0.0–0.3) |
0.26 |
Results presented as median (25th–75th percentiles)
ranksum test comparing difference between pre and post measurements between BS and AS groups
Discussion
In this first randomized double blind, placebo controlled trial of whole broccoli sprouts in atopic asthmatic adults, we showed that pulmonary inflammation, as measured by exhaled nitric oxide is not reduced. Ingestion of broccoli sprouts also fails to induce cytoprotective anti-oxidant gene expression, improve lung function or reduce asthma symptoms. We confirmed that consumption of BS leads to increases in serum SFN levels, and in spite of this increase there is no apparent effect on anti-oxidant genes in PBMCs or nasal epithelial cells, systemic oxidative stress or inflammatory markers. These findings suggest that although short-term ingestion of 100g of BS increased circulating SFN, it is not sufficient to induce protective biologic or clinical effects.
Several studies, in animal models and in humans, have reported that consumption of SFN-rich BS results in effective induction of phase II enzymes10, 24, which results in an increased capacity to fend off oxidative insults. BS are highly enriched for precursors of SFN, which activates a key regulator of the antioxidant response, Nrf2. Pharmacologic activation of Nrf2 signaling has been shown to reduce airway hyper-responsiveness and eosinophilic inflammation in the murine airways after allergen challenge25, 26. BS and SFN have been shown to induce Phase-II antioxidants primarily by activation of Nrf2 signaling. Riedl et al reported that oral SFN consumption in healthy volunteers for 3 days lead to induction of phase II enzymes in nasal lavage cells reflective of dose dependent induction in the upper airways10. Another group reported that the consumption of SFN rich BSE orally for 4 days followed by intranasal challenge with diesel exhaust particles significantly attenuated the inflammatory response and increase in total white blood count in nasal lavage cells following diesel exhaust particle challenge11. Similarly, another trial reported protective effects of BS homogenate against inflammation induced by live attenuated influenza virus. BS ingestion was associated with reduced viral titres and inflammation in smokers without any significant or sustained activation of Phase-II antioxidant genes12.
In contrast, our study found no effect on either antioxidant gene expression in PBMCs or nasal epithelial cells and no effect on FENO, a marker of airway inflammation, among atopic adults with asthma. Although the reasons for the differing results are not clear, there are several notable differences between our study and the studies above. First, our study’s intervention was ingestion of whole broccoli sprouts, rather than an extract or homogenate as has been used in other studies. It is possible that delivery of SFN precursors and conversion to SFN is less efficient with whole BS than with the other preparations that are designed to maximize delivery of SFN. However, there were marked increases in serum SFN levels following ingestion of whole BS, although it is possible that the concentrations of SFN were not sufficiently high to have biologic activity. The total SFN conjugate levels detected in our study were similar to some of the published studies, although a lot of variability exists in the SFN conjugate/metabolite measured as well as timing and methods of detection, making it difficult to compare systemic SFN levels among studies10, 11, 27. Second, our study was conducted in adults with atopic asthma and the other studies were all conducted in healthy subjects, so it is possible that the dose and/or duration of treatment needed among a population of asthmatics, who may have impaired activation of Nrf2 and induction of the antioxidant pathway, may be greater than that needed for healthy adults. It is important to note, though, that our intervention was similar in dose and duration to the intervention in the Riedl study (3 days of 100g of BS in our study compared to 3 days of 25–200g BS homogenate in the Riedl study). Last, our study evaluated lower airway outcomes, while the studies above did not, so it is possible that the effect of BS on lower airway inflammation is less pronounced than its biologic and antioxidant effects on the upper airways. However, our study also did not see biologic effects in circulating PBMCs or upper airway epithelial cell gene expression, suggesting that this is an unlikely explanation for the differences between our study and the others. Although there was no observed effect of BS on symptom-based outcomes, the tools used capture symptoms in the previous 4 weeks (ACT) and previous 1week (mini-RQLQ) and the study design included an intervention that lasted three days, a much shorter period of time than captured by these tools.
One study, however, has evaluated the effect of BS extract on airway hyper-responsiveness among adults with asthma. In this study, there was no overall effect of daily ingestion of BSE for 14 days on airway hyper-responsiveness. A post-hoc analysis, however, indicated that BSE ameliorated the bronchoconstrictor effects of methacholine in 60% of the participants, but aggravated the effects among 20% of the participants28. Although the study lacked a placebo group, leaving the findings difficult to interpret, the heterogeneity of the lower airways response to BSE suggests that there are genetic factors that determine whether an individual is likely to have a therapeutic or adverse response to BSE. Although we did not observe any asthma-related adverse events associated with ingestion of BS, we cannot exclude the possibility that some subpopulation of adults with atopic asthma could have a beneficial – or even harmful - effect from ingestion of whole BS. For example, polymorphisms in Glutathione S transferases could affect responsiveness to an antioxidant such as whole BS29, 30. It is also possible that long-term corticosteroid use may have affected responsiveness to the biologic effects of whole BS. However, we excluded asthmatics who had taken oral corticosteroids in the two weeks prior to BS ingestion.
In terms of dosing of BS, Atwell et al recently reported that consuming BS twice in a single day results in a higher steady-state SFN levels in humans than consuming an equivalent dose all at once. In that study, subjects consumed whole BS each providing 200 μmol of SFN (which is approximately equivalent to 127gm of BS) daily as a single dose or as two 100 μmol doses taken 12h apart27. Two doses, 12h apart compared to consuming a single dose, resulted in higher plasma concentrations of SFN metabolites 24 h post-consumption. However, neither 200 μmol once daily or 100 μmol twice daily induced significant antioxidant gene expression in blood27, suggesting that more frequent dosing of whole BS would not result in biologic activity. This study, like ours, suggests that whole BS, despite resulting in marked increases in serum SFN, has little biologic activity in doses that could reasonably be consumed by an adult. Furthermore, unlike our study, several studies of BS rely on pre- and post-measurements of biomarkers in the same participants without a blinded placebo controlled study.
