Abstract
The members of the TGF-β superfamily play a key role in regulating developmental and homeostasis programs by controlling differentiation, proliferation, polarization and survival of different cell types. Although the role of TGF-β1 in inflammation and immunity is well evident, the contribution of other TGF-β family cytokines in the modulation of the antitumor immune response remains less documented. Here we show that activin A triggers SMAD2 and ERK1/2 pathways in dendritic cells (DC) expressing type I and II activin receptors, and up-regulates production of the TNF-α family cytokines BAFF (TALL-1, TNFSF13B) and APRIL (TALL-2, TNFSF13A), which is blocked by SMAD2 and ERK1/2 inhibitors, respectively. BAFF and APRIL derived from activin A-treated DC up-regulate proliferation and survival of T cells expressing the corresponding receptors - BAFF-R and TACI. In vivo, activin A stimulated DC demonstrate a significantly increased ability to induce tumor-specific CTL and inhibit the growth of melanoma and lung carcinoma, which relays on DC-derived BAFF and APRIL since knockdown of the BAFF and APRIL gene expression in activin A-treated DC blocks augmentation of their antitumor potential. Though systemic administration of activin A, BAFF or APRIL for the therapeutic purposes is not likely due to the pluripotent effects on malignant and non-malignant cells, our data open a novel opportunity for improving the efficacy of DC vaccines. In fact, a significant augmentation of the antitumor activity of DC pre-treated with activin A and the proven role of DC-derived BAFF and APRIL in the induction of antitumor immunity in vivo support this direction.
Keywords: antitumor immunity, DC vaccines, melanoma, lung cancer, activin receptors
Introduction
The TGF-β superfamily is a group of ~30 structurally related polypeptides: TGF-β, bone morphogenetic proteins, activins, inhibins, nodals, growth differentiation factors and glial-derived neurotrophic factors. They are expressed in different tissues and function during the early development and in adults regulating stem cell differentiation, organ morphogenesis, tissue remodeling and reproduction (1). The TGF-β family members act as the dimeric ligands by binding to type I and II receptors, forming hetero-tetrameric complexes and signaling via a cytoplasmic Ser/Thr kinase domain in the canonical SMAD and non-canonical SMAD-independent pathways (2). TGF-β1 is a well-known immunomodulatory cytokine that, in the tumor microenvironment, inhibits the antitumor activity of NK cells, cytotoxic T-cells, macrophages and dendritic cells (DC) and up-regulates the tumor-promoting activity of regulatory T (Treg) cells and myeloid-derived suppressor cells (3–10). However, significantly less is known about the immunomodulatory activity of other members of the TGF-β family.
Activin A (ActA), a member of the TGF-β superfamily sharing the SMAD signaling pathway with TGF-β, was identified as gonadal protein that stimulated FSH release from pituitary (11), but now known to be involved in neuroprotection, wound healing, apoptosis, tissue repair and inflammation (12–14). ActA is a key regulator of B-cell development and, similar to TGF-β, can induce apoptosis in B lymphoid cells. It inhibits NK cells by suppressing cell proliferation, IFN-γ production and chemokine expression (15). ActA can induce the synthesis of prostanoids, NO and IL-1β in resting macrophages, but in the presence of LPS, ActA down-regulates iNOS and release of IL-1β (14). ActA can also function as a Th2 cytokine promoting the alternative activation of M2 macrophages (16).
Less is known about the effect of ActA on functioning of DC, the most powerful antigen-presenting cells responsible for initiating adaptive immunity. ActA affects the generation of human DC and impairs the induction of T-cell proliferation, similar to the effect of TGF-β and dexamethasone (17). Under inflammatory conditions, ActA promotes differentiation of human monocyte into DC and Langerhans cells similarly to TGF-β (18,19). It can also counteract the expression of HLA-DR on human DC induced by IL-1β, IL-6 and TNF-α (17). However, neither the effect nor the mechanism of ActA action on the hematopoietic precursor-derived DC and the ability of DC to induce antitumor immune responses have been investigated.
Here we show that bone marrow-derived DC express type I and II ActA receptors and upon treatment with ActA produce and release the TNF family ligands APRIL and BAFF, which is mediated by ERK1/2 and SMAD2 signaling, respectively. DC-derived APRIL via TACI receptors up-regulates proliferation of T-cells while DC-derived BAFF via TACI and BAFF receptors up-regulates survival of activated T-cells. In both melanoma and lung carcinoma models, administration of ActA-treated DC significantly inhibits tumor growth and prolongs animal survival, which is blocked if BAFF and APRIL production in DC is precluded by specific shRNA. These findings provide the first evidence for a novel role of ActA in augmenting the immunoprotective and immunostimulating effects of DC on T-cells in vitro and in vivo via the TNF family ligands, as well as the antitumor potential of DC.
MATERIAL AND METHODS
Animals
6–8-week old male C57BL/6 mice (Taconic) were housed under the standard controlled conditions with food and water available at libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee and performed in accordance with the U.S. Public Health Service policy.
Cell cultures and treatments
Murine 3LL Lewis lung carcinoma and B16 melanoma cell lines (ATCC) were received in 2015. For cell line authentication, ATCC utilizes Short Tandem Repeat (STR) profiling by amplifying 17 STR loci plus Amelogenin using Promega’s PowerPlex 18D System. Tumor cells were maintained in a complete RPMI 1640 medium (CM) (GIBCO BRL) supplemented with non-essential amino acids, 10% FBS, 2 mM L-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin.
DC were generated from bone marrow hematopoietic precursors isolated from femur and tibia after depletion of erythrocytes, T and B lymphocytes and monocytes/macrophages. The enriched population of DC precursors (1×106 cells/ml) was cultured in CM containing rmGM-CSF and rmIL-4 (1000 U/ml, Miltenyi Biotec). Day5 DC were treated for 48h with ActA (100 and 500 nM, R&D Systems) and a solvent (control). DC were harvested on Day7 and used in other experiments.
For phenotyping, DC were washed in FACS medium (HBSS containing 0.1% BSA and 0.1% NaN3), stained with appropriately diluted antibodies and fixed in 2% paraformaldehyde. The following antibodies were used: anti-mouse MHC class II, CD80, CD86, F4/80, CD40 and CD11c (PharMingen).
Cytokines, chemokines and growth factors were measured in control and treated DC cultures using Luminex-based Multiplexed assay. BAFF and APRIL secretion by DC was determined by specific ELISA (MyBioSource).