Taken together, our findings suggest that consuming a large serving of whole BS on a daily basis has no biologic effect on airway antioxidant gene expression or inflammation in adults with atopic asthma. Our study addressed the question of the effect of whole BS on biologic markers among adults with established asthma, and not the question of their effect on the risk of developing asthma, so this latter question remains to be answered. Future studies of the effect of diet on asthma should focus on other dietary components or patterns, while additional studies focused on BS (or its active ingredients) should aim to reproduce previous work that has found that they upregulate anti-oxidant gene expression in airway epithelium.
Highlights.
What is already known about this topic?
Broccoli sprouts are rich source of a potent antioxidant, sulforaphane, and a robust antioxidant response is thought to protect the airways from inflammation.
What does this article add to our knowledge?
In this double-blind, placebo-controlled trial of broccoli sprouts in adults with asthma, short-term ingestion of 100 grams of broccoli sprouts increased blood sulforaphane levels, but did not reduce airway inflammation or induce protective biologic effects.
How does this study impact current management guidelines?
Although broccoli sprouts are commercially available and purported by some to have beneficial health effects, eating large amounts for three consecutive days had no effects on the airway despite increasing blood sulforaphane levels.
Acknowledgments
The trial was registered at Clinicaltrials.gov: NCT01183923
Funding: P01 ES018176; R01 ES023447; K24 AI114769; P50ES015903
Abbreviations
- ACT
Asthma control test
- AS
Alfalfa Sprouts
- BS
Broccoli Sprouts
- BSE
Broccoli Sprouts extract
- ED
Emergency department
- FENO
Fractional exhaled nitric oxide
- FEV
Forced expiratory volume
- FVC
Forced vital capacity
- Gclc
Glutamate-cysteine ligase catalytic subunit
- Gclm
Glutamate-cysteine ligase modifier subunit
- HMOX1
Hemeoxygenase-1
- ICS
inhaled corticosteroids
- IL4
Interleukin-4
- IL-6
Interleukin 6
- IL13
Interleukin -13
- LABA
Long acting beta agonist
- mini RQLQ
mini Rhinitis Quality of Life Questionnaire
- Nrf2
Nuclear factor erythroid-2-related factor 2
- NQO1
NADPH Quinone Oxidoreductase 1
- PBMC
peripheral blood mononuclear cells
- PPB
parts per billion
- SFN
Sulforaphane
- TBARS
Thiobarbituric acid reactive substances
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet. 2002;360:1313–22. doi: 10.1016/s0140-6736(02)11312-2. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 4.Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15:308–22. doi: 10.1038/nri3830. [DOI] [PubMed] [Google Scholar]
- 5.Scott HA, Jensen ME, Wood LG. Dietary interventions in asthma. Curr Pharm Des. 2014;20:1003–10. doi: 10.2174/13816128113190990421. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med. 1990;9:235–43. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- 7.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–3. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 8.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruiz NI, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–9. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 9.Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96:534–43. doi: 10.3945/ajcn.111.032623. [DOI] [PubMed] [Google Scholar]
- 10.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–51. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, et al. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 2014;5:35–41. doi: 10.1039/c3fo60277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN, et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One. 2014;9:e98671. doi: 10.1371/journal.pone.0098671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu M, Seltzer TF. Myxedema coma induced by ingestion of raw bok choy. N Engl J Med. 2010;362:1945–6. doi: 10.1056/NEJMc0911005. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005;115:1130–6. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Cardinale F, de Benedictis FM, Muggeo V, Giordano P, Loffredo MS, Iacoviello G, et al. Exhaled nitric oxide, total serum IgE and allergic sensitization in childhood asthma and allergic rhinitis. Pediatr Allergy Immunol. 2005;16:236–42. doi: 10.1111/j.1399-3038.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 18.Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60:215–8. doi: 10.1136/thx.2004.023374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 20.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–57. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen M, Klopping-Ketelaars IW, van den Berg R, Vaes WH. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–9. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 22.Oliviero T, Verkerk R, Vermeulen M, Dekker M. In vivo formation and bioavailability of isothiocyanates from glucosinolates in broccoli as affected by processing conditions. Mol Nutr Food Res. 2014;58:1447–56. doi: 10.1002/mnfr.201300894. [DOI] [PubMed] [Google Scholar]
- 23.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009;2:353–60. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 25.Sussan TE, Gajghate S, Chatterjee S, Mandke P, McCormick S, Sudini K, et al. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am J Physiol Lung Cell Mol Physiol. 2015;309:L27–36. doi: 10.1152/ajplung.00398.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH, Kim JW, Lee CM, Kim YD, Chung SW, Jung ID, et al. Sulforaphane inhibits the Th2 immune response in ovalbumin-induced asthma. BMB Rep. 2012;45:311–6. doi: 10.5483/bmbrep.2012.45.5.311. [DOI] [PubMed] [Google Scholar]
- 27.Atwell LL, Hsu A, Wong CP, Stevens JF, Bella D, Yu TW, et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol Nutr Food Res. 2015;59:424–33. doi: 10.1002/mnfr.201400674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RH, Reynolds C, Brooker A, Talalay P, Fahey JW. Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res. 2015;16:106. doi: 10.1186/s12931-015-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–91. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 30.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]