T-cell analysis
T-cells were enriched from splenocytes by passage through the nylon wool columns (Polyscience) (pilot experiments) or cell sorted by CD3-based negative selection (Miltenyi Biotec kits) followed by the 48h treatment with: (i) control DC or DC/ActA, (ii) 20, 100 and 500 ng/ml rmBAFF (R&D Systems), (iii) 20, 100 and 500 ng/ml rmAPRIL (R&D Systems). The purity of cell sorted T cells, analyzed by flow cytometry, was >98%. T-cell phenotype, function, proliferation and apoptosis were then assessed. B-cells, used as a control in receptor expression experiments, were cell sorted from splenocytes using Miltenyi Biotec kits.
APC-labeled anti-mouse CD25, FITC-labeled CD4 and CD8 antibody and PE FoxP3 flow Kit (BioLegend) were used to determine Treg cells. APC-labeled anti-BAFFR and anti-TACI antibody (BioLegend) were used to determine the expression of BAFF and APRIL receptor on T cells. APC and FITC RatIgG1 isotype controls were used at the concentrations (1μg/1×106T cells/100μl). For apoptosis studies, Con A (2.5μg/ml, 5h, Sigma) pre-activated T-cells were washed and treated with DC, BAFF and APRIL and double stained with FITC-Annexin V (PharMingen) and APC-conjugated CD3, CD4 or CD8 (Biolegend). Early apoptosis was determined as the percentage of Annexin V+APC+ cells by FACScan with Cell Quest software package (Becton Dickinson).
T-cell proliferation was assessed after the treatment with DC, BAFF and APRIL and activation with Con A. T-cells were seeded at 2×105/100μl in 96-well U-bottom plates. DC were added in triplicates at 1:30 and 1:100 DC:T cell ratio to the total volume of 200 μl. To neutralize APRIL and BAFF, rmBAFF R/TNFRSF13C Fc chimera (R&D Systems) and rmTACI-Fc chimera (Biolegend) were used (2.5 and 10 ng/ml). T-cell proliferation was measured by uptake of 3H-thymidine (1 μCi/well, 5 Ci/mmol; DuPont-NEN) pulsed for 16–18h after two days in cultures. Cells were harvested on GF/C glass fiber filters (Whatman Intl. Ltd) using MACH III harvester (Tomtec). 3H-thymidine incorporation was determined on MicroBeta TRILUX scintillation counter (WALLAC) and expressed as count per minute (cpm).
RT-PCR
RNA was extracted from DC and T-cells using TRIzol LS reagent (Molecular Research Center). First-strand cDNA was reverse transcribed with MMLV reverse transcriptase (Gibco) and oligo d(T)16 (Roche Applied Science) from 1μg of total RNA. PCR was performed using specific primers (IDT Integrated DNA Technologies) for ALK2, ALK4, ACTRIIB (ActA receptors), SMAD2 and SMAD4 expression in DC. Specific primers for TACI and BAFF-R were used to measure the expression of BAFF and APRIL receptors in T and B-cells. GAPDH transcript was used as an internal control. The PCR products were analyzed in 1.2% agarose gel containing ethidium bromide. A DNA ladder II (GeneChoice, Inc.) was used as a size marker.
Western blot
The following primary antibodies were used: Total SMAD2 and Phospho-SMAD2 (1:1000, Cell Signaling), Total ERK1/2 and Phospho-ERK1/2 (1:1000, Cell Signaling), APRIL (2μg/ml, Cell Signaling), BAFF (0.5μg/ml, EMD Millipore), ALK2 (ActRIA) (1μg/ml), ALK4 (ActRIB) (1μg/ml), ActRIIB (1:200, Abcam) and β-actin (1:50000, Sigma). For detection of soluble BAFF and APRIL, DC culture medium was precipitated with 1.5% sodium desoxycholate/60% trichloracetic acid (Sigma) and re-suspended in lysis buffer and Laemmli sample buffer. Samples from 1×106 cells were normalized by protein concentration, resolved by electrophoresis on 4–12% SDS-PAGE and transferred to PVDF membranes (Invitrogen). For quantitation, gels were scanned and analyzed by UN-SCAN-IT Gel software (Silk Scientific, Inc.). The bands were quantified by pixel density using β-actin as a control.
Experimental design in vivo
B16 and 3LL cells (5×104/100μl) were inoculated s.c. in flank of mice in three groups: (i) control (tumor cells, Day1), (ii) tumor cells+control DC (1×106cells/mouse, s.c., Day1 and Day7), (iii) tumor cells+DC/ActA (1×106cells/mouse, s.c., Day1 and Day7). The tumor size was expressed as the tumor area (mm2). Survival of animals was also determined. To evaluate the role of DC-derived APRIL and BAFF in vivo, the APRIL and BAFF genes were knockdown in DC with 1 and 5 MOI of APRIL/TALL-2 and BAFF/TALL-1 shRNA Lentiviral particles (Santa Cruz Biotechnology) and Polybrene (5 μg/ml) as a transfection agent. shRNA Lentiviral particles encoded a scrambled shRNA served as a control. Transfection was conducted according to the manufacturer’s protocol, and its efficacy was determined by Western blot. DC/ActA cells (1×106cells/mouse, s.c.) with the knockdown APRIL and BAFF genes were injected as described above (group four). All studies consisted of 5–7 mice per group and were repeated two times.
IFN-γ assay
For evaluation of tumor-specific CTL response, splenic T-cells (1×106/ml) from control and DC-vaccinated tumor-bearing mice were stimulated with irradiated (300 Gy) 3LL or B16 cells (0.2×106/ml). Cell-free supernatants were collected 48h later, and IFN-γ levels were determined by ELISA (R&D Systems). Supernatants from non-stimulated T-cells were used for assessing spontaneous cytokine production.
Statistical analysis
For a standard comparison of two groups, the Student t-test was used after evaluation of normality. If data distribution was not normal, a Mann-Whitney rank sum test was performed. For the comparison of multiple groups, analysis of variance was applied. SigmaStat Software was used for data analysis (SyStat Software, Inc.). For all statistical analyzes, p<0.05 was considered significant. All experiments were repeated at least two times. Data are presented as mean±SEM.
RESULTS
DC express activin A receptors and respond to activin A stimulation
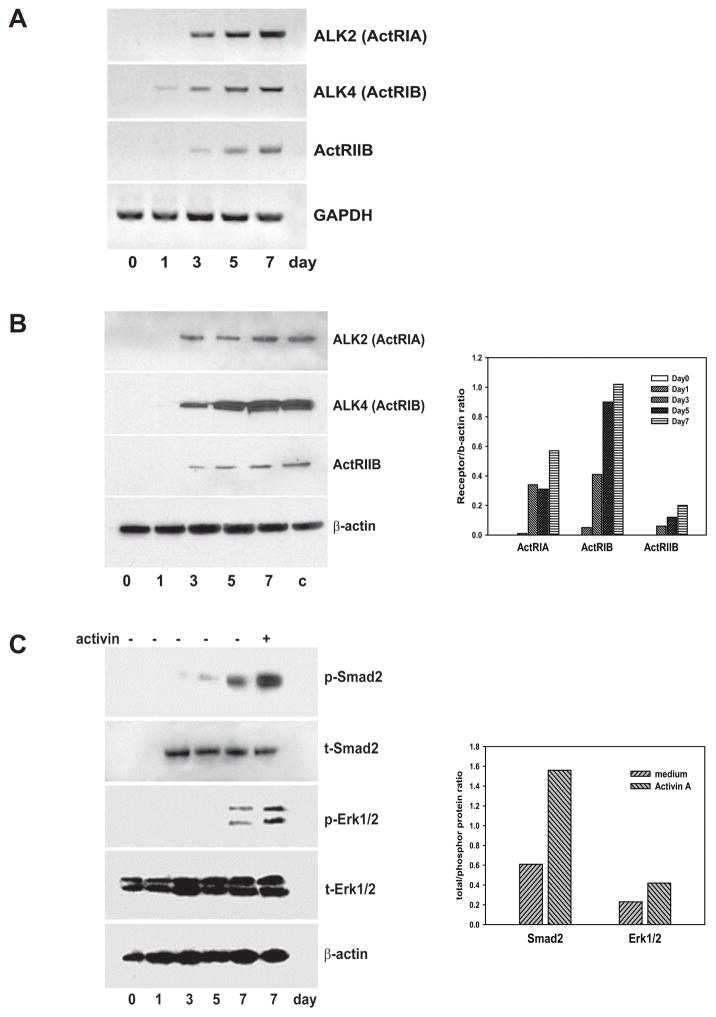
Analysis of ActA receptor expression during DC differentiation revealed the expression of both activin type I (ALK4 and ALK2) and type II (ACTRIIB) receptor mRNA on DC starting from Day3 in DC cultures (Fig. 1A). These results were confirmed by Western blot analysis showing expression of both types I and II receptor proteins in cultured DC (Fig. 1B). Importantly, DC isolated from the spleen expressed compatible levels of ActA receptors (Fig. 1B). Next, treatment of DC with ActA significantly activated both SMAD2 and ERK1/2 signaling determined by increased levels (up to 2–3 folds based on the densitometric enumeration, p<0.05) of phosphorylated SMAD2 and ERK1/2 proteins in treated DC (Fig. 1C).
Figure 1. DC express activin A receptors and respond to activin A stimulation by up-regulating phosphorylation of SMAD2 and ERK1/2.
DC cultures were initiated from the bone marrow precursors, and cells were harvested on Days 0,1,3,5 and 7. A. Expression of type I and II ActA receptor ALK2, ALK4 and ACTRIIB mRNA was measured by RT-PCR. GAPDH mRNA was used as an internal control. The results of a representative experiment are shown (n=3). B. Expression of type I and II ActA receptors were determined by Western blot (left panel) and evaluated by densitometric analysis (right panel). DC isolated from the spleen (c) served as a control. C. Total and phosphorylated SMAD2 and ERK1/2 proteins were analyzed by Western blot in non-treated Day0–7 DC and Day7 ActA-treated DC (100ng/ml, 48h) (left panel). Thirty μg of cell lysate protein were used for each probe. Quantitative analysis of relative band density and relative alteration of the proportion of phosphorylated protein (right panel) was performed as described in M&M. β-actin served as a housekeeping control. The results of one representative experiment out of three independent experiments showing similar results are shown.
These results demonstrate that bone marrow-derived DC express ActA receptors and respond to ActA treatment by increasing SMAD2 and ERK1/2 phosphorylation, which suggests that ActA may modulate functional activity of DC.
Activin A-treated DC up-regulate proliferation and survival of T-cells
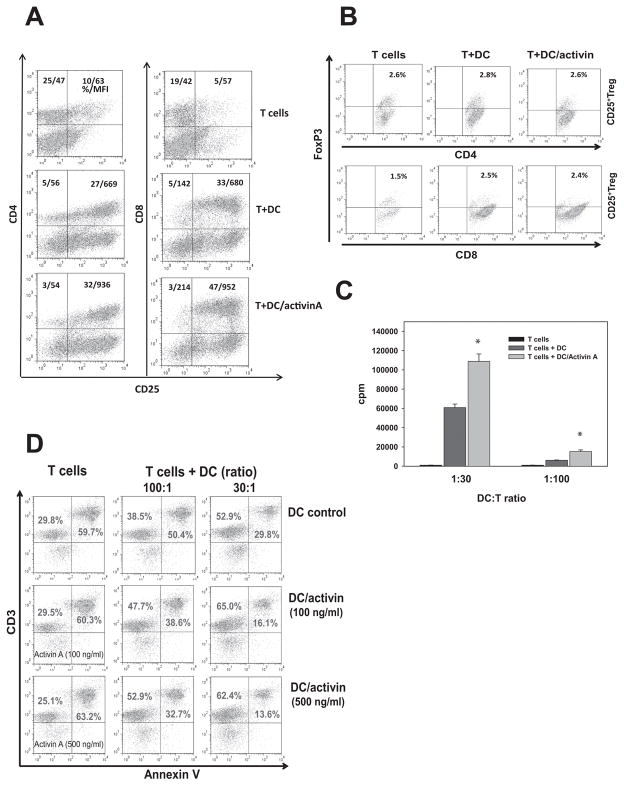
To determine whether ActA-treated DC affect T-cells, pre-activated T-cells were mixed with control and treated DC for 48h. The results demonstrated that ActA-treated DC significantly up to 40% (p<0.05) increased expression of the IL-2 receptor α-chain CD25 (MFI and the percent of positive cells) on CD8+ and CD4+ T-cells (Fig. 2A). This effect was not associated with up-regulation of CD25+FoxP3+ regulatory T-cells (Fig. 2B), as could be expected if the effect of ActA on DC is similar to the effect of TGF-β1. ActA added directly to T-cells did not change the expression of CD25 on CD8+ and CD4+ T-cells (supplemental Fig. S1).
Figure 2. Activin A-treated DC up-regulate activation (A), proliferation (C) and survival (D) of T cells in vitro.
Bone marrow-derived Day5 DC were treated with ActA (100 ng/ml, 48h) and mixed with ConA (2.5μg/ml) pre-activated splenic T-cells for 48h. Expression of CD25 (A) on CD4+ and CD8+ T-cells and expression of FoxP3 (B) on gated CD25+CD4+ and CD25+CD8+ T-cells was determined by flow cytometry. The results of one representative out of three independent experiments are shown. C. Proliferation of T-cells mixed with control and ActA-treated DC at different ratios was assessed by 3H-thymidine incorporation and expressed as a count per minute (cpm). *, p<0.05 (ANOVA, mean ± SEM, n=3). D. Control and ActA-treated (100 and 500ng/ml, 48h) DC were co-incubated with ConA pre-activated and washed T-cells at different ratios. Untreated and ActA-treated T-cells without DC (left column) served as a control. The level of CD3+ T-cell apoptosis was measured by Annexin V binding. The results from a representative experiment are shown (n=3).
Analysis of T-cells revealed that ActA-treated DC significantly increased proliferation of T-cells (p<0.05; Fig. 2C). Examination of T-cell survival revealed that ActA-DC were 2-fold stronger protectors of T-cells from activation-induced cell death than control DC. Figure 2D shows that the percentage of Annexin V+CD3+ cells in the non-treated and ActA-treated T-cells was the same and reached 63%. The addition of control DC decreased the level of apoptotic T-cells by 2.1-fold, while ActA-DC reduced T-cell death by 4-fold (DC:T ratio 1:30, p<0.05, n=3). Importantly, phenotypic analysis of ActA-DC revealed no changes in the expression of CD80, CD86, CD40 or MHC class II molecules, suggesting no alterations in DC maturation; no changes in CD11c or F4/80 were detected, suggesting the absence of DC de-differentiation into macrophages (supplemental Fig. S2).
Thus, ActA enhances the ability of DC to stimulate proliferation and decrease apoptosis of T-cells in vitro, which raises the question about factors and mechanisms responsible for these effects.
Activin A stimulates DC to produce BAFF and APRIL via SMAD2 and ERK1/2 pathways
To determine DC-derived factors responsible for up-regulation of T-cell proliferation and survival, production of G-CSF, Eotaxin, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-7, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IP-10, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNF-α and VEGF was assessed in control and ActA-DC. No significant differences in tested cytokine, chemokine or growth factor production between control and treated DC were detected (supplemental Table I).
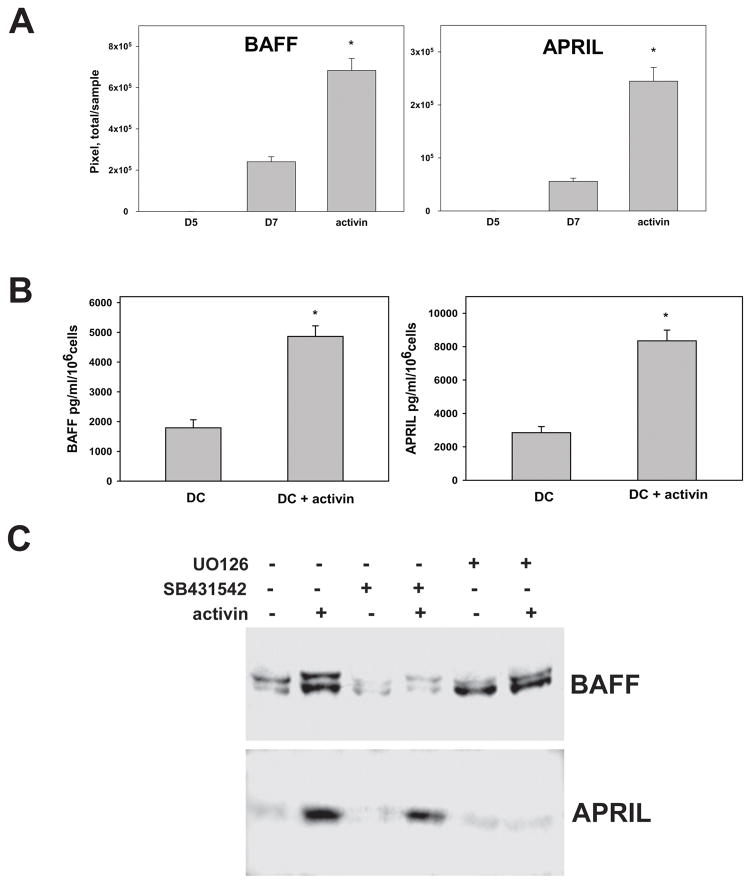
However, analysis of control and ActA-treated DC revealed that ActA increased secretion of the TNF superfamily proteins BAFF (TALL-1, TNFSF13B) and APRIL (TALL-2, TNFSF13A) up to 3–4 folds (p<0.05), as was determined by Western blot and ELISA (Fig. 3A,B). Importantly, pretreatment of DC with an inhibitor of SMAD2 phosphorylation SB431542 abrogated ActA-induced secretion of BAFF. An inhibitor of ERK1/2 activation UO126 blocked ActA-induced secretion of APRIL but did not change BAFF secretion from DC (Fig. 3C). These results suggest that ActA stimulates DC to release BAFF via SMAD2 and APRIL via ERK1/2 pathways.
Figure 3. Activin A up-regulates production of BAFF and APRIL in DC via SMAD2 and ERK1/2 pathways, respectively.
A. Cell-free culture medium from control (Day5 and Day7) and ActA-treated (100ng/ml, 48h) DC were precipitated with 1.5% sodium deoxycholate/60% trichloroacetic acid. The levels of BAFF and APRIL were measured by Western blot. BAFF and APRIL bands were quantified by densitometry as pixel/sample, and the results are presented as mean±SEM (*, p<0.05, ANOVA, n=4). B. DC were treated with medium and ActA (100ng/ml, 48h) and concentrations of BAFF and APRIL in cell-free culture supernatants were determined by ELISA (*, p<0.05, Student t-test, n=2). C. DC were pre-treated with an inhibitor of SMAD2 phosphorylation SB431542 (10μM), an inhibitor of ERK1/2 phosphorylation UO126 (20μM) or culture medium for 1h prior to the treatment with ActA (100ng/ml, 48h) or medium. DC supernatants were collected, precipitated and the levels of BAFF and APRIL were detected by Western blot. The representative results from three independent experiments are shown.
DC-derived BAFF and APRIL regulate T-cell activity
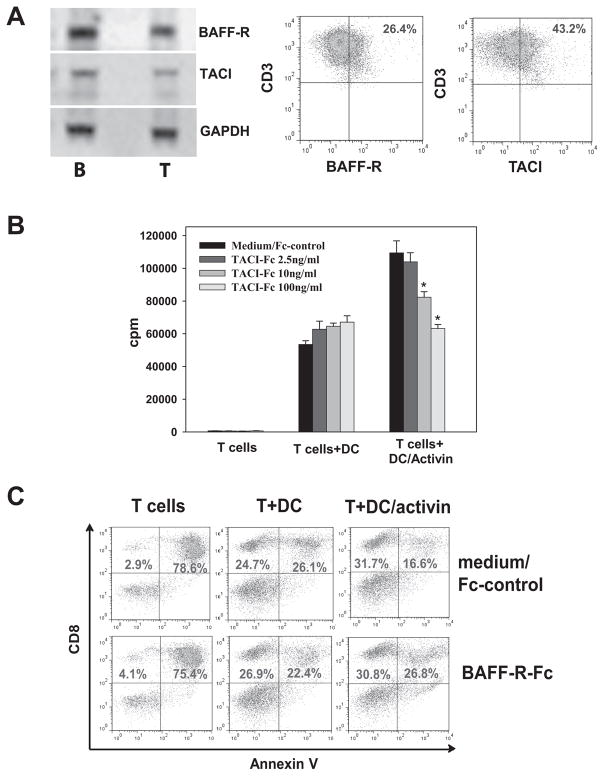
To test whether ActA-induced DC-derived BAFF and APRIL affect T-cells, expression of their receptors on T-cells was determined. Figure 4A shows that T-cells express two BAFF receptors (BAFF-R and transmembrane activator and calcium modulator ligand interactor (TACI)) and APRIL receptor (TACI) mRNA and protein. Next, treatment of T-cells with rBAFF and rAPRIL (20–500ng/ml, 48h) demonstrated that rAPRIL (500ng/ml), but not rBAFF, increased proliferation of T-cells up to 2-fold (p<0.05, supplemental Fig. S3). However, rBAFF decreased apoptosis of both CD4+ and CD8+ T-cells by ~40% in low concentrations (20ng/ml), while rAPRIL only moderately decreased CD8+ T-cell apoptosis in significantly higher concentrations (500ng/ml) (supplemental Fig. S4). These data suggest that T-cells express BAFF and APRIL receptors, and APRIL stimulates T-cells proliferation while BAFF serves as a T-cell survival factor.
Figure 4. BAFF and APRIL derived from activin A-treated DC up-regulate T-cell proliferation and survival in vitro.
A. Expression of BAFF receptor mRNA (BAFF-R and TACI) and APRIL receptor mRNA (TACI) was assessed in splenic ConA-activated T-cells by RT-PCR. Splenic B-cells served as a control. Expression of BAFF-R and TACI on splenic CD3+ T-cells was determined by flow cytometry (right panels). B. DC-derived APRIL up-regulates proliferation of T-cells. DC were treated with ActA (100ng/ml, 48h) or medium, washed and co-cultures with ConA pre-activated T-cells for 48h. To neutralize DC-derived APRIL, different concentrations of TACI-Fc chimera were added to DC/T-cell co-cultures. T-cell proliferation was assessed by 3H-thymidine incorporation and expressed as a count per minute (cpm). *, p<0.05 (ANOVA, mean±SEM, n=3). Addition of medium and control Fc served as controls and resulted in identical alterations of T-cell proliferation. C. DC-derived BAFF protects T-cells from activation-induced cell death. DC were treated with ActA (100ng/ml, 48h) or medium, washed and co-cultures with ConA pre-activated T-cells for 48h. T-cell apoptosis was initiated by removing ConA mitogen from T-cell cultures prior to their co-culture with DC. To neutralize DC-derived BAFF, BAFF-R-Fc chimera was added to DC/T-cell co-cultures. Apoptosis was assessed by flow cytometry as the percentage of Annexin V+ cells among CD8+ T-cells. Addition of medium and control Fc served as controls and resulted in identical alterations of T-cell apoptosis. Results of one representative experiment out of three are shown.
The role of ActA-induced DC-derived APRIL and BAFF in up-regulation of T-cell activity was tested in the neutralizing experiments. The addition of TACI fusion proteins (TACI-Fc, 2.5–100ng/ml) to DC/T-cell co-cultures to neutralize secreted APRIL did not change the T-cell stimulatory capacity of control DC, but dose-dependently decreased the effect of ActA-DC on T-cell proliferation (p<0.05, Fig. 4B). This suggests that APRIL released from ActA-DC is responsible for up-regulation of T-cell proliferation in vitro.
Furthermore, addition of BAFF-R-Fc (10ng/ml) to the same cultures to neutralize DC-derived BAFF abrogated the ability of ActA-DC to further decrease activation-induced CD8+ T-cell apoptosis compared to control DC (Fig. 4C): 76.3±6.9% of AnnexinV+ cells in control T-cells, 24.8±4.6% in control DC/T-cells, 14.5±2.4% in ActA-DC/T-cells and 25.3±4.1% in ActA-DC/T-cells in the presence of BAFF-R-Fc protein (p<0.05). Similar results were obtained with CD4+ T-cells (supplemental Fig. S5). This suggests that BAFF released from ActA-DC is responsible for up-regulation of T-cell survival in vitro.
Together, our data demonstrate that ActA stimulates DC to express APRIL and BAFF, which in turn increase proliferation and survival of T-cells in vitro, respectively. Because the biological role of DC-derived BAFF and APRIL induced by ActA is unknown, we next tested whether BAFF and APRIL released from ActA-DC may contribute to the immunostimulatory potential of DC in vivo.
BAFF and APRIL from ActA-treated DC enhance the antitumor potential of DC in vivo
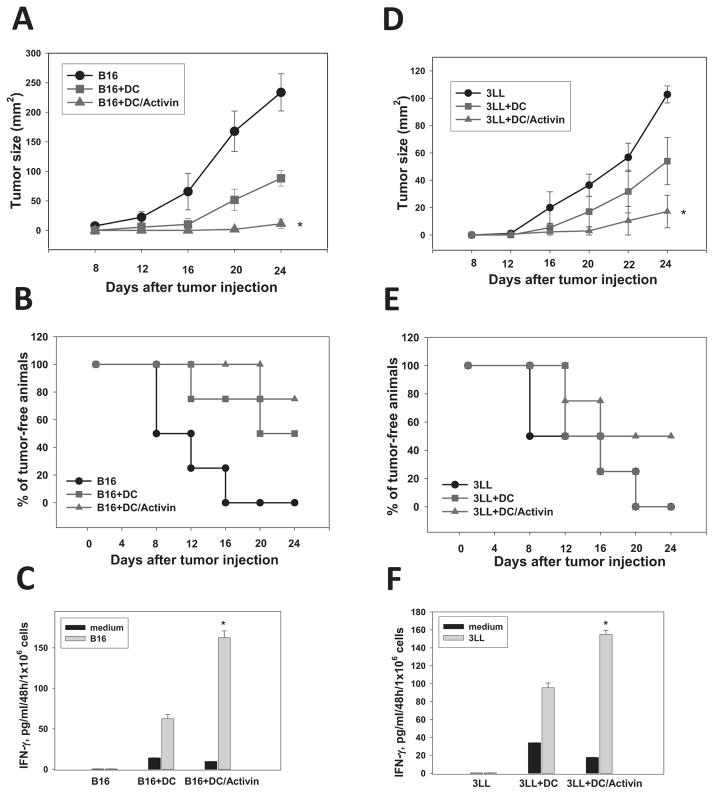
Evaluation of the antitumor potential of ActA-treated DC revealed that ActA significantly augmented the ability of DC to inhibit tumor growth in melanoma and lung carcinoma models (Fig. 5A,D). For example, on Day24 after B16 cell injection, the mean tumor size was 233.7±31.4 mm2 in non-treated mice, 88.2±13.3 mm2 in mice receiving control DC and 11.3±2.4 mm2 in animals injected with ActA-DC (p<0.05 vs all groups, n=3, Fig. 6A). Similar results were observed in the lung carcinoma model (Fig. 5D).
Figure 5. Activin A augments the antitumor efficacy of DC in melanoma and lung carcinoma models.
B16 (A,B,C) and 3LL (D,E,F) tumor cells (0.05×106) were inoculated s.c. in flank of C57BL/6 mice in three groups (5–7 mice/group) on Day1: (i) control (tumor cells only), (ii) control DC, (iii) ActA-DC. Control or ActA-treated DC (100ng/ml, 48h) (1×106cells/mouse) were injected s.c. on Day1 and Day7. The tumor size was expressed as the tumor area (mm2) and shown as mean±SEM (A, D). *, p< 0.05 vs groups (i) and (ii) (Two way ANOVA, n=3). Survival of animals was also determined (B,E). For evaluation of tumor-specific CTL, splenic T-cells were isolated on Day24 and stimulated with medium (control) or irradiated (30,000Rad) B16 or 3LL cells at 5:1 T-cell:tumor cell ratio. Supernatants were collected 48h later, and IFN-γ levels were determined by ELISA. Supernatants from non-stimulated T-cells were used for assessing spontaneous cytokine production (C,F) (*, p<0.05 vs. control DC, Two way ANOVA, n=3).
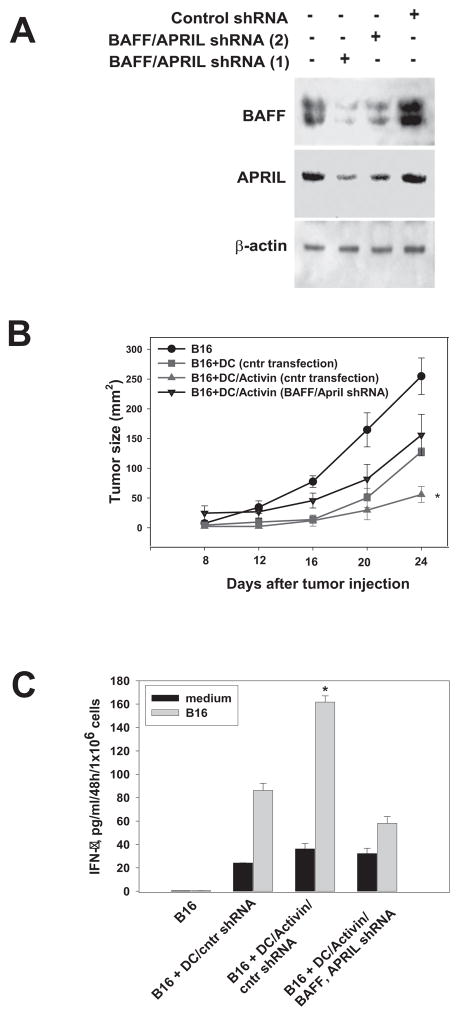
Figure 6. Knockdown of the BAFF and APRIL gene expression blocks augmentation of the antitumor potential of activin A-pretreated DC in vivo.
The APRIL and BAFF genes were knockdown in DC with 1 and 5 MOI of APRIL/TALL-2 and BAFF/TALL-1 shRNA Lentiviral particles and Polybrene (5μg/ml). shRNA Lentiviral particles encoded a scrambled shRNA served as a control. The efficacy of transfection was determined by the levels of protein expression using Western blot (A). (i) Control DC (scrambled shRNA), (ii) DC (scrambled shRNA) treated with ActA (100 ng/ml) and (iii) DC (BAFF+APRIL shRNA) treated with ActA were harvested 48h later, washed and injected in B16-bearing mice (see Fig. 5 legend). Control tumor-bearing mice did not receive DC injections. The tumor size was expressed as the tumor area (mm2) and shown as mean±SEM (B). *, p< 0.05 vs all other groups (two-way ANOVA). All studies consisted of 5–7 mice per group and were independently repeated two times. Tumor-specific CTL were evaluated as described in Fig. 5 legend, and IFN-γ levels were determined by ELISA. (C) (*, p < 0.05 vs other groups, Two way ANOVA, n=3).
Importantly, the strongest inhibition of tumor growth by ActA-DC correlated with animal survival and the generation of tumor-specific IFN-γ producing T-cells (Fig. 5B,C,D,E). For instance, on Day24, the percent of live animals in B16- and 3LL-bearing mice treated with ActA-DC reached 75% and 50%, respectively, compared to 50% and 0% in B16- and 3LL-bearing mice treated with control DC (p<0.05 for both models). 0% of mice were alive by Day 24 without DC injection (Fig. 5B,E). Similarly, Figures 5C,F show that splenic T-cells isolated from tumor-bearing mice treated with ActA-DC released the highest levels of IFN-γ upon stimulation with irradiated tumor cells: 162.5±8.4 pg/ml (B16) and 155.2±4.5 pg/ml (3LL) versus 62.5±6.2 pg/ml (B16) and 110.5±5.4 pg/ml (3LL) released by T-cells in mice treated with control DC (p<0.05 for both models). The level of IFN-γ production by T-cells isolated from tumor-bearing mice treated with saline was only 0.5±0.03 pg/ml/48h/106cells.
Next, the role of DC-derived BAFF and APRIL in the increased antitumor activity of ActA-DC was tested in B16 melanoma model using DC with blocked BAFF and APRIL expression. Figure 6A demonstrates four- and five-fold decrease in BAFF and APRIL protein expression, respectively, in ActA-DC after lentiviral transfection with BAFF and APRIL shRNA, but not after control shRNA transfection. Analysis of tumor growth revealed that transfection of ActA-DC with BAFF+APRIL shRNA completely abrogated their superior antitumor potential (Fig. 6B). For example, on Day24 after tumor cells injection, the mean tumor size in mice receiving saline was 261.2±38.5 mm2; in mice treated with ActA-DC/control shRNA the mean tumor size was 10-fold lower, but in mice treated with ActA-DC/BAFF+APRIL shRNA tumor size was 2.5 times higher than in the last group (p<0.05) and was similar to the tumor size in mice treated with control DC (Fig. 6B). Furthermore, BAFF and APRIL knockdown in injected ActA-DC resulted in 3-fold (p<0.05) decrease of tumor-specific CTL response (Fig. 6C). Thus, these data show that BAFF and APRIL gene silencing in ActA-DC blocks the ActA-induced up-regulation of the antitumor activity of DC in vivo in tumor-bearing animals.
In summary, the results of our in vitro and in vivo studies suggest that ActA via type I and II activin receptors on DC activates SMAD2 and ERK1/2 pathways resulting in up-regulated expression of BAFF and APRIL, which, in turn, up-regulate proliferation and survival of T-cells expressing BAFF-R and TACI; in vivo, ActA-stimulated DC demonstrate a significantly increased ability to induce tumor-specific CTL and inhibit tumor growth, which relays on DC-derived BAFF and APRIL.
Discussion
Here, we uncover an unexpected role for the TNF family cytokines BAFF and APRIL derived from DC treated with the TGF-β family member ActA as crucial drivers of DC-induced antitumor immune response in melanoma- and lung carcinoma-bearing hosts.
Two ligands, BAFF and APRIL, and three receptors, BAFF-R (TNFRS13C), BCMA (TNFRS17, only on B cells) and TACI (TNFRS13B) form the BAFF/APRIL system. BAFF/BAFF-R interaction is essential for both survival and maturation of immature B-cells. BAFF-deficient mice lack mature B-cells and are immunodeficient (20), whereas mice transgenic for BAFF have high numbers of mature B-cells and antibodies, including autoantibodies (21). APRIL/BAFF/TACI interaction is critical for T-cell-independent responses of B-cells to type I and II antigens and class-switch recombination of B-cells (22). Altered expression of BAFF or APRIL or their receptors has been reported in various B-cell malignancies including non-Hodgkin’s and Hodgkin’s lymphoma, CLL and multiple myeloma (23). Overexpression of BAFF and APRIL has been reported in solid tumors, including breast, colon and pancreatic cancers (24–26). In contrary, in prostate cancer, epithelial cell-derived BAFF protects lymphocyte survival and limits tumor expansion (27).
BAFF and APRIL are produced by macrophages, neutrophils, DC, B-cells, activated T- and NK cells, and also by intestinal epithelial cells, keratinocytes and osteoclasts (28–31). Although stimulation of BAFF and APRIL expression by IFN-α and IFN-γ in DC has been reported (32–34), up-regulation of BAFF expression by ActA has been shown only in the mouse macrophage and DC cell lines (35) and no data on the effect of ActA on APRIL expression in DC are available. Here we showed that bone marrow-derived DC express both types of ActA receptors and respond to ActA by producing BAFF and APRIL in SMAD2- and ERK1/2-dependent manner, respectively (Fig. 3). Our findings also revealed that DC-derived BAFF and APRIL may target T-cells by up-regulating their proliferation and longevity via BAFF-R and TACI expressed on T-cells. These new data suggest that, in addition to B-cells, T-cells should be considered as important targets for BAFF and APRIL. However, molecular signaling downstream of BAFF-R and TACI are only partly characterized and signaling in T-cells has not been investigated. Survival seems to be mediated by upregulation of the Bcl-2 family members through NF-κB activation and degradation of the pro-apoptotic Bim protein (30,36). Nothing is known about other signaling events associated with receptor engagement by BAFF and APRIL that lead for example to CD8+ T-cell priming or CD4+ T-cell polarization.
Our in vivo data revealed that prevention of BAFF and APRIL production in ActA-DC completely abrogated up-regulation of the antitumor potential of DC, which suggests that the local delivery of these cytokines by DC, presumably to T-cells, may stimulate T-cell priming and activation leading to augmented antitumor immune response. It is possible that the antitumor potential of DC-derived BAFF and APRIL in vivo is not limited by a direct activation of effector T-cells. Because BAFF and APRIL share two receptors – TACI and BCMA, and BCMA is expressed on B-cells, but not T-cells, one can suggest a potential role for B-cells in the antitumor effect of ActA-treated DC. B-cells may be involved in CTL priming, as TACI or BCMA on B-cells can bind to membrane-bound BAFF expressed on DC, and through a postulated reverse BAFF signaling (37), DC may gain the ability to prime CD8+ T-cells.
Involvement of BAFF and APRIL in the antitumor activity of ActA-treated DC is a new finding suggesting a new approach to enhancing the efficacy of DC vaccines. Interestingly, ActA has both oncogenic and tumor suppressor roles in cancer. For instance, in prostate and breast cancer ActA demonstrated tumor suppressive effects, while in lung and HNSCC, ActA expression correlated with increased proliferation and poor prognosis (38). ActA is also an anti-lymphangiogenic factor in melanoma (39). Although ActA levels were reported to be increased in patients with breast cancer (40) and in some mouse tumor models (41), new data showed that ActA protein in lung adenocarcinoma tissue was significantly lower than in normal lung tissue (42) and ActA may inhibit proliferation of breast cancer cell lines (43,44). It is likely that ActA can activate autocrine and paracrine signaling affecting crosstalk between the epithelial compartment and the surrounding microenvironment (45) in a cell-type and context-dependent manner supporting or inhibiting tumor development (38). Without better understanding the controversial role of ActA in cancer, the use of ActA as a systemic pharmacological agent appears not suitable (39). At the same time, this justifies investigations into utilization of ActA potential to modulate cancer vaccines ex vivo for improving their efficacy. It will be important to test the effect of ActA on DC activation in the presence of DC-stimulating agents commonly used in pre-clinical and clinical trials, since the effect of ActA on immature and mature DC might be different.
In summary, although inhibition of BAFF and APRIL or their receptors has been a strong focal point for therapeutic development, currently no data on the clinical activity in cancer are available (22). Systemic administration of ActA, BAFF or APRIL for the therapeutic purposes is not likely dues to a wide expression of their receptors on a variety of cells. However, as shown here, significant augmentation of the antitumor activity of DC treated with ActA and the proven role of DC-derived BAFF and APRIL in the induction of antitumor immunity open novel opportunity for improving the efficacy of DC vaccines.
Supplementary Material
Acknowledgments
This work was supported in part by NIH NCI RO1 CA154369 (to M.R.S.) and BSF award (to M.R.S.).
Footnotes
The authors state that there is no an actual, potential, or perceived conflict of interest with regard to the manuscript submitted for review.
References
- 1.Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley interdisciplinary reviews Developmental biology. 2013;2(1):47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- 2.Massague J. TGFbeta signalling in context. Nature reviews Molecular cell biology. 2012;13(10):616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends in immunology. 2010;31(6):220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nature reviews Cancer. 2013;13(5):328–41. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(9):2078–93. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine & growth factor reviews. 2010;21(1):49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(18 Pt 1):5262–70. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 8.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annual review of immunology. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 9.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25(3):455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. The Journal of experimental medicine. 2005;201(7):1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robson NC, Phillips DJ, McAlpine T, Shin A, Svobodova S, Toy T, et al. Activin-A: a novel dendritic cell-derived cytokine that potently attenuates CD40 ligand-specific cytokine and chemokine production. Blood. 2008;111(5):2733–43. doi: 10.1182/blood-2007-03-080994. [DOI] [PubMed] [Google Scholar]
- 12.Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood) 2006;231(5):534–44. doi: 10.1177/153537020623100507. [DOI] [PubMed] [Google Scholar]
- 13.Phillips DJ, Woodruff TK. Inhibin: actions and signalling. Growth Factors. 2004;22(1):13–8. doi: 10.1080/08977190410001688687. [DOI] [PubMed] [Google Scholar]
- 14.Aleman-Muench GR, Soldevila G. When versatility matters: activins/inhibins as key regulators of immunity. Immunology and cell biology. 2012;90(2):137–48. doi: 10.1038/icb.2011.32. [DOI] [PubMed] [Google Scholar]
- 15.Robson NC, Wei H, McAlpine T, Kirkpatrick N, Cebon J, Maraskovsky E. Activin-A attenuates several human natural killer cell functions. Blood. 2009;113(14):3218–25. doi: 10.1182/blood-2008-07-166926. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J Immunol. 2006;177(10):6787–94. doi: 10.4049/jimmunol.177.10.6787. [DOI] [PubMed] [Google Scholar]
- 17.Segerer SE, Muller N, Brandt J, Kapp M, Dietl J, Reichardt HM, et al. The glycoprotein-hormones activin A and inhibin A interfere with dendritic cell maturation. Reproductive biology and endocrinology : RB&E. 2008;6:17. doi: 10.1186/1477-7827-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scutera S, Riboldi E, Daniele R, Elia AR, Fraone T, Castagnoli C, et al. Production and function of activin A in human dendritic cells. European cytokine network. 2008;19(1):60–8. doi: 10.1684/ecn.2008.0121. [DOI] [PubMed] [Google Scholar]
- 19.Musso T, Scutera S, Vermi W, Daniele R, Fornaro M, Castagnoli C, et al. Activin A induces Langerhans cell differentiation in vitro and in human skin explants. PloS one. 2008;3(9):e3271. doi: 10.1371/journal.pone.0003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15(2):289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 21.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. The Journal of experimental medicine. 1999;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nature reviews Rheumatology. 2014;10(6):365–73. doi: 10.1038/nrrheum.2014.33. [DOI] [PubMed] [Google Scholar]
- 23.Kern C, Cornuel JF, Billard C, Tang R, Rouillard D, Stenou V, et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood. 2004;103(2):679–88. doi: 10.1182/blood-2003-02-0540. [DOI] [PubMed] [Google Scholar]
- 24.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. The Journal of experimental medicine. 1998;188(6):1185–90. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreaux J, Veyrune JL, De Vos J, Klein B. APRIL is overexpressed in cancer: link with tumor progression. BMC cancer. 2009;9:83. doi: 10.1186/1471-2407-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Sun L, Lin S, Zhao R, Zhou L, Fang D, et al. BlyS is up-regulated by hypoxia and promotes migration of human breast cancer cells. Journal of experimental & clinical cancer research : CR. 2012;31:31. doi: 10.1186/1756-9966-31-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Carlo E, D’Antuono T, Pompa P, Giuliani R, Rosini S, Stuppia L, et al. The lack of epithelial interleukin-7 and BAFF/BLyS gene expression in prostate cancer as a possible mechanism of tumor escape from immunosurveillance. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(9):2979–87. doi: 10.1158/1078-0432.CCR-08-1951. [DOI] [PubMed] [Google Scholar]
- 28.Vincent FB, Morand EF, Mackay F. BAFF and innate immunity: new therapeutic targets for systemic lupus erythematosus. Immunology and cell biology. 2012;90(3):293–303. doi: 10.1038/icb.2011.111. [DOI] [PubMed] [Google Scholar]
- 29.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 30.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Seminars in immunology. 2006;18(5):263–75. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine & growth factor reviews. 2013;24(3):203–15. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. The Journal of experimental medicine. 1999;189(11):1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97(1):198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 34.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature immunology. 2002;3(9):822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Seo GY, Kim PH. Activin A Stimulates Mouse APCs to Express BAFF via ALK4-Smad3 Pathway. Immune network. 2011;11(4):196–202. doi: 10.4110/in.2011.11.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Molecular immunology. 2005;42(7):763–72. doi: 10.1016/j.molimm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 37.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107(2):594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loomans HA, Andl CD. Intertwining of Activin A and TGFbeta Signaling: Dual Roles in Cancer Progression and Cancer Cell Invasion. Cancers. 2015;7(1):70–91. doi: 10.3390/cancers7010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinz M, Niederleithner HL, Puujalka E, Soler-Cardona A, Grusch M, Pehamberger H, et al. Activin A is anti-lymphangiogenic in a melanoma mouse model. The Journal of investigative dermatology. 2015;135(1):212–21. doi: 10.1038/jid.2014.328. [DOI] [PubMed] [Google Scholar]
- 40.Reis FM, Cobellis L, Tameirao LC, Anania G, Luisi S, Silva IS, et al. Serum and tissue expression of activin a in postmenopausal women with breast cancer. The Journal of clinical endocrinology and metabolism. 2002;87(5):2277–82. doi: 10.1210/jcem.87.5.8512. [DOI] [PubMed] [Google Scholar]
- 41.Matsuyama T, Ishikawa T, Okayama T, Oka K, Adachi S, Mizushima K, et al. Tumor inoculation site affects the development of cancer cachexia and muscle wasting. International journal of cancer Journal international du cancer. 2015 doi: 10.1002/ijc.29620. [DOI] [PubMed] [Google Scholar]
- 42.Shan Y, Li S. Expression of Cripto-1 gene protein and Activin-A in human lung adenocarcinoma tissue. Pakistan journal of pharmaceutical sciences. 2015;28(2 Suppl):739–43. [PubMed] [Google Scholar]
- 43.Wilson C, Ottewell P, Coleman RE, Holen I. The differential anti-tumour effects of zoledronic acid in breast cancer - evidence for a role of the activin signaling pathway. BMC cancer. 2015;15:55. doi: 10.1186/s12885-015-1066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razanajaona D, Joguet S, Ay AS, Treilleux I, Goddard-Leon S, Bartholin L, et al. Silencing of FLRG, an antagonist of activin, inhibits human breast tumor cell growth. Cancer research. 2007;67(15):7223–9. doi: 10.1158/0008-5472.CAN-07-0805. [DOI] [PubMed] [Google Scholar]
- 45.Le Bras GF, Loomans HA, Taylor CJ, Revetta FL, Andl CD. Activin A balance regulates epithelial invasiveness and tumorigenesis. Laboratory investigation; a journal of technical methods and pathology. 2014;94(10):1134–46. doi: 10.1038/labinvest.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.